저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
i
Identification of Undifferentiated
Human Embryonic Stem
Cell-Specific Genes
by
Dong Chul Kim
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Identification of Undifferentiated
Human Embryonic Stem Cell-Specific
Genes
by
Dong Chul Kim
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Master of Neuroscience
Supervised by
Myung Ae Lee, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
iii
This certifies that the dissertation
of Dong Chul Kim is approved.
SUPERVISORY COMMITTEE
Myung Ae Lee
Hye Sun Kim
Joon Kyu Lee
The Graduate School, Ajou University
December, 21st, 2009
-
ABSTRACT
-Identification of Undifferentiated Human Embryonic Stem
Cell-Specific Genes
Embryonic stem cells are derived from mammalian blastocysts and self-renewal and maintain pluripotency, an ability to differentiate into all types of somatic and germ cells. Recent reports showed that several genes such as Pou5F1 (Oct4, Oct3/4), Sox2, Nanog, LIN28, KLF4, Myc(c-Myc) maintained a pluripotent undifferentiated state. As we know, both stem cells and cancer cells have self-renewal ability. To investigate for the mechanism that maintain the self-renewal and proliferation ability, we identified 11 genes that are specifically and commonly expressed in human ES cells and various cancer cells by digital differential display of expressed sequence tag databases. Then, we confirmed differential expression of these genes by RT-PCR in human embryonic stem cell, neural stem cells and normal tissues, and identified that 3 genes, KIAA1922, SELV, and OR56B1. These genes are strongly expressed human embryonic stem cells in their undifferentiated state and represent the core key mammalian stemness factors. Understanding the molecular mechanism by which self-renewal is maintained in human embryonic stem cells is important for the development of improved methods to derive, culture and differentiate these into cells of potential therapeutics.
Key words: Embryonic stem cell, self-renewal, stemness, KIAA1922, SELV, OR56B1
v
TABLE OF CONTENTS
ABSTRACT ……….... i
TABLE OF CONTENTS ……….……...…ii
LIST OF FIGURE ………..……….iii
LIST OF TABLE ……….…...…iv
I. INTRODUCTION ……….……….1
II. MATERIALS AND METHODS ………..2
1. Digital differential display ……….…………..2
2. Cell culture ………..….……2
3. RNA preparation and reverse transcription-polymerase chain reaction ……….….……2
4. Construction of the expression vector ………...……….………..3
5. Transfection ……….……….……3
6. Cell cycle analysis ……….………..……….3
III. RESULTS ……….…………..………..5
1. Selection of highly expression genes in human embryonic stem cell …….……….5
2. mRNA expression pattern of selected genes ……….……..…….5
IV. DISCUSSION ………...…….9
V. CONCLUSION…..………...….………..11
REFERENCE ………...12
LIST OF FIGURES
Fig. 1. Browsing data from the Digital Differential Display database··· 6
vii
LIST OF TABLES
Table 1. Oligonucleotide primers used for RT-PCR analysis …….………. 3
Table 2. Identification of top 10 genes with highest enrichment in hES cells
I. INTRODUCTION
Human embryonic stem cells (hESCs) derived from the inner cell masses (ICM) of blastocysts are pluripotent cells, which have the capacity to self-renew and to differentiate into a wide variety of tissues exhibiting characteristics of all three germ layers in vitro and in
vivo and yet still retain a normal karyotype(Thomson, 1998; Reubinoff, 2000). These unique
properties make them exceptionally valuable for cell replacement therapies, drug discovery and regenerative medicine. Since the development of this technique, several variations for the derivation and maintenance of hESC were reported( Xu, 2001; Xu, 2005; Amit, 2004; Klimanskaya, 2005; Stojkovic, 2005). In order to use like this ability it stands, we need for understand it's ability of undifferentiation and proliferation. Recent reports showed that several genes such as Pou5F1 (Oct4, Oct3/4), Sox2, Nanog, LIN28, KLF4, Myc(c-Myc) maintained a pluripotent undifferentiated state. These genes are also known as important factor of IPS(Induced pluripotent stem cell) generation(Kazutoshi, 2007; Junying, 2007).
According to recently studies, stem cells, including emberyonic stem cells, are similar to cancer cells(Dick JE, 2008). Futhermore, Human embryonic stem cell genes(OCT4, NANOG) are expressed in several cancer cells (Ezeh, 2005). Therefore, we compared gene expression between human embryonic stem cells and cancer cells except other tissue cells. There were 11 genes selected in common expression between two cells. Then we confirmed that 11 genes are expressed in human embryonic stem cell with other cells. Finally we choose 3 genes which is confirmed that expressed in human embryonic stem cells.
In this study, we expect that the 3 genes regulate self-renewal and proliferation ability in human embryonic stem cells and control of tumor formation when human embryonic stem cells are directly injected for stem cell therapy.
- 2 -
II. MATERIAL AND METHOD
1. Digital differential displayIn a typical DDD experiment the user must select which tissue libraries are to be assinged to each pool. The pools will then be compared. DDD compares the EST constituents of various tisue types, depending on which libraries are selected thereby determining the relative representation of each sequence in the libraries being compared. The DDD output is in the form of a web file that has links to Unigene clusters that correspond to the EST's that are differentially expressed between the two tissues
2. Cell culture
Immotalized human neural stem cell lines(NSCs) HB1.F3, F4, F5, human embeyonic kidney cell line (HEK293), and human neuroblastoma cell line(SH-SY5Y) was maintained and passaged on uncoated culture dishes in Dulbecco's modified medium (DMEM, Sigma-aldrich) with 10% Fetal bovine serum (FBS, Hyclone), 10 μg/ml penicillin-streptomycin (Gibco). PA317 (Mouse amphotropic packaging cell line) were cultured in DMEM(Sigma-aldrich) with 10% FBS (Hyclone) and 10 μg/ml penicillin-streptomycin (Gibco).
3. RNA preparation and reverse transcription-polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen) from cell lines and human tissues as described previously(Bonny, 1995). cDNA was synthesized using 2 μg total RNA in the presence of Superscript II and oligo(dT) 12-18 (Invitrogen). The PCR was performed in a 50 μl reaction solution containing 5 μl 10x PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 10 pmol primer, 10x diluted cDNA, and 1U of Taq DNA Polymerase (Invitrogen). The PCR condition were as follow : 30 - 34 cycle as 94°C for 45 seconds, 58 - 65°C for 45 seconds,
72°C for 45 seconds and a final extension for 10 minutes as 72°C. PCR products were separated in a 1% agarose gel with TAE buffer(Table. 1).
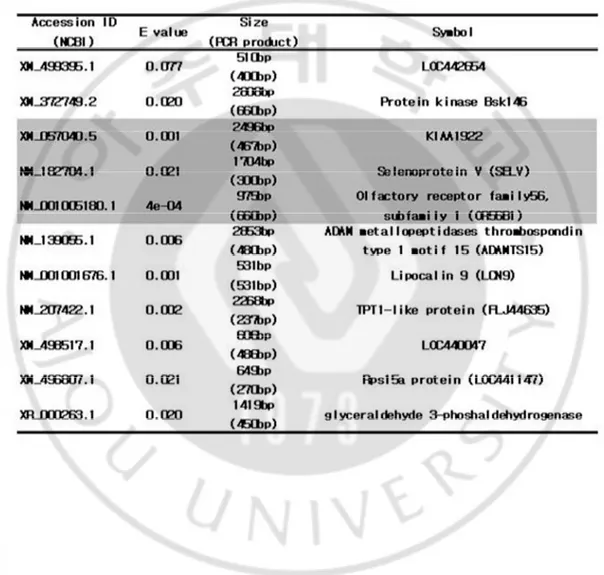
Table 1. Oligonucleotide primers used for RT-PCR analysis
Gene Foward primer Reverse primer Product size
KIAA1922 CAGACCACAGACCTCTGGAC GTGGCCTCCTGCTTCGCCTGCC 467bp
SELV CCCAGATTCCCACTCTGGTCC TCCTCGGGCAACAAAGGCAGC 300bp
OR56B1 ATGAATCATATGTCTGCATCTCTC TAGATCACTCCCCATTCCAAGC 660bp
4. Construction of the expression vector
The cDNA of 3 genes, KIAA1922(RZPD, Germany), SELV, OR56B1(Origene, USA) were cloned into the pLPCX(promega) expression vector. The resulting constructs were confirmed by nucleotide sequencing.
5. Transfection
Transfection were done using LIPOFECTAMINE PLUS reagent(Invitrogen) according to the menufacturer's instructions. Total 2μg of DNA was used in transfection. Cells were transfected at 60~80% confluency in 35mm dishes. 24hr later, transfected cells were transferred to a medium containing puromycin (Invitrogen) at a final concentration of 2.5 μg/mL. After this selection, cells were maintained in DMEM(Sigma-aldrich) with 10% FBS (Hyclone) and 10 μg/ml penicillin-streptomycin (Gibco).
6. Cell cycle analysis
Cells were seeded onto 35mm dishes. After transfection, cells were harvested and fixed in 70% ethanol and stored overnight at 4°C. Followed by PBS washing, the pellet was
- 4 -
dissolved in RNaseA solution(0.5mg/ml) and incubated at 37°C for 15 minutes, stained with propidium iodide(10 μg/ml) (Sigma) for 30 minutes in the dark at 37°C. For each sample at least 1 × 105 cells were analyzed using a FACS-Calibur cytometer (Becton Dickinson, San
III. RESULT
Selection of highly expression genes in human embryonic stem cell
As mentioned above, human embryonic stem cells are maintained there pluripotency by
several important genes. Such as Oct4, Nanog, Sox2, Klf4 genes are already well known factors of human embryonic stem cell. Except these genes, we are searched for novel genes which is regulation of human embryonic stem cell undifferentiation state. So, we select high expressed genes by digital differential display of expressed sequence tag databases(Fig. 1). Selected genes were not only expressed in human embryonic stem cells but also expressed in cancer cells(Fig.1D). Because, these two cells have similar cell mechanisms and human embryonic stem cells have potential ability of tumor formation.
mRNA expression pattern of selected genes
According to our result, we selected 10 highly expressed genes both human embryonic
stem cells and cancer cells except other tissue cells by computer database(Table. 2). After this result, we confirmed that these 10 genes were really expressed in human embryonic stem cells. Finally, we identify 3 genes expression in human embryonic stem cell. Next, we compared expression pattern of 3 genes with various human tissues(Fig.2A) and cell lines(Fig.2B).
We expect that these genes are playing a important role in human embryonic stem cells especially self-renewal and tumor formation.
- 6 -
Fig.1 Browsing data from the Digital Differential Display database. (A) Tissues were
chosen as an interesting target. (B) The system returns the numbers of UniGenes of different specificities in each tissue. (C) After clicking a number on the last page, the system returns target tissues UniGenes matching the chosen criteria. (D) After then, users can set parameters for comparisons with secondary tissues.
Table 2. Identification of top 11 genes with highest enrichment in hES cells by digital differential display.
- 8 -
Fig.2 Identification of selected gene expression by RT-PCR. (A) mRNA expression of 3
genes were compared in human nomal tissues. (B) Neural stem cell lines and cancer cell lines.
IV. DISCUSSION
Prior to this study, several marker genes are investigated in human embryonic stem cells. As we know that, Oct4, Nanog, Sox2, Klf4 genes are highly expressed in human embryonic stem cells. These genes are also used for reprogramming somatic cells and turn into pluripotent stem cells. These results indicated that stemness regulated by several important key factors. However, Oct4, Nanog genes are also expressed in cancer cell lines(Ezeh, 2005). Moreover, embryonic stem cell still have a potential of tumor formation(Yang, 2008; Lensch, 2007; Prokhorova, 2008). Because of these facts, we selected genes which expressed both of human embryonic stem cells and cancer cells but does not expressed in other tissues. The antibodies of seleted genes are not exist yet, so we confirmed expression pattern by RT-PCR. According to our result, we sorted 11 genes that expressed in both human embryonic stem cells and cancer cells by digital differential display of expressed sequence tag databases. Then we performed RT-PCR for confirming of gene expression pattern. There are 3 genes which expressed highly in human embryonic stem cells compared with other tissue and neural stem cells. We expect that these genes are regulating of stemness in human embryonic stem cells. We think that these genes are not only regulated self-renewal and proliferation but also tumor formation ability. One of the difficult part for applied human embryonic stem cells to stem cell therapy is tumor formation possibility. If these genes are regulation of these potential, it is possible that human embryonic stem cells treat directly to patients without fear of tumor formation. These important genes belong to complex transcription network(Babaie, 2007). It means that maintain of stemness is regulated by activity of many related genes. We expect that selected genes are member of this network.
- 10 -
Next, we established expression retroviral vector with 3 genes. cDNA of these genes were cloned into pLPCX retroviral vector. Then, we transfected into neural stem cell line(HB1.F3) and check the cell alteration. After transfection, we found cell morphology was significant changed during 1 month but after 1month, this changes were disappeared. This situation maybe caused by loss of CMV promoter activity. But we discovered that one of the 3 genes caused necrosis cell morphology. In addition, transfected cells are showed cell cycle arrest or activation. So, we performed cell cycle analysis and confirmed this situation.
There are several studies that maintaining pluripotency caused by various of signaling pathways(Humphrey, 2004; Xiao, 2006; Smith, 2007; Li, 2007). We expect that there are some related pathways with our genes and need more furher studies of these cell mechanisms. If these mechanisms are identified, it is very useful for studies in stem cell research and clinical application.
V. CONCLUSION
In this study, we confirmed highly expression genes in human embryonic stem cells and these genes had some kind of effect to other cells. Therefore, these genes have important role in human embryonic stem cells. Especially, OR56B1 gene seems to activate cell cycle in neural stem cell and induce there morphological change.
This result provide a basic platform for further characterizing human embryonic stem cell self-renewal and differentiation using genomic, proteomic, and functional high-throughput screening approaches, and may ultimately lead to the development of novel therapies.
- 12 -
REFERENCE
1. Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod 70(3):837-845, 2004
2. Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol Reprod 68(6):2150-2156, 2003
3. Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, Groth D, Lehrach H, Burdon T, Adjaye J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells 25(2):500-510, 2007
4. Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol Reprod Dev 75(5):818-827, 2008
5. Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase.
J Cell Physiol 209(3):883-893, 2006
6. Becker KA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J Cell
Physiol 210(2):517-526, 2007
7. Cai L, Ye Z, Zhou BY, Mali P, Zhou C, Cheng L. Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res 17(1):62-72, 2007
8. Chiao E, Kmet M, Behr B, Baker J. Derivation of human embryonic stem cells in standard and chemically defined conditions. Methods Cell Biol 86:1-14, 2008
9. Darr H, Mayshar Y, Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development 133(6):1193-1201, 2006
10. Dick JE. Stem cell concepts renew cancer research. Blood 112(13):4793-4807, 2008
11. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma.
Cancer 104(10):2255-2265, 2005
12. Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells 26(8):1931-1938, 2008
- 14 -
effect of actin disrupting agents on contact guidance of human embryonic stem cells.
Biomaterials 28(28):4068-4077, 2007
14. Heins N, Englund MC, Sjöblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells.
Stem Cells 22(3):367-376, 2004
15. Hovatta O, Mikkola M, Gertow K, Strömberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andäng M, Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum
Reprod 18(7):1404-1409, 2003
16. Hong-mei P, Gui-an C. Serum-free medium cultivation to improve efficacy in establishment of human embryonic stem cell lines. Hum Reprod 21(1):217-222, 2006
17. Humphrey RK, Beattie GM, Lopez AD, Bucay N, King CC, Firpo MT, Rose-John S, Hayek A. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells 22(4):522-530, 2004
18. James D, Noggle SA, Swigut T, Brivanlou AH. Contribution of human embryonic stem cells to mouse blastocysts. Dev Biol 295(1):90-102, 2006
pluripotency of embryonic stem cells. Cell 132(6):1049-1061, 2008
20. Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet 365(9471):1636-1641, 2005
21. Lam H, Patel S, Wong J, Chu J, Lau A, Li S. Localized decrease of beta-catenin contributes to the differentiation of human embryonic stem cells. Biochem Biophys Res
Commun 372(4):601-606, 2008
22. Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell 1(3):253-258, 2007
23. Li O, Li J, Dröge P. DNA architectural factor and proto-oncogene HMGA2 regulates key developmental genes in pluripotent human embryonic stem cells. FEBS Lett 581(18):3533-3537, 2007
24. Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal.
Differentiation 75(4):299-307, 2007
25. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of
- 16 -
pluripotency in mouse epiblast and ES cells. Cell 113(5):631-642, 2003
26. Navarro-Alvarez N, Soto-Gutierrez A, Yuasa T, Yamatsuji T, Shirakawa Y, Nagasaka T, Sun SD, Javed MS, Tanaka N, Kobayashi N. Long-term culture of Japanese human embryonic stem cells in feeder-free conditions. Cell Transplant 17(1-2):27-33, 2008
27. Nakayama M, Iida M, Koseki H, Ohara O. A gene-targeting approach for functional characterization of KIAA genes encoding extremely large proteins. FASEB J 20(10):1718-1720, 2006
28. Nieto A, Cabrera CM, Catalina P, Cobo F, Barnie A, Cortés JL, Barroso del Jesus A, Montes R, Concha A. Effect of mitomycin-C on human foreskin fibroblasts used as feeders in human embryonic stem cells: immunocytochemistry MIB1 score and DNA ploidy and apoptosis evaluated by flow cytometry. Cell Biol Int 31(3):269-278, 2007
29. Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19(3):193-204, 2001
30. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 448(7151):313-317, 2007
31. Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH. In vitro and in vivo
analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem 92(5):1265-1276, 2005
32. Pébay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells 23(10):1541-1548, 2005
33. Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Burns JS, Schroeder HD, Kassem M. Teratoma Formation by Human Embryonic Stem Cells is site-dependent and enhanced by the presence of Matrigel. Stem Cells Dev 2008
34. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18(4):399-404, 2000
35. Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 21(5):546-556, 2003
36. Sartipy P, Strehl R, Björquist P, Hyllner J. Low molecular weight compounds for in vitro fate determination of human embryonic stem cells. Pharmacol Res 58(2):152-157, 2008
- 18 -
37. Salli U, Fox TE, Carkaci-Salli N, Sharma A, Robertson GP, Kester M, Vrana K. Propagation of undifferentiated human embryonic stem cells with nano-liposomal ceramide. Stem Cells Dev 2008
38. Schmiedeberg K, Shirokova E, Weber HP, Schilling B, Meyerhof W, Krautwurst D. Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. J Struct Biol 159(3):400-412, 2007
39. Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci U S A 105(12):4820-4825
40. Sjögren-Jansson E, Zetterström M, Moya K, Lindqvist J, Strehl R, Eriksson PS. Large-scale propagation of four undifferentiated human embryonic stem cell lines in a feeder-free culture system. Dev Dyn 233(4):1304-1413, 2005
41. Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol 313(1):107-117, 2008
42. Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, Murdoch A, Strachan T, Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells 23(3):306-314, 2005
43. Synnergren J, Giesler TL, Adak S, Tandon R, Noaksson K, Lindahl A, Nilsson P, Nelson D, Olsson B, Englund MC, Abbot S, Sartipy P. Differentiating human embryonic stem cells express a unique housekeeping gene signature. Stem Cells 25(2):473-480, 2007
44. Tan SM, Wang ST, Hentze H, Dröge P. A UTF1-based selection system for stable homogeneously pluripotent human embryonic stem cell cultures. Nucleic Acids Res 35(18):e118, 2007
45. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861-872, 2007
46. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145-1147, 1998
47. Valdimarsdottir G, Mummery C. Functions of the TGFbeta superfamily in human embryonic stem cells. APMIS 113(11-12):773-789, 2005
48. Vieyra DS, Goodell MA. Pluripotentiality and conditional transgene regulation in human embryonic stem cells expressing insulated tetracycline-ON transactivator. Stem Cells 25(10):2559-2266, 2007
- 20 -
49. Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, Brimble SN, McLean A, Galeano MJ, Uhl EW, D'Amour KA, Chesnut JD, Rao MS, Blau CA, Robins AJ. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110(12):4111-4119, 2007
50. Walker E, Ohishi M, Davey RE, Zhang W, Cassar PA, Tanaka TS, Der SD, Morris Q, Hughes TR, Zandstra PW, Stanford WL. Prediction and testing of novel transcriptional networks regulating embryonic stem cell self-renewal and commitment. Cell Stem Cell 1(1):71-86, 2007
51. Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, Meng S, Feng J, Miao C, Ding M, Li D, Deng H. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem Biophys Res Commun 330(3):934-942, 2005
52. Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state.
Nature 448(7151):318-324, 2007
53. Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem
cells. Stem Cells 24(6):1476-1486, 2006
54. Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19(10):971-974, 2001
55. Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2(3):185-190, 2005
56. Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O'Sullivan C, Delavan-Boorsma K, Mok M, Bronstein A, Carpenter MK. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem
Cells 23(3):315-323, 2005
57. Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A 103(18):6907-6912, 2006
58. Yang S, Lin G, Tan YQ, Zhou D, Deng LY, Cheng DH, Luo SW, Liu TC, Zhou XY, Sun Z, Xiang Y, Chen TJ, Wen JF, Lu GX. Tumor progression of culture-adapted human embryonic stem cells during long-term culture. Genes Chromosomes Cancer 47(8):665-679, 2008
- 22 -
59. Yeo S, Jeong S, Kim J, Han JS, Han YM, Kang YK. Characterization of DNA methylation change in stem cell marker genes during differentiation of human embryonic stem cells. Biochem Biophys Res Commun 359(3):536-542, 2007
60. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917-1920, 2007
61. Zhao M, Ren C, Yang H, Feng X, Jiang X, Zhu B, Zhou W, Wang L, Zeng Y, Yao K. Transcriptional profiling of human embryonic stem cells and embryoid bodies identifies HESRG, a novel stem cell gene. Biochem Biophys Res Commun 362(4):916-922, 2007
62. Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells.
- 국문요약 -
미분화상태의 인간배아줄기세포에서 특이적으로 발현하는 유전
자의 동정과 기능 연구
아주대학교 대학원 의생명과학과 김 동 철 (지도교수 : 이 명 애) 포유류의 배반포에서부터 유래한 배아줄기세포는 늙지않고 다능성을 지니며 체세포나 생식세포등의 모든 형태로 분화가 가능하다. 최근 연구결과에 의하면 Pou5F1(Oct4, Oct3/4), Sox2, Nanog, LIN28, KLF4, Myc(c-Myc) 과 같은 유전자들이 배아줄기세포의 미분화상태를 유지시켜준다. 어떠한 메커니즘으로 인 해 미분화상태가 유지되어지는지 확인하기위해 우리는 digital differential display of expressed sequence tag databases를 통하여 인간배아줄기세포와 여러가지 다양한 암세포들에서 공통적으로 발현하는 유전자 10개를 골라내었다. 그리고 RT-PCR을 통하여 실제로 이들 유전자가 발현하는지 인간배아줄기세포, 신경줄기세포, 정상조직들로 확인해보았다. 그중 KIAA1922, SELV, OR56B1 유전자의 발현을 확인할수 있었다. 이들 유전자는 미분화상태의 인간배아줄기세 포에서 강하게 발현하며 이것은 세포의 줄기성을 유지하는데에 핵심적인 역할을- 24 -
하고있다는 것을 나타낸다. 인간배아줄기세포의 다능성 유지에 관한 분자적 메커 니즘을 밝혀낸다면 추후 이들 세포에 관한 연구나 치료목적을 위한 연구분야에 큰 공헌을 할 것이라 기대된다.
핵심어 : 배아줄기세포, 다능성, 줄기성, KIAA1922, SELV, OR56B1