Master's Thesis of Science in Medicine
Therapeutic hypothermia after recanalization in
acute ischemic stroke patients:
Analyses of multicenter, consecutively-enrolled,
observational, retrospective endovascular treatment
registry (ASIAN-KR)
Ajou University Graduate School
Science in Medicine Major
Therapeutic hypothermia after recanalization in acute
ischemic stroke patients: Analyses of a multicenter,
consecutively-enrolled, observational, retrospective
endovascular treatment registry (ASIAN-KR)
Ji Man Hong, Advisor
I submit this thesis as the
Master's thesis of Science in Medicine.
Feb, 2019
Ajou University Graduate School
Science in Medicine Major
i
-ABSTRACT-
Therapeutic hypothermia after recanalization in acute ischemic stroke
patients: Analyses of a multicenter, consecutively-enrolled, observational,
retrospective endovascular treatment registry (ASIAN-KR)
The results of large randomized studies showed that endovascular therapy (EVT) is effective in patients with acute ischemic stroke patients with large vessel occlusion, and recanalization therapy have become an indispensable treatment for the acute ischemic stroke. However, it is unclear whether EVT would be beneficial for stroke involving the whole territory of the middle cerebral artery (MCA), called ‘malignant MCA infarction’. Malignant middle cerebral artery (MCA) infarction is associated with a dismal prognosis regardless of maximum intensive care and timely carrying decompressive hemicranietomy.
Due to evolution of treatment modality about EVT, the treatment direction to reduce reperfusion injury has come to a new horizon. After recanalization, reperfusion injury, which is expressed by edema and hemorrhagic transformation occurs, and several medical therapies that minimize such neuronal damage have been studied. One of them is a therapeutic hypothermia, and there are not many studies that have been verified as a multi-center trial. Therefore, we aimed to evaluate the efficacy of therapeutic hypothermia in acute ischemic stroke patients after endovascular recanalization therapy. We also investigated the therapeutic hypothermia effect in subgroups that included only malignant MCA infarct patients.
The ASIAN KR, a multi-center, retrospective, neurointervention registry in South Korea was analyzed from January 2011 to May 2016. We investigated baseline demographics, risk factors, endovascular treatment parameters, and outcome. Clinical and radiological outcomes were compared between therapeutic hypothermia (TH) and no therapeutic hypothermia (NH) group. Patients with anterior circulation were selected from all patients, malignant MCA infarction is determined as (1) baseline Alberta Stroke program early CT (ASPECT) below 6 and (2) diffusion-weighted imaging (DWI) lesion volume measurement (>82 ml) or National Institutes of Health Stroke Scale (NIHSS) score >20 with item Ia >0.
Among 721 consecutive patients, 548 patients with anterior circulation infarction were selected, 91 patients had therapeutic hypothermia, and 457 patients had not. TH group (vs. NH group) significantly had
ii
a low value of baseline ASPECT score (p<0.001) and a higher DWI volume (p<0.001). In order to overcome the differences in the groups, we used a propensity score matching. Even after adjusting baseline demographics as a conventional propensity method of 1:2 matching, the bias of baseline DWI volume (55.22±47.60 [n=91] vs. 37.82±64.73 [n=182], p<0.001) and EVT method (stent retriever: n=62, 68.1% vs. n=75, 41.2%; aspiration: n=19, 20.9% vs. n=74, 40,7%; others: n=10, 11.0% vs. n=33, 18.1%; p<0.001) did not balance between TH and NH groups. TH group (vs. NH group) had a lower prevalence of good outcome at 3 month (modified Rankin Scale [mRS] 1; n=22, 24.2% vs. n=70, 38.5%, p=0.019, mRS 0-2; n=35, 38.5% vs. n=101, 55.5%, p=0.008).
In subgroup analysis, 80 patients were diagnosed as malignant MCA infarction. Twenty-eight patients had received therapeutic hypothermia. Age, diabetes, baseline NIHSS score, stroke etiology were unbalanced between TH and NH groups. TH group (vs. NH group) had a better clinical outcome (32.1% vs. 7.7% p=0.009) and a lower frequency of hemorrhagic transformation (none vs. any hemorrhage, p=0.007). After adjusting potential confounders to predict good outcome, therapeutic hypothermia (OR 4.63; CI 1.20-17.89; p=0.026) and hypertension (OR 0.18; CI 0.04-0.74; p=0.018) were independent determinants.
Due to the difficulty in statistical interpretation from not balanced baseline characteristic between the TH and NH group, our current data was not to be conclusive on clinical benefit of therapeutic hypothermia in acute ischemic stroke. However, our data demonstrated that therapeutic hypothermia reduced impending hemorrhagic transformation and leaded to an improvement of short-term clinical outcome in patients with malignant MCA infarction who received EVT.
Key words: stroke, endovascular therapy, reperfusion injury, therapeutic hypothermia, malignant MCA
iii
TABLE OF CONTENTS
ABSTRACT --- i
TABLE OF CONTENTS --- iii
LIST OF FIGURES --- iv
LIST OF TABLES --- --- v
I. INTRODUCTION --- 1
II. PATIENTS AND METHODS --- 4
A. Patients and protocols --- 4
B. Endovascular treatment --- 6
C. Hypothermia treatment protocol --- 6
D. Clinical and radiological evaluation --- 6
E. Propensity score matching --- 7
F. Statistical analysis --- 8
III. RESULTS --- 9
A. Comparison between the hypothermia and no hypothermia groups --- 9
B. Non-malignant MCA group and hypothermia --- 14
C. Malignant MCA group and hypothermia --- 19
IV. DISCUSSION --- 26
A. Unmet need for additional treatment of acute ischemic stroke --- 26
B.
Neuroprotective mechanism and effect of therapeutic hypothermia on various conditions-- 26C. Therapeutic hypothermia in non-malignant MCA infarction --- 27
D. Therapeutic hypothermia in malignant MCA infarction --- 28
E. Limitations --- 29
V. CONCLUSION --- 30
REFERENCES --- 31
iv
LIST OF FIGURES
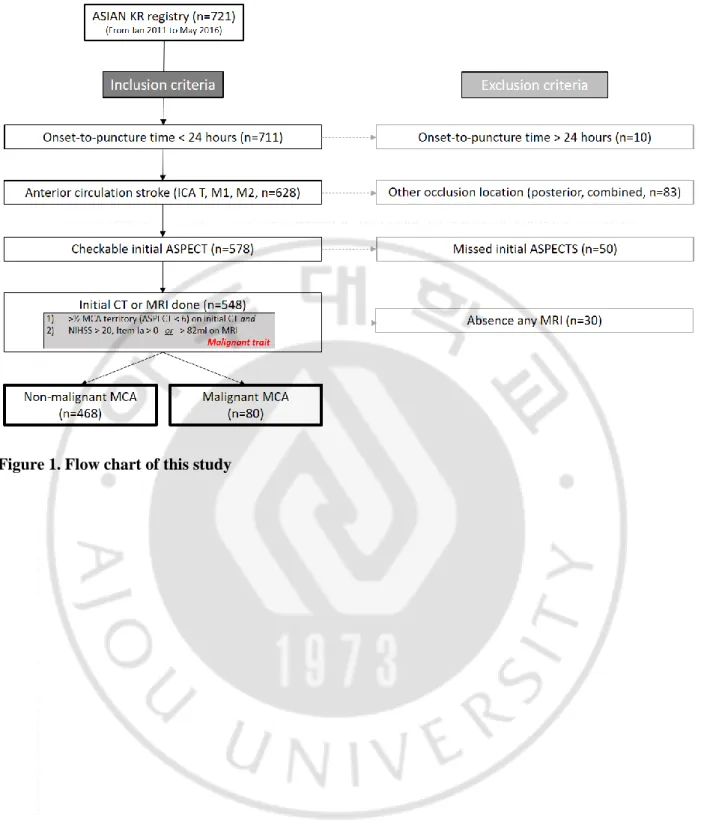
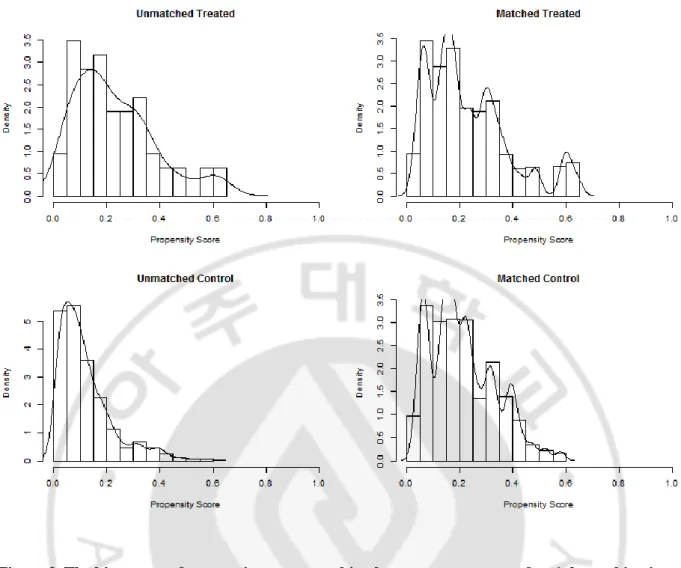
Fig 1.Flow chart of this study --- 5 Fig 2. The histogram of propensity score matching between two groups after 1:2 matching in all-included patient --- 10
Fig 3. The histogram of propensity score matching between two groups after 1:2 matching in non-malignant MCA infarction --- 15 Fig 4. Distribution of hemorrhagic transformation between the hypothermia and no hypothermia groups with malignant MCA infarction --- 23
Fig 5. Distribution of 3-month modified Rankin Scale between the hypothermia and no hypothermia groups with malignant MCA infarction --- 24
v
LIST OF TABLES
Table 1. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups ---11
Table 2. Comparison of laboratory, radiological and treatment parameters between the hypothermia and no hypothermia groups --- ---12
Table 3. Clinical outcome between the hypothermia and no hypothermia groups --- 13
Table 4. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups after 1:2 matching of non-malignant MCA group (before and after 1:2 matching) -- 16
Table 5. Comparison of radiological and treatment parameters between the hypothermia and no hypothermia groups after 1:2 matching of non-malignant MCA group (before and after 1:2 matching) -- 17
Table 6. Clinical outcome between the hypothermia and no hypothermia groups after 1:2 matching of non-malignant MCA group (before and after 1:2 matching) --- 18
Table 7. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups with malignant MCA infarction --- 20
Table 8. Comparison of radiological and treatment parameters between the hypothermia and no hypothermia groups with malignant MCA infarction --- 21
Table 9. Clinical outcome between the hypothermia and no hypothermia groups with malignant MCA infarction --- 22
1
I.
INTRODUCTION
Endovascular treatment (EVT) is now a standard treatment option in acute ischemic stroke (AIS) with a large vessel occlusion (Berkhemer et al., 2015; Goyal et al., 2015; Campbell et al., 2015; Saver et al, 2015; Jovin et al, 2015).Based on several trials, the clinical guideline is also changing, (Powers et al., 2018) and in recent years, the time window to consider EVT is also getting longer (Nogueira et al., 2018). In the meta-analysis of EVT, AIS patients with large vessel occlusion in the anterior circulation were found to be generally beneficial, but mortality and morbidity were still high (Goyal et al., 2016).
The stroke involving the whole territory of the middle cerebral artery (MCA), due to its devastating outcome with a profound fatality up to 70%, called 'malignant MCA infarction' (Hacke et al., 1996). Diffusion-weighted imaging (DWI) of magnetic resonance imaging (MRI) volume larger than 82mL predicted malignant MCA infarction with high specificity (Thomalla G et al., 2010). Because of the poor prognosis of malignant MCA infarction, decompressive surgery is often performed in addition to medical therapy including maximal intensive care. Decompressive hemicraniectomy has been used for several patients based on studies that reduce mortality at 6 and 12 months and improve functional outcome (Hofmeijer et al., 2009; Juttler et al., 2007; Vahedi et al., 2007). However, there is controversy over whether decompression surgery is performed by combining the time of operation, patient age, and the ethical limitations of previous studies (Wartenberg KE., 2012; Huttner et al., 2009). Because all patients cannot undergo surgery, maximal intensive therapy is a very important part. In addition to the poor prognosis of malignant MCA infarct itself, increased endovascular therapy has made medical therapy more significant. It is true that EVT is a new treatment for patients who cannot be recanalized with intravenous thrombolysis (IVT) with alteplase alone or who do not meet the standard of IVT. However, it is unclear whether EVT would be beneficial for this clinical entity (LC Rebello., 2017). EVT may cause excessive tissue injury due to reperfusion injury, cerebral edema, and hemorrhagic transformation.
Therefore, numerous physicians contemplate adjuvant treatment focused on neuroprotection (Tymianski. 2017). The mechanism of neuronal injury due to ischemia and reperfusion has been explained in various ways. Excitotoxicity, oxidative and nitrosative stress, and inflammation are involved in the
2
process of neuronal death (Chamorro et al., 2016; Neuhaus et al., 2017). Thus, understanding the pathway associated with each was considered a target of neuroprotection.
For decades, neuroprotection therapeutics for acute stroke showed successful results in animals but failed in humans (Gladstone et al., 2002). By 2007, there were more than 100 clinical trials and studies on experimental neuroprotective treatments targeting each part of more than 1000 ischemic cascades. However, none of them proved effectiveness (Goldstein et al., 2007). These failures can be resulted from clinical trial design including patient selection, drug dosing, treatment windows, outcome measures, and data analysis. Also, that can be resulted from the difference of human and animal model, combined injury mechanism. However, the development of EVT has caused a new phase of neuroprotection because human stroke attain ischemia and reperfusion model. In addition, research is being implemented with the combination of existing drugs and targeting specific mechanisms (Chamorro. 2018).
Multi-target neuroprotection strategies have been drawing attention in this context. Neu2000, a derivative of sulfasalazine currently undergoing phase II trials, is one of the multi-target neuroprotectants (Hong et al. 2018). Therapeutic hypothermia (TH), a non-pharmacological strategy, is also a multi-target neuroprotective strategy. TH is recognized as a standard therapy for post-cardiac arrest brain injury, the representatives of ischemia-reperfusion injury (Hypothermia after Cardiac Arrest Study Group. 2002; Holzer et al., 2005). There is debate about the use of TH in patients with ischemic stroke, but some studies have shown better outcome and safety (Rizwan et al., 2016).
HARIS study (Hong et al., 2014) showed that TH after recanalization which is similar to post-cardiac arrest may reduce risk of cerebral edema and hemorrhagic transformation, and lead to better clinical outcomes. Patients who underwent hypothermia in the study also had characteristics close to malignant MCA infarction. And, ReCCLAIM I study (Horn CM et al., 2014) showed hypothermia can be safely performed after definitive intra-arterial reperfusion therapy in patients with large pretreatment core infarcts. In recent post-reperfusion cooling study, favorable outcome (mRS ≤2) at 3 months was observed in 10 (55.6%) patients who had average DWI volume of 127.1mL (Hwang YH et al., 2017). However, there has been no study that reaffirms the effect of TH in the present state of EVT as a standard therapy. Also, recent
3
successful trials with widened EVT time criteria, patients who had a large diffusion volume were excluded (Nogueira RG et al., 2018, GW Albers et al., 2018).
In this context, we aimed to evaluate the efficacy of therapeutic hypothermia in patients of multi-center with acute ischemic stroke after endovascular therapy. We investigated the possibility that therapeutic hypothermia could have a positive effect on malignant MCA infarction which have not yet established standard therapy.
4
II.
PATIENTS AND METHOD
A. Patients and protocols
The patient data was obtained from the observational ASIAN KR registry, which included patients 18 years of age or older who underwent EVT due to an acute ischemic stroke with occlusion of intracranial and/or extracranial large vessel occlusion (Lee et al., 2018). Between January 2011 and May 2016, patients were enrolled from three comprehensive stroke centers (Ajou University Hospital [center A, Suwon], Kyungpook National University Hospital [center B, Daegu], and Keimyung University Dongsan Hospital [center C, Daegu]) in South Korea. The data was applied in accordance with the 1964 Helsinki Declaration and subsequent amendments and approved by the Institutional Review Board of each participating hospital for registry. Informed consent was waivered because this study had retrospective nature.
Seven hundred and twenty-one consecutive acute stroke patients who received endovascular treatment enrolled in this period. All centers used computed tomography (CT) and CT angiography (CTA) for baseline screening. If applicable, intravenous recombinant tissue plasminogen activator (IV-rtPA) was used before endovascular treatment. Of these, 10 were excluded due to longer onset to puncture time over 24 hours, 83 due to other occlusion location (posterior, combined). Fifty were excluded due to unavailable Alberta Stroke program early CT (ASPECT) score, and 30 patients excluded due to absence of any MRI. Therefore, the total number of cases except for exclusion criteria was 548, of which 80 were in the malignant MCA group and 468 in the non-malignant MCA group. Malignant trait were revised by several previous studies (Kim et al., 2015). First, initial CT showed >1/2 MCA territory infarction (ASPECTS<6) and the National Institutes of Health Stroke Scale (NIHSS) score >20, Item Ia > 0 or DWI volume>82mL were enrolled (Thomalla G et al., 2010). Figure 1 showed a flow chart of this study.
5
6
B. Endovascular treatment
EVT was performed by experienced neurointerventionists. The direct aspiration method, stent retrieval technique, or a combination of both methods was used for intracranial mechanical thrombectomy, according the perfrence of neurointerventionists. According to decision making, direct aspiration method (Penumbra, Alameda, CA, USA) or stent retrievers such as Solitaire AB/FR (Medtronic, Irvine, CA, USA) or Trevo (Stryker, Kalamazoo, MI, USA) were used. Sometimes, balloon guide catheters such as Cello (Fuji System Corp., Tokyo, Japan) or Optimo (Tokai Medical Products, Aichi, Japan) were used to prevent clot migration (Lee et al., 2018). If necessary, angioplasty and/or stenting of intracranial or extracranial vessels were allowed.
C. Therapeutic hypothermia protocol
After recanalization, patients expected to benefit from TH were cooled as soon as possible according to the clinician’s decision. TH were conducted using with a surface cooling device (Arctic Sun, Bard Medical, Covington, GA) (Heard KJ et al., 2010) or an endovascular cooling catheter (CoolGard, Alsius Corporation, Irvine, CA) (Al-Senani FM et al., 2004). A maximal cooling rate was applied with a target temperature of 34.5℃, and the hypothermia treatment was maintained for 48 hours. The maintenance time can be prolonged to 72 hours depending on the patients’ condition. The rewarming process was controlled as the rate of 0.5℃ elevation per every 12 hours. During hypothermia therapy, all patients were mechanically ventilated to provide airway protection and prevent aspiration pneumonia. The IV infusion of sedative drugs, anti-shivering strategies with oral buspirone and IV muscle relaxant were maintained and adjusted according to the severity of shivering.
D. Clinical and radiological evaluation
Patients’ characteristics were compared between hypothermia and no hypothermia groups in patients with non-malignant MCA infarction and malignant MCA infarction, respectively. Clinical data were
7
comprised of demographic features, stroke risk factors, premorbid status, severity and clinical outcomes, occlusion location, subtypes of stroke, use of IVT, and use of TH. The baseline neurologic assessment was performed by stroke neurologists, including the National Institutes of Health Stroke Scale (NIHSS) score. Subtypes of stroke were determined according to the classification of the Trial of Org 10172 in Acute Stroke Treatment (Adams et al., 1993) by stroke neurologists.
Baseline NIHSS score and modified Rankin scale (mRS) of patients were evaluated and clinical outcomes is assessed by the mRS at months. Mostly, the mRS score 0-2 at 3 months were classified as good outcome. Radiological data includes CT and CTA as well as initial brain magnetic resonance imaging (MRI) and some follow-up MRs have also been evaluated. The volume of stroke were measure with diffusion-weighted imaging (DWI) of MRI.
Interventional data includes time parameters-onset to puncture time, door to puncture time, median procedure time, onset to reperfusion time, and door to reperfusion time. The reperfusion status was evaluated by the modified TICI score, (Zaidat et al., 2013) and the presence of hemorrhagic transformation was investigated. Modified TICI score 2b or 3 was defined as successful reperfusion. Hemorrhagic transformation were classified in accordance with the criteria defined by the European Cooperative Acute Stroke Study (Fiorelli et al., 1999).
E. Propensity score matching
Propensity score is a tool for estimating causal effects of treatments in the presence of imbalance in baseline covariates distributions between treatment groups due to lack of randomization (Rosenbasum et al., 1983). To reduce the imbalance between hypothermia and no hypothermia groups, propensity score were calculated using multivariable logistic regression model. The model included age, sex, risk factors (hypertension, diabetes mellitus, atrial fibrillation), stroke characteristics (NIHSS, etiology, vessel occlusion site), and treatment parameters (use of IV-rtPA, onset to reperfusion time, successful recanalization). In the analysis of patients with non-malignant MCA infarction, we matched hypothermia group with no hypothermia group in a 1:2 ratio with generalized estimating equations method. The
8
standardized difference of means and ratio of the variances between the propensity scores of both groups were checked to determine the quality of the matched sample. In addition, individual covariates between groups in the matched sample were confirmed (Iacus et al., 2011).
F. Statistical analysis
Baseline characteristics were presented as means (with standard deviation) or medians (with range) in numeric variables, and frequencies and percentage in categorical variables. To test for significant differences between groups, the χ2 or Fisher exact test was used for categorical variables, and the independent t test and Mann-Whitney U test were used for numeric variables. In order to compare the two groups with different baseline characteristics, matching was attempted using the propensity score. In the analysis of malignant MCA infarction group, binary logistic regression analysis was performed to identify hypothermia therapy was an independent factor of good outcome at 3 months. Variables were selected based on the results of univariate analyses (p<0.05). Statistical analyses were performed by using SPSS for Windows (Version 18.0; IBM, Armonk, New York). P<0.005 was considered statistical significant.
9
III. RESULTS
A. Comparison between the all included patient
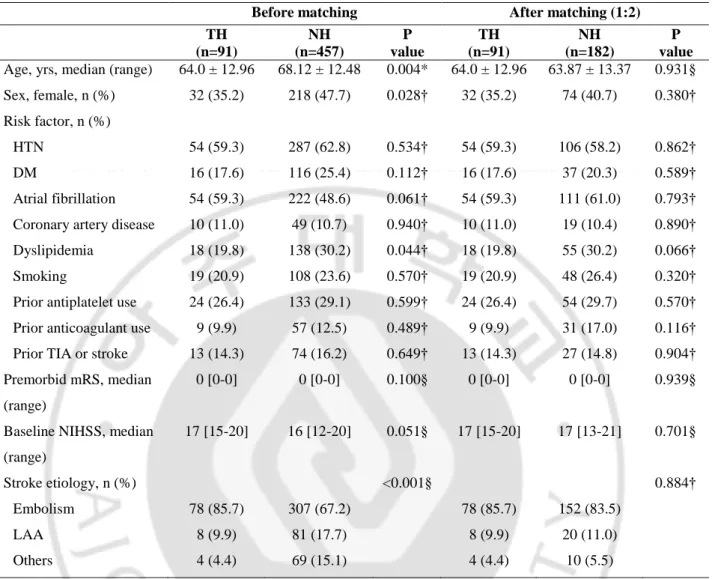
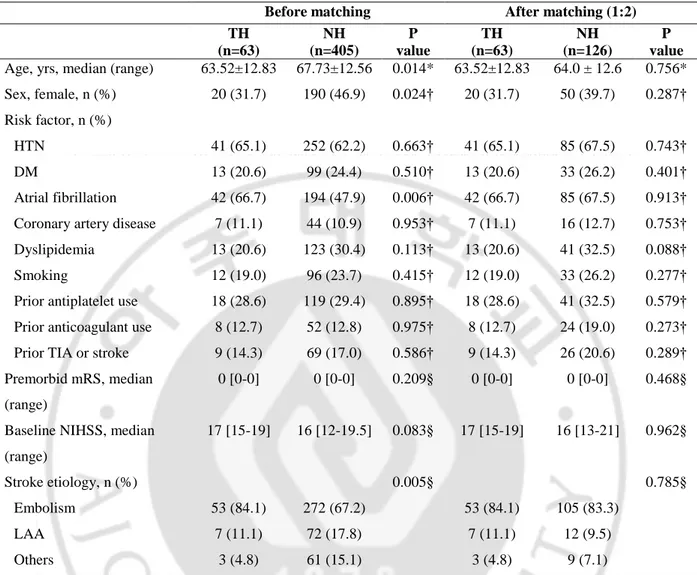
548 patients with acute ischemic stroke with anterior circulation fulfilled the selection criteria and were included in this study. 91 were included in the hypothermia group, and 457 were in the untreated group. Table 1 showed demographics and clinical characteristics between the hypothermia and no hypothermia groups. Age and sex were unbalanced between the two groups, so additional statistical matching was required and will be introduced in the next part.
General stroke risk factors and the ratio of patients with prior antiplatelet or anticoagulant use were similar in the two groups. There was no difference in premorbid mRS and initial NIHSS in both groups. The TH group (vs. NH group) showed significantly different distribution of stroke etiology (p=0.001), 78 embolism patients (86.2%), 8 ICAS patients (9.9%), and 4 other etiology patients (4.4%).
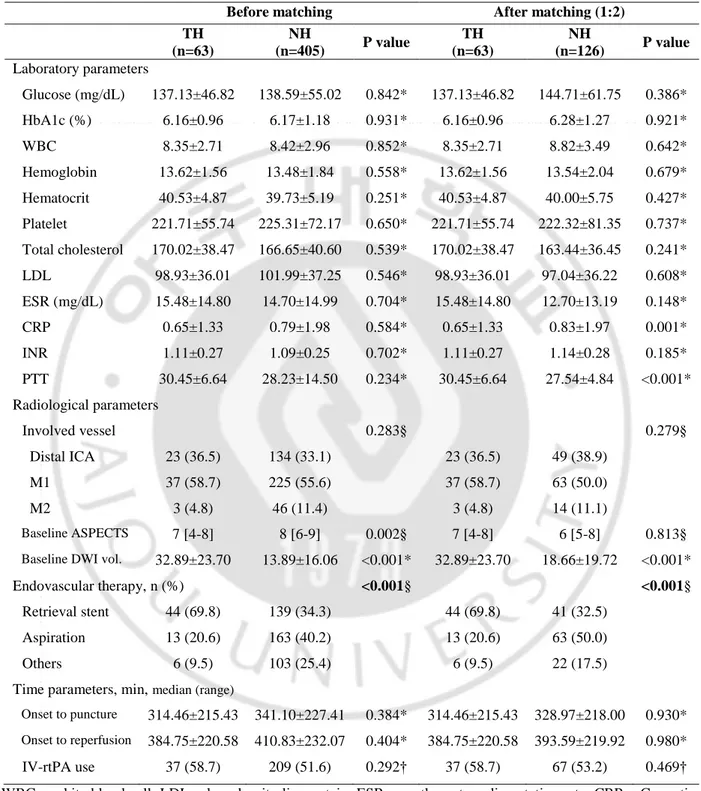
Laboratory, radiological and treatment related parameters were presented in Table 2. In laboratory parameters, other results of patients were similar in the two groups. Baseline ASPECT score and DWI volume was significantly different between groups. TH group (vs. NH group) was worse ASPECT score (5 vs. 7, p<0.001) and large DWI volume (55.22±47.60 vs. 24.59±47.79, p<0.001).
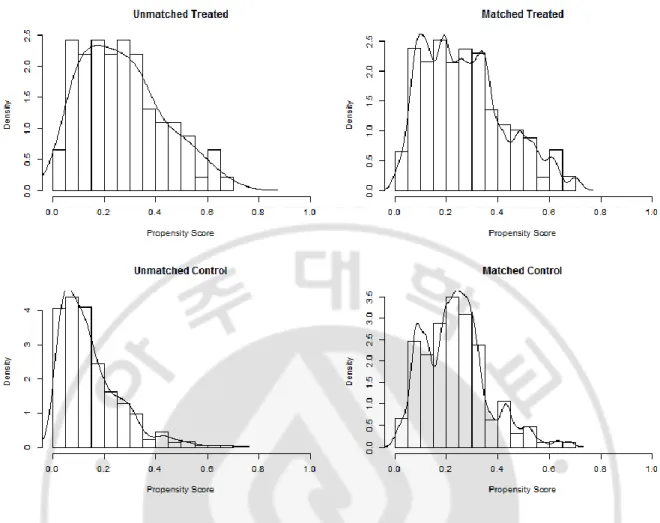
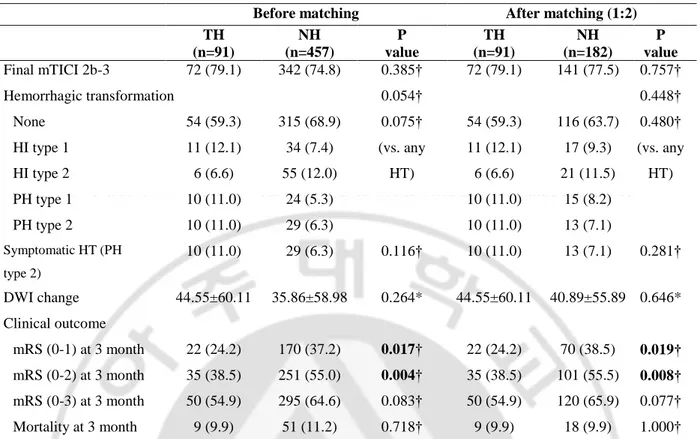
Time parameters of EVT were unbalanced also, onset to puncture time, onset to reperfusion time were different between two groups. As for the EVT method, stent retrieval was used in the TH group (68.1%) vs. 36.8%). In addition, the HT group (vs. NH group) had more IV-rtPA use (64.8% vs. 52.1%, p=0.026). In the clinical outcome (Table 3), good (mRS 0-2) outcome at 3 month were found to be worse in the hypothermia group (38.5% vs. 55.0%, p=0.004). These results suggest that the baseline characteristics are largely affected. There is a mismatch of baseline characteristics, so matching is performed for comparison of the clinical outcomes. Even after adjusting baseline demographics as a conventional propensity method of 1:2 matching, clinical outcome was the same as before the matching.
10
Figure 2. The histogram of propensity score matching between two groups after 1:2 matching in all-included patient
11
Table 1. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups
HTN = hypertension, DM = diabetes mellitus, TIA = Transient ischemic attack, mRS = modified Rankin scale, NIHSS = National Institutes of Health Stroke Scale, LAA = large artery atherosclerosis;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Before matching After matching (1:2)
TH (n=91) NH (n=457) P value TH (n=91) NH (n=182) P value Age, yrs, median (range) 64.0 ± 12.96 68.12 ± 12.48 0.004* 64.0 ± 12.96 63.87 ± 13.37 0.931§ Sex, female, n (%) 32 (35.2) 218 (47.7) 0.028† 32 (35.2) 74 (40.7) 0.380† Risk factor, n (%)
HTN 54 (59.3) 287 (62.8) 0.534† 54 (59.3) 106 (58.2) 0.862†
DM 16 (17.6) 116 (25.4) 0.112† 16 (17.6) 37 (20.3) 0.589†
Atrial fibrillation 54 (59.3) 222 (48.6) 0.061† 54 (59.3) 111 (61.0) 0.793† Coronary artery disease 10 (11.0) 49 (10.7) 0.940† 10 (11.0) 19 (10.4) 0.890† Dyslipidemia 18 (19.8) 138 (30.2) 0.044† 18 (19.8) 55 (30.2) 0.066† Smoking 19 (20.9) 108 (23.6) 0.570† 19 (20.9) 48 (26.4) 0.320† Prior antiplatelet use 24 (26.4) 133 (29.1) 0.599† 24 (26.4) 54 (29.7) 0.570† Prior anticoagulant use 9 (9.9) 57 (12.5) 0.489† 9 (9.9) 31 (17.0) 0.116† Prior TIA or stroke 13 (14.3) 74 (16.2) 0.649† 13 (14.3) 27 (14.8) 0.904† Premorbid mRS, median
(range)
0 [0-0] 0 [0-0] 0.100§ 0 [0-0] 0 [0-0] 0.939§
Baseline NIHSS, median (range) 17 [15-20] 16 [12-20] 0.051§ 17 [15-20] 17 [13-21] 0.701§ Stroke etiology, n (%) <0.001§ 0.884† Embolism 78 (85.7) 307 (67.2) 78 (85.7) 152 (83.5) LAA 8 (9.9) 81 (17.7) 8 (9.9) 20 (11.0) Others 4 (4.4) 69 (15.1) 4 (4.4) 10 (5.5)
12
Table 2. Comparison of laboratory, radiological and treatment parameters between the hypothermia and no hypothermia groups
WBC = white blood cell, LDL = low-density lipoprotein, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, INR = international normalized ratio, PTT = partial thromboplastin time, ASPECT = Alberta Stroke program early CT score;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Before matching After matching (1:2)
TH (n=91) NH (n=457) P value TH (n=91) NH (n=182) P value Laboratory parameters Glucose (mg/dL) 134.01±42.69 140.27±55.99 0.313* 134.01±42.69 138.26±54.95 0.540* HbA1c (%) 6.116±0.93 6.19±1.19 0.553* 6.116±0.93 6.160±1.28 0.637* WBC 8.35±2.69 8.53±3.13 0.617* 8.35±2.69 8.66±3.44 0.752* Hemoglobin 13.58±1.56 13.39±1.88 0.340* 13.58±1.56 13.58±1.97 0.933* Hematocrit 40.41±4.78 39.54±5.30 0.150* 40.41±4.78 40.12±5.51 0.653* Platelet 214.58±58.99 225.70±71.95 0.167* 214.58±58.99 224.10±71.90 0.220* Total cholesterol 166.89±38.00 166.48±40.00 0.928* 166.89±38.00 168.37±39.40 0.963* LDL 97.81±35.56 102.08±36.74 0.312* 97.81±35.56 102.41±36.89 0.329* ESR (mg/dL) 15.74±16.66 15.17±15.48 0.755* 15.74±16.66 12.45±13.82 0.034* CRP 0.55±1.13 0.82±2.01 0.075* 0.55±1.13 0.56±1.28 0.002* INR 1.09±0.25 1.09±0.24 0.976* 1.09±0.25 1.10±0.24 0.568* PTT 30.13±5.88 28.02±13.74 0.150* 30.13±5.88 26.89±4.74 <0.001* Radiological parameters Involved vessel 0.175† 0.510† Distal ICA 37 (40.7) 162 (35.4) 37 (40.7) 75 (41.2) M1 49 (53.8) 246 (53.8) 49 (53.8) 90 (49.5) M2 5 (5.5) 49 (10.7) 5 (5.5) 17 (9.3) Baseline ASPECTS 5 [3-8] 7 [5-9] <0.001§ 5 [3-8] 6 [5-8] 0.318§ Baseline DWI vol. 55.22±47.60 24.59±47.79 <0.001* 55.22±47.60 37.82±64.73 <0.001*
Endovascular therapy, n (%) <0.001§ <0.001§
Retrieval stent 62 (68.1) 168 (36.8) 62 (68.1) 75 (41.2)
Aspiration 19 (20.9) 175 (38.3) 19 (20.9) 74 (40.7)
Others 10 (11.0) 114 (24.9) 10 (11.0) 33 (18.1)
Time parameters, min, median (range)
Onset to puncture 285.01±193.72 342.35±228.61 0.014* 285.01±193.72 302.57±195.91 0.548* Onset to reperfusion 354.65±199.52 415.32±324.35 0.011* 354.65±199.52 375.53±207.82 0.453* IV-rtPA use 59 (64.8) 238 (52.1) 0.026† 59 (64.8) 114 (62.6) 0.722†
13
Table 3. Clinical outcome between the hypothermia and no hypothermia groups
mTICI = modified thrombolysis in cerebral infarction, HI = hemorrhagic infarct, PH = parenchymal hematoma, HT = hemorrhagic transformation, DWI = diffusion-weighted imaging, mRS = modified Rankin Scale;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Before matching After matching (1:2)
TH (n=91) NH (n=457) P value TH (n=91) NH (n=182) P value Final mTICI 2b-3 72 (79.1) 342 (74.8) 0.385† 72 (79.1) 141 (77.5) 0.757† Hemorrhagic transformation 0.054† 0.448† None 54 (59.3) 315 (68.9) 0.075† 54 (59.3) 116 (63.7) 0.480† HI type 1 11 (12.1) 34 (7.4) (vs. any HT) 11 (12.1) 17 (9.3) (vs. any HT) HI type 2 6 (6.6) 55 (12.0) 6 (6.6) 21 (11.5) PH type 1 10 (11.0) 24 (5.3) 10 (11.0) 15 (8.2) PH type 2 10 (11.0) 29 (6.3) 10 (11.0) 13 (7.1) Symptomatic HT (PH type 2) 10 (11.0) 29 (6.3) 0.116† 10 (11.0) 13 (7.1) 0.281† DWI change 44.55±60.11 35.86±58.98 0.264* 44.55±60.11 40.89±55.89 0.646* Clinical outcome mRS (0-1) at 3 month 22 (24.2) 170 (37.2) 0.017† 22 (24.2) 70 (38.5) 0.019† mRS (0-2) at 3 month 35 (38.5) 251 (55.0) 0.004† 35 (38.5) 101 (55.5) 0.008† mRS (0-3) at 3 month 50 (54.9) 295 (64.6) 0.083† 50 (54.9) 120 (65.9) 0.077† Mortality at 3 month 9 (9.9) 51 (11.2) 0.718† 9 (9.9) 18 (9.9) 1.000†
14
B. Non-malignant MCA group and hypothermia
A total of 468 patients were included in the non-malignant MCA infarction group except for 80 patients who met the malignant trait described previously. Propensity score matching was performed to overcome the bias of the baseline covariates of the two groups. Figure 3 presented with histogram of propensity score matching between two groups after matching. It is seen that the distribution of the two groups is relatively even compared with that of the former group.
Table 4 showed demographics and clinical characteristics, Table 5 showed laboratory, radiological and treatment related parameters between the hypothermia and no hypothermia groups of non-malignant MCA infarction patients. The mismatch of age, gender, atrial fibrillation, stroke etiology, and baseline ASPECTS was resolved through propensity score matching. However, after 1:2 matching, the bias of baseline DWI volume (p<0.001) and EVT method (p<0.001) could not be resolved.
Clinical outcome between the TH and NH groups of non-malignant MCA infarction assessed by various methods (Table 6). Successful reperfusion and symptomatic hemorrhagic transformation (PH type2) was not differ between the two groups. But, TH group (vs. NH group) was worse when compared no hemorrhage than any types of hemorrhagic transformation (57.1 % vs. 69.8%, p=0.083). And, good (mRS 0-2) outcome at 3 month were found to be worse in the TH group (41.3% vs. 65.2%, p=0.002).
15
Figure 3. The histogram of propensity score matching between two groups after 1:2 matching in non-malignant MCA infarction
16
Table 4. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups of non-malignant MCA group (before and after 1:2 matching)
HTN = hypertension, DM = diabetes mellitus, TIA = Transient ischemic attack, mRS = modified Rankin scale, NIHSS = National Institutes of Health Stroke Scale, LAA = large artery atherosclerosis;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Before matching After matching (1:2)
TH (n=63) NH (n=405) P value TH (n=63) NH (n=126) P value Age, yrs, median (range) 63.52±12.83 67.73±12.56 0.014* 63.52±12.83 64.0 ± 12.6 0.756* Sex, female, n (%) 20 (31.7) 190 (46.9) 0.024† 20 (31.7) 50 (39.7) 0.287† Risk factor, n (%)
HTN 41 (65.1) 252 (62.2) 0.663† 41 (65.1) 85 (67.5) 0.743†
DM 13 (20.6) 99 (24.4) 0.510† 13 (20.6) 33 (26.2) 0.401†
Atrial fibrillation 42 (66.7) 194 (47.9) 0.006† 42 (66.7) 85 (67.5) 0.913† Coronary artery disease 7 (11.1) 44 (10.9) 0.953† 7 (11.1) 16 (12.7) 0.753† Dyslipidemia 13 (20.6) 123 (30.4) 0.113† 13 (20.6) 41 (32.5) 0.088† Smoking 12 (19.0) 96 (23.7) 0.415† 12 (19.0) 33 (26.2) 0.277† Prior antiplatelet use 18 (28.6) 119 (29.4) 0.895† 18 (28.6) 41 (32.5) 0.579† Prior anticoagulant use 8 (12.7) 52 (12.8) 0.975† 8 (12.7) 24 (19.0) 0.273† Prior TIA or stroke 9 (14.3) 69 (17.0) 0.586† 9 (14.3) 26 (20.6) 0.289† Premorbid mRS, median
(range)
0 [0-0] 0 [0-0] 0.209§ 0 [0-0] 0 [0-0] 0.468§
Baseline NIHSS, median (range) 17 [15-19] 16 [12-19.5] 0.083§ 17 [15-19] 16 [13-21] 0.962§ Stroke etiology, n (%) 0.005§ 0.785§ Embolism 53 (84.1) 272 (67.2) 53 (84.1) 105 (83.3) LAA 7 (11.1) 72 (17.8) 7 (11.1) 12 (9.5) Others 3 (4.8) 61 (15.1) 3 (4.8) 9 (7.1)
17
Table 5. Comparison of laboratory, radiological and treatment parameters between the hypothermia and no hypothermia groups of non-malignant MCA group (before and after 1:2 matching)
Before matching After matching (1:2)
TH (n=63) NH (n=405) P value TH (n=63) NH (n=126) P value Laboratory parameters Glucose (mg/dL) 137.13±46.82 138.59±55.02 0.842* 137.13±46.82 144.71±61.75 0.386* HbA1c (%) 6.16±0.96 6.17±1.18 0.931* 6.16±0.96 6.28±1.27 0.921* WBC 8.35±2.71 8.42±2.96 0.852* 8.35±2.71 8.82±3.49 0.642* Hemoglobin 13.62±1.56 13.48±1.84 0.558* 13.62±1.56 13.54±2.04 0.679* Hematocrit 40.53±4.87 39.73±5.19 0.251* 40.53±4.87 40.00±5.75 0.427* Platelet 221.71±55.74 225.31±72.17 0.650* 221.71±55.74 222.32±81.35 0.737* Total cholesterol 170.02±38.47 166.65±40.60 0.539* 170.02±38.47 163.44±36.45 0.241* LDL 98.93±36.01 101.99±37.25 0.546* 98.93±36.01 97.04±36.22 0.608* ESR (mg/dL) 15.48±14.80 14.70±14.99 0.704* 15.48±14.80 12.70±13.19 0.148* CRP 0.65±1.33 0.79±1.98 0.584* 0.65±1.33 0.83±1.97 0.001* INR 1.11±0.27 1.09±0.25 0.702* 1.11±0.27 1.14±0.28 0.185* PTT 30.45±6.64 28.23±14.50 0.234* 30.45±6.64 27.54±4.84 <0.001* Radiological parameters Involved vessel 0.283§ 0.279§ Distal ICA 23 (36.5) 134 (33.1) 23 (36.5) 49 (38.9) M1 37 (58.7) 225 (55.6) 37 (58.7) 63 (50.0) M2 3 (4.8) 46 (11.4) 3 (4.8) 14 (11.1) Baseline ASPECTS 7 [4-8] 8 [6-9] 0.002§ 7 [4-8] 6 [5-8] 0.813§ Baseline DWI vol. 32.89±23.70 13.89±16.06 <0.001* 32.89±23.70 18.66±19.72 <0.001*
Endovascular therapy, n (%) <0.001§ <0.001§
Retrieval stent 44 (69.8) 139 (34.3) 44 (69.8) 41 (32.5)
Aspiration 13 (20.6) 163 (40.2) 13 (20.6) 63 (50.0)
Others 6 (9.5) 103 (25.4) 6 (9.5) 22 (17.5)
Time parameters, min, median (range)
Onset to puncture 314.46±215.43 341.10±227.41 0.384* 314.46±215.43 328.97±218.00 0.930* Onset to reperfusion 384.75±220.58 410.83±232.07 0.404* 384.75±220.58 393.59±219.92 0.980* IV-rtPA use 37 (58.7) 209 (51.6) 0.292† 37 (58.7) 67 (53.2) 0.469† WBC = white blood cell, LDL = low-density lipoprotein, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, INR = international normalized ratio, PTT = partial thromboplastin time, ASPECT = Alberta Stroke program early CT score; *independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
18
Table 6. Clinical outcome between the hypothermia and no hypothermia groups and no hypothermia groups of non-malignant MCA group (before and after 1:2 matching)
mTICI = modified thrombolysis in cerebral infarction, HI = hemorrhagic infarct, PH = parenchymal hematoma, HT = hemorrhagic transformation, DWI = diffusion-weighted imaging, mRS = modified Rankin Scale;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Before matching After matching (1:2)
TH (n=63) NH (n=405) P value TH (n=63) NH (n=126) P value Final mTICI 2b-3 51 (81.0) 308 (76.0) 0.392† 51 (81.0) 101 (80.2) 0.897† Hemorrhagic transformation 0.008§ 0.457§ None 36 (57.1) 298 (73.6) 0.007† 36 (57.1) 88 (69.8) 0.083† HI type 1 9 (14.3) 30 (7.4) (vs. any HT) 9 (14.3) 12 (9.5) (vs. any HT) HI type 2 6 (9.5) 39 (9.6) 6 (9.5) 10 (7.9) PH type 1 6 (9.5) 14 (3.5) 6 (9.5) 6 (4.8) PH type 2 6 (9.5) 24 (5.9) 6 (9.5) 10 (7.9) Symptomatic HT (PH type 2) 6 (9.5) 24 (5.9) 0.278† 6 (9.5) 10 (7.9) 0.712† DWI change 42.09±59.24 31.86±53.63 0.216 42.09±59.24 27.08±36.18 0.245 Clinical outcome mRS (0-1) at 3 month 18 (28.6) 169 (41.7) 0.047† 18 (28.6) 55 (43.7) 0.045† mRS (0-2) at 3 month 26 (41.3) 247 (61.1) 0.003† 26 (41.3) 82 (65.2) 0.002† mRS (0-3) at 3 month 38 (6.03) 281 (69.4) 0.151† 38 (6.03) 95 (75.4) 0.032† Mortality at 3 month 3 (4.8) 36 (8.9) 0.268† 3 (4.8) 10 (7.9) 0.549†
19
C. Malignant MCA infarction and hypothermia
Among anterior circulation ischemic stroke patients, 80 patients were diagnosed as malignant MCA infarction. Twenty-eight patients had received TH. Table 7 showed demographics and clinical characteristics between the TH and NH groups of malignant MCA infarct. Demographics and baseline characteristics differed in several variables. Age, diabetes, baseline NIHSS score, stroke etiology were unbalanced between the two groups. Table 8 showed laboratory, radiological and treatment related parameters. In laboratory parameters, other results of patients were similar in the two groups except glucose, C-reactive protein (CRP) and partial thromboplastin time (PTT). In time parameters, TH group (vs. NH group) had significantly short onset to puncture time (218.75±109.26 vs. 352.04±239.80, p=0.001) and onset to reperfusion time (286.93±118.72 vs. 450.23±251.05, p<0.001).
Clinical outcome between the TH and NH groups were meaningful (Table 9). TH group (vs. NH group) had a better clinical outcome (32.1% vs. 7.7% p=0.009) and a lower frequency of hemorrhagic transformation (none vs. any hemorrhage, p=0.007). Decompressive hemicraniectomy rate (n=5, 17.9% vs. n=6, 11.5%, p=0.503) and mortality (n=6, 21.4% vs. n=15, 28.8%, p=0.472) were not different (Table 9).
To find prognostic factors for good clinical outcome, potential factors that were significant in the univariate analyses entered multivariate logistic regression with stepwise-backward conditional mode (Table 10). After adjusting potential confounders to predict good outcome, therapeutic hypothermia (OR 4.63; CI 1.20-17.89; p=0.026) and hypertension (OR 0.18; CI 0.04-0.74; p=0.018) were independent determinants.
20
HTN = hypertension, DM = diabetes mellitus, TIA = Transient ischemic attack, mRS = modified Rankin scale, NIHSS = National Institutes of Health Stroke Scale, LAA = large artery atherosclerosis;
*independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
Table 7. Comparison of demographics and baseline characteristics between the hypothermia and no hypothermia groups with malignant MCA infarction
TH (n=28)
NH
(n=52) P value
Age, yrs, median (range) 65.07±13.43 71.1 ±11.47 0.038*
Sex, female, n (%) 12 (42.9) 28 (53.8) 0.348†
Risk factor, n (%)
HTN 13 (48.4) 35 (67.3) 0.069†
DM 3 (10.7) 17 (32.7) 0.030†
Atrial fibrillation 12 (42.9) 28 (53.8) 0.348†
Coronary artery disease 3 (10..7) 5 (9.6) 0.876†
Dyslipidemia 5 (17.9) 15 (28.8) 0.279†
Smoking 7 (25.0) 12 (23.1) 0.847†
Prior antiplatelet use 6 (21.4) 14 (26.9) 0.588†
Prior anticoagulant use 1 (3.6) 5 (9.6) 0.328†
Prior TIA or stroke 4 (14.3) 5 (9.6) 0.712†
Premorbid mRS, median (range) 0 [0-0] 0 [0-0] 0.209§
Baseline NIHSS, median (range) 19 [15.3-21] 21 [18-22] 0.013§
Stroke etiology, n (%) 0.030§
Embolism 25 (89.3) 35 (67.3)
LAA 2 (7.1) 9 (17.3)
21
Table 8. Comparison of laboratory, radiological and treatment parameters between the hypothermia and no hypothermia groups with malignant MCA infarction
TH (n=28) NH (n=52) P value Laboratory parameters Glucose (mg/dL) 127.0 ± 31.13 153.40 ± 62.09 0.013* HbA1c (%) 6.029 ± 0.88 6.41 ± 1.32 0.179* WBC 8.36 ± 2.69 9.35 ± 4.18 0.262* Hemoglobin 13.48 ± 1.57 12.78 ± 2.10 0.131* Hematocrit 40.14 ± 4.64 38.13 ± 5.93 0.125* Platelet 198.54 ± 63.89 228.75 ± 70.88 0.064* Total cholesterol 159.86 ± 36.62 165.16 ± 35.34 0.531* LDL 95.32 ± 35.04 102.82 ± 32.68 0.349* ESR (mg/dL) 16.32 ± 20.53 18.96 ± 18.69 0.565* CRP 0.30 ± 0.33 0.98 ± 2.23 0.037* INR 1.05 ± 0.19 1.06 ± 0.147 0.742* PTT 29.43 ± 3.64 26.33 ± 4.29 0.002* Radiological parameters Involved vessel 0.724§ Distal ICA 14 (50.0) 28 (53.8) M1 12 (42.9) 21 (40.4) M2 2 (7.1) 3 (5.8) Baseline ASPECTS 3 [2-4] 3 [2-4] 0.245§
Baseline DWI vol. 104.84±50.14 112.62±102.28 0.714§
Endovascular therapy, n (%) 0.417§
Retrieval stent 18 (64.3) 29 (55.8)
Aspiration 6 (21.4) 12 (23.1)
Others 4 (14.3) 11 (21.2)
Time parameters, min, median (range)
Onset to puncture 218.75±109.26 352.04±239.80 0.001*
Onset to reperfusion 286.93±118.72 450.23± 251.05 <0.001*
IV-rtPA use 22 (78.6) 29 (55.8) 0.043†
WBC = white blood cell, LDL = low-density lipoprotein, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, INR = international normalized ratio, PTT = partial thromboplastin time, ASPECT = Alberta Stroke program early CT score; *independent t-test, †Pearson’s chi-square test, §Mann-Whitney U test
22
Table 9. Clinical outcome between the hypothermia and no hypothermia groups with malignant MCA infarction TH (n=28) NH (n=52) P value Final mTICI 2b-3 21 (75.0) 34 (65.4) 0.376† Hemorrhagic transformation 0.069§ None 18 (64.3) 17 (32.7) 0.007† HI type 1 2 (7.1) 4 (7.7) (vs. any HT) HI type 2 0 (0.0) 16 (30.8) PH type 1 4 (14.3) 10 (19.2) PH type 2 4 (14.3) 5 (9.6) Symptomatic HT (PH type 2) 4 (14.3) 5 (9.6) 0.712† DWI change 50.82±63.39 89.84±94.15 0.127* Clinical outcome mRS (0-1) at 3 month 4 (14.3) 1 (1.9) 0.048† mRS (0-2) at 3 month 9 (32.1) 4 (7.7) 0.009† mRS (0-3) at 3 month 12 (42.9) 14 (26.9) 0.147† Mortality at 3 month 6 (21.4) 15 (28.8) 0.472† Medical complications Pneumonia 10 (35.7) 16 (30.7) 0.652† Electrolyte imbalance 9 (32.1) 5 (9.6) 0.028‡
Deep vein thrombosis 1 (3.6) 0 (0.0) 0.350‡
mTICI = modified thrombolysis in cerebral infarction, HI = hemorrhagic infarct, PH = parenchymal hematoma, HT = hemorrhagic transformation, DWI = diffusion-weighted imaging, mRS = modified Rankin Scale;
23
Figure 4. Distribution of hemorrhagic transformation between the hypothermia and no hypothermia groups with malignant MCA infarction
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
Non-TH (n=52) TH (n=28)
24
Figure 5. Distribution of 3-month modified Rankin Scale between the hypothermia and no hypothermia groups with malignant MCA infarction
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
Non-TH (n=52) TH (n=28)
25
Table 10. Multivariate Logistic Regression Analysis for Prediction of Good Outcome (mRS, 0–2)
Characteristics Good outcome (n=13) Poor outcome (n=67) P value Crude OR (95% CI) P value Adjusted OR (95% CI) P value General demographics Age, y 65.00±14.14 69.76±12.06 0.209 0.97 (0.93-1.02) 0.211 … … Women, n (%) 4 (30.8) 36 (53.7) 0.130 2.61 (0.73-9.32) 0.139 NIHSS 18 [14-21] 21 [17-22] 0.034 0.84 (0.72-0.98) 0.030 … … ASPECTS 3 [2-3.5] 3 [2-4] 0.846 1.03 (0.68-1.57) 0.873 … … DWI volume 93.62±34.52 113.25±93.46 0.46 0.99 (0.98-1.00) 0.457 Hypothermia 9 (69.2) 19 (28.4) 0.009 5.68 (1.56-20.69) 0.008 4.63 (1.20-17.89) 0.026 Risk factors, n (%) HTN 3 (23.1) 45 (67.2) 0.003 0.15 (0.04-0.59) 0.007 0.18 (0.04-0.74) 0.018 DM 1 (7.7) 19 (28.4) 0.167 0.21 (0.03-1.73) 0.147 A-fib 4 (30.8) 36 (53.7) 0.130 0.38 (0.11-1.37) 0.139 Hyperlipidemia 2 (15.4) 18 (26.9) 0.500 0.49 (0.10-2.45) 0.495 Smoking 5 (38.5) 14 (20.9) 0.283 2.37 (0.67-8.37) 0.181 Involved vessels 0.209 ICA 5 (38.5) 37 (55.2) ref … M1 6 (46.2) 27 (40.3) 1.64 (0.45-5.95) 0.449 M2 2 (15.4) 3 (4.5) 4.93 (0.66-37.12) 0.121 Embolism etiology 12 (92.3) 48 (71.6) 0.167 4.75 (0.58-39.10) 0.147 Treatment parameters IV-rtPA 9 (69.2) 42 (62.7) 0.760 1.34 (0.38-4.81) 0.654 Onset to puncture 217.62±100.75 322.42±224.86 0.105 0.99 (0.99-1.00) 0.110 Onset to reperfusion 275.00±118.93 415.99±236.58 0.040 0.99 (0.98-1.00) 0.042 … … mTICI (2b,3) 10 (76.9) 45 (67.2) 0.745 1.63 (0.41-6.53) 0.490 EVT method
Stent retriever 7 (53.8) 40 (59.7) 0.842 ref …
Direct aspiration 4 (30.8) 14 (20.9) 1.63 (0.41-6.43) 0.483
Other 2 (15.4) 13 (19.4) 0.88 (0.16-4.77) 0.881
ASPECTS = Alberta Stroke Program Early CT Score; CI = confidence interval; CT = computed tomography; DWI = diffusion-weighted imaging; HT = hemorrhagic transformation; ICA = internal carotid artery; NIHSS = National Institutes of Health Stroke Scale; OR = odds ratio; TICI, thrombolysis in cerebral ischemia; IV-rtPA = intravenous recombinant tissue-type plasminogen activator, and ICAS = intracranial arterial stenosis.
26
IV.
DISCUSSION
This study demonstrated that therapeutic hypothermia led to reduction of hemorrhagic transformation and improvement of clinical outcomes in malignant MCA infarction patients who treated with EVT. In addition, therapeutic hypothermia was an independent predictor of good outcome after adjustment of potential confounding factors.
A. Unmet need for additional treatment of acute ischemic stroke
Even after the remarkable achievement of EVT, a considerable number of stroke patients still disabled despite a higher reperfusion rate. In addition, the usefulness of EVT in patients with large core infarct was still under discussion. Several previous studies in patients with a malignant MCA infarct trait represented by low ASPECT have shown poor outcomes despite reperfusion (Goyal M et al., 2011; Yoo AJ et al., 2014; Inoue M et al., 2014). On the contrary, EVT was found to be useful in patients with a large core volume greater than 70 mL, and reperfusion rate was a predictor of good outcome (Chen Z et al., 2018). These results demonstrates that reperfusion itself does not always guarantee a good functional outcome, especially in patients with ‘malignant MCA infarction trait’. To improve clinical outcome, neuroprotection after recanalization is drawing attention in the era of EVT. The previous failure of neuroprotective drugs can be overcome by the achievement of recanalization same as preclinical ischemic-reperfusion model (Chamorro et al., 2016). Among the neuroprotective strategies, immediate post-reperfusion cooling is encouraging as an option to minimize reperfusion-related complications.
B. Neuroprotective mechanism and effect of therapeutic hypothermia on various conditions
TH is used as neuroprotective therapy in various situations such as traumatic brain injury, brain damage after cardiac arrest, as well as stroke. TH has a multi-target neuroprotective effect. It is involved in the ischemic phase where excitotoxicity, apoptosis, inflammation and free radical production occur, or it
27
affects neurogenesis, gliogenesis and angiogenesis after injury (Yenari et al., 2012). After ischemic insult, decrease of cerebral blood flow ensues ionic homeostasis disruption and calcium influx (Khanna et al., 2014; Lai et al., 2014). Intracellular calcium permit the release of excitatory neurotransmitters, mitochondrial dysfunction, and increase of reactive oxygen species generation (Globus et al., 1995; Gonzalez-Ibarra et al., 2011). The cerebral metabolic rate is important in ischemia-reperfusion status. Hypothermia reduces oxygen and energy demands as body temperature decreases by 1℃, resulting 6-7% decrease in cerebral metabolic rate (Polderman et al., 2009). It also reverse transport of glutamate by suppression of excitatory neurotransmitter release and reduction in glutamate receptor expression.(Kim et al., 2011; Wang et al., 2013) In addition, hypothermia attenuates the inflammatory response by suppression of astrocyte and microglial activation (Xiong et al., 2011).
Numerous preclinical and clinical studies have proved the neuroprotective efficacy of TH on various conditions. TH is applied as standard treatment in patients with coma after cardiac arrest (Bernard et al., 2002). In experimental stroke models, TH has been shown to be helpful as a potent neuroprotectant, especially in ischemia-reperfusion models (Yenari et al., 2012). These results were in accordance with several clinical researches in acute ischemic stroke (Hong et al., 2014; Hwang et al., 2017). However, the efficacy of TH in ischemic stroke is still open to question (Liu et al., 2016) because some studies have reported a harmful systemic effect of TH, such as pneumonia (Lyden et al., 2016), cardiopulmonary problems (De Georgia et al., 2004), and immune suppression (Liu et al., 2016).
C. Therapeutic hypothermia in non-malignant MCA infarction
In this study, we focused on determining the population who can benefit from neuroprotection. Subgroup analysis revealed that ischemic stroke patients who had non-malignant trait showed a poor prognosis in hypothermia group. This can be explained for the following reasons. First, baseline characteristics between the hypothermia and no hypothermia groups were considerably different. The hypothermia group had more common sources of cardioembolism, more severe ischemic change in ASPECTS, and more prevalent hemorrhagic transformation. It means that clinicians conducted TH in
28
patients who had severe reperfusion injury. We executed propensity score matching to overcome these imbalance, however, substantial differences of the baseline characteristics were not balanced after matching.
Second, TH can be associated with various medical complications. Low temperature can predispose stroke patients to systemic infections by inhibiting the cytokine response and stimulating anti-inflammatory cytokines (Lee et al., 2001; Russwurm et al., 2002). Although hypothermia treatment has not been reported to increase the risk of infection in systemic review and meta-analysis study, (Den Hertog et al., 2009) several clinical studies described the higher incidence of pneumonia in patients treated with hypothermia (De Georgia et al., 2004; Polderman et al., 2009). Hemodynamic changes, including decreased heart rate and arrhythmias, can be problematic during hypothermia treatment (De Georgia et al., 2004; Staikou et al., 2011). This phenomenon is related to the temperature level, the degree of hypothermia treatment should be controlled with caution. Various other side effects including electrolyte imbalance, cold diuresis, and alterations in coagulation cascade can affect the clinical outcome of patients treated with hypothermia treatment.
D. Therapeutic hypothermia in malignant MCA infarction
Our study showed that TH can lead to good clinical outcome in patients with malignant MCA infarction. There are efforts to apply the clinical effect of TH to malignant MCA infarction (Georgiadis et al., 2002; Milhaud et al., 2005). Current treatment options for malignant MCA infarction have been immersed in surgical decompression (Huttner et al., 2009; Treadwell et al., 2010). However, there is still debate about the timing and subjects of surgery, and intensive medical treatment including osmotherapy is not sufficient for some patients. TH can be a candidate therapy as an additional treatment strategy, although further investigation for the efficacy of treatment in malignant MCA infarction is needed (Wartenberg KE., 2012). Therefore, the tendency of good clinical outcome and low hemorrhagic transformation represented in the hypothermia group of the malignant MCA infarct group in this study may be very encouraging.
29
The reasons for the good outcomes of malignant MCA infarction in this study are as follows. First, hypothermia can alleviate reperfusion injury such as hemorrhagic transformation and edema. In patients with substantial ischemic changes (ASPECTS ≤5 on baseline imaging), the functional outcome usually cannot reach the desired level. Additional strategies that emphasize reperfusion injury can be beneficial in malignant MCA infarct trait because reperfusion injury after EVT can be responsible for poor functional recovery. Our results demonstrated the hypothermia group occurred in hemorrhagic transformation less frequent than no hypothermia group, especially in malignant MCA infarct patients. In addition, relative long-term hypothermia therapy (>48 hours) has been performed to prevent rebound cerebral edema.
Second, the critical care might enhance the effectiveness of hypothermia treatment. Previous trials on hypothermia in acute stroke patients usually conducted conscious hypothermia therapy without mechanical ventilation (De Georgia et al., 2004; Polderman et al., 2009). In case of conscious hypothermia, shivering and aspiration can cause various complications. This study is a retrospective study, however, general critical care (sedation with mechanical ventilation, protocolized medical critical care, and timely hemicraniectomy) has been executed. Therefore, patient selection for effective hypothermia and intensive critical care can improve the functional outcome of patients with malignant MCA infarction.
E. Limitations
This study has several limitations. Due to the retrospective nature, the number of hypothermia group was relatively small. We could not investigate the extent of cerebral edema that may reflect reperfusion injury. The inclusion of malignant MCA infarction can be controversial because there are no definitive diagnostic criteria. We classified the patients without DWI lesion within 6 hours as malignant MCA infarct using only baseline severity (NIHSS>20, Item Ia>0). To confirm our perspective of neuroprotection with TH, a prospective study with an appropriate definition of ‘malignant MCA infarction’ should be needed. Our findings can generalize in applying future neuroprotective agent development.
30
V.
CONCLUSION
This study shows the effect of hypothermia among the anterior circulation ischemic stroke patients who treated with EVT. The statistical comparison between the hypothermia and no hypothermia groups was impracticable due to the imbalance of baseline characteristics. However, in patients with malignant MCA infarction undergoing EVT, therapeutic hypothermia may reduce the risk of hemorrhagic transformation and lead to an improved clinical outcome. This study suggests that therapeutic hypothermia can be considered as a treatment modality for malignant MCA infarction.
31
REFERENCES
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, Investigators MC: A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11-20, 2015
2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET: Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019-1030, 2015
3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, Investigators E-I: Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009-1018, 2015
4. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, Investigators SP: Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285-2295,
32
2015
5. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez-Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A, Investigators RT: Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296-2306, 2015
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association.Stroke 49:e46–e99, 2018
7. Nogueira RG, Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21.
8. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, collaborators H: Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387: 1723-1731, 2016
9. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R: 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 53(4):309-15. 1996
33
10. Wartenberg KE: Malignant middle cerebral artery infarction. Curr Opin Crit Care 18(2):152-63. 2012
11. Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol 8(10):949-58. 2009
12. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB; HAMLET investigators. Surgical decompression for spaceoccupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 8:326–333. 2009
13. Juttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, Witte S, Jenetzky E, Hacke W; DESTINY Study Group. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke 38:2518–2525. 2007 14. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, Boutron C, Couvreur G, Rouanet F, Touzé E, Guillon B, Carpentier A, Yelnik A, George B, Payen D, Bousser MG; DECIMAL Investigators. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 38:2506–2517. 2007
15. Chamorro Á , Dirnagl U, Urra X, Planas AM; Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 15(8):869-881. 2016 16. Neuhaus AA, Couch Y, Hadley G, Buchan AM; Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 1;140(8):2079-2092. 2017
17. Gladstone DJ, Black SE, Hakim AM; Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery; Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33(8):2123-36. 2002
18. Chamorro A; Neuroprotectants in the Era of Reperfusion Therapy. J Stroke 20(2):197-207. 2018 19. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 21;346(8):549-56. 2002
20. Hong JM, Lee JS, Song HJ, Jeong HS, Choi HA, Lee K; Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke 45(1):134-40. 2014
34
21. Lee JS, Lee SJ, Hong JM, Choi JW, Hong JH, Chang HW, Kim CH, Kim YW, Kang DH, Kim YS, Ovbiagele B, Demchuk AM, Hwang YH, Sohn S; Temporal Changes in Care Processes and Outcomes for Endovascular Treatment of Acute Ischemic Stroke: Retrospective Registry Data from Three Korean Centers. Neurointervention 13(1):2-12. 2018
22. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS, Cerebral Angiographic Revascularization Grading C, group SRw, Force STiCIT: Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44: 2650-2663, 2013
23. Thymianski M. Combining Neuroprotection With Endovascular Treatment of Acute Stroke: Is There Hope? Stroke 48(6):1700-1705. 2017
24. Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Köhrmann M, Kersten JF, Krützelmann A, Humpich MC, Sobesky J, Gerloff C, Villringer A, Fiehler J, Neumann-Haefelin T, Schellinger PD, Röther J; Clinical Trial Net of the German Competence Network Stroke. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol 68(4):435-45. 2010
25. Lee JS, Lee SJ, Yoo JS, Hong JH, Kim CH, Kim YW, Kang DH, Kim YS, Hong JM, Choi JW, Ovbiagele B, Demchuk AM, Sohn SI, Hwang YH. Prognosis of Acute Intracranial Atherosclerosis-Related Occlusion after Endovascular Treatment. J Stroke 20(3):394-403. 2018
26. Iacus SM, King G, Porro G. Causal inference without balance checking: Coarsened Exant Matching. Political Analysis 20:1-24. 2011
27. Liu K, Khan H, Geng X, Zhang J, Ding Y. Pharmacological hypothermia: a potential for future stroke therapy? Neurol Res 38(6):478-90. 2016
28. Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, Parker S, Concha M, Hussain S, Agarwal S, Meyer B, Jurf J, Altafullah I, Raman R; Collaborators. Results of the ICTuS 2 Trial
35
(Intravascular Cooling in the Treatment of Stroke 2). Stroke 47(12):2888-2895. 2016
29. De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID). Neurology 27;63(2):312-7. 2004
30. Hwang YH, Jeon JS, Kim YW, Kang DH, Kim YS, Liebeskind DS. Impact of immediate post-reperfusion cooling on outcome in patients with acute stroke and substantial ischemic changes. J Neurointerv Surg 9(1):21-25. 2017
31. Treadwell SD, Thanvi B. Malignant middle cerebral artery (MCA) infarction: pathophysiology, diagnosis and management. Postgrad Med J 86(1014):235-42. 2010
32. Georgiadis D, Schwarz S, Aschoff A, Schwab S. Hemicraniectomy and moderate hypothermia in patients with severe ischemic stroke. Stroke 33(6):1584-8. 2002
33. Milhaud D, Thouvenot E, Heroum C, Escuret E. Prolonged moderate hypothermia in massive hemispheric infarction: clinical experience. J Neurosurg Anesthesiol 17(1):49-53. 2005
34. Keller E, Steiner T, Fandino J, Schwab S, Hacke W. Changes in cerebral blood flow and oxygen metabolism during moderate hypothermia in patients with severe middle cerebral artery infarction. Neurosurg Focus. 15;8(5):e4. 2000
35. Lakhan SE, Pamplona F. Application of mild therapeutic hypothermia on stroke: a systematic review and meta-analysis. Stroke Res Treat. 2012
36. Kim DH, Ko SB, Cha JK, Hong KS, Yu KH, Heo JH, Kwon SU, Bae HJ, Lee BC, Yoon BW, Kim JE, Kang HS, Seo DH, Park SQ, Sheen SH, Park HS, Kang SD, Kim JM, Oh CW, Park IS, Rha JH. Updated Korean Clinical Practice Guidelines on Decompressive Surgery for Malignant Middle Cerebral Artery Territory Infarction. J Stroke. 17(3):369-76. 2015
37. Wan YH, Nie C, Wang HL, Huang CY. Therapeutic hypothermia (different depths, durations, and rewarming speeds) for acute ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis. 23(10):2736-47. 2014
38. Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: Meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab.
36(7):1157-36
64. 2016
39. Horn CM, Sun CH, Nogueira RG, Patel VN, Krishnan A, Glenn BA, Belagaje SR, Thomas TT, Anderson AM, Frankel MR, Schindler KM, Gupta R. Endovascular Reperfusion and Cooling in Cerebral Acute Ischemia (ReCCLAIM I). J Neurointerv Surg. 6(2):91-5. 2014
40. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 22;378(8):708-718. 2018
41. Neugebauer H, Jüttler E. Hemicraniectomy for malignant middle cerebral artery infarction: current status and future directions. Int J Stroke. 9(4):460-7. 2014
42. Inoue M, Olivot JM, Labreuche J, Mlynash M, Tai W, Albucher JF, Meseguer E, Amarenco P, Mazighi M. Impact of diffusion-weighted imaging Alberta stroke program early computed tomography score on the success of endovascular reperfusion therapy. Stroke. 45(7):1992-8. 2014
43. Chen Z, Zhang R, Zhou Y, Gong X, Zhang M, Shi F, Yu X, Lou M. Patients With Ischemic Core ≥70 ml Within 6 h of Symptom Onset May Still Benefit From Endovascular Treatment. Front Neurol ;9:933. 2018
44. Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM; Penumbra Pivotal Stroke Trial Investigators, Calgary Stroke Program, and the Seaman MR Research Center. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 42(1):93-7. 2011
45. Yoo AJ, Zaidat OO, Chaudhry ZA, Berkhemer OA, González RG, Goyal M, Demchuk AM, Menon BK, Mualem E, Ueda D, Buell H, Sit SP, Bose A; Penumbra Pivotal and Penumbra Imaging Collaborative Study (PICS) Investigators. Impact of pretreatment noncontrast CT Alberta Stroke Program Early CT Score on clinical outcome after intra-arterial stroke therapy. Stroke. 45(3):746-51. 2014
46. Heard KJ, Peberdy MA, Sayre MR, Sanders A, Geocadin RG, Dixon SR, Larabee TM, Hiller K, Fiorello A, Paradis NA, O'Neil BJ. A randomized controlled trial comparing the Arctic Sun to standard