저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in Medicine
Prediction of postoperative
complication after gastric cancer
surgery using artificial neural
network (ANN)
Ajou University Graduate School
Major in Medicine
Prediction of postoperative
complication after gastric cancer
surgery using artificial neural
network (ANN)
Sang-Uk Han, M.D., Ph.D., Advisor
I submit this thesis as doctoral thesis in medicine.
August
2017
Ajou University Graduate School
Major in Medicine
The doctoral thesis of Long-Hai Cui
in Medicine is hereby approved.
Thesis Defense Committee President
Wook-Hwan Kim Seal
Member Sang-Uk Han Seal
Member Rae-Woong Park Seal
Member Hyung-Ho Kim Seal
Member Hoon Hur Seal
The Graduate School, Ajou University
i
- ABSTRACT -
Prediction of postoperative complication after gastric cancer surgery using
artificial neural network
Background: Incidence of gastric cancer is very high in Asia, and then gastrectomy
and perigastric lymphadenectomy is commonly performed surgical procedure. An artificial neuronal network (ANN) had been applied to predict survivals or postoperative complications in some cancers and it showed a higher level of accuracy compared with the conventional linear regression analysis. However, it has not been validated whether this prediction model can be applied in the far and away time periods from the learning time, thus this study was conducted to evaluate the feasibility of previously developed ANN model for predicting severe postoperative complications after gastric cancer surgery in the recent year data.
Methods: From March to September 2010, the data of 186 patients who underwent
curative gastric cancer surgery were prospectively collected for 42 prognostic variables for postoperative complications. A three-layer ANN model was constructed with Clementine 12.0 and trained by a three-layer feed forward neural network using back-propagation learning algorithm. Then, the trained model was applied to the data between 2011 (n=389), 2012 (n=313), 2013 (n=356), 2014 (n=350) and 2015 (n=329) for validating its accuracy in prediction of severe postoperative complications.
ii
Results: For the development of an ANN model, 70% of 2010 data were used in
training and 30% of 2010 data were used in the internal validation. In this model, the postoperative complication could be predicted by 20 top scored variables with an accuracy of 83.8% and an area under the curve (AUC) of 0.873, which was higher than that of POSSUM scoring system (AUC of 0.637). In the interval validation using the recent year data, the accuracy rate was 82.9% in 2011, 79.5% in 2012, 75.8% in 2013, 67.8% in 2014 data and 86.7% in 2015 data; however, AUC was 0.600 in 2011, 0.650 in 2012, 0.580 in 2013, 0.620 in 2014 data and 0.540 in 2015 data, respectively; it tended to decrease over the years.
Conclusions: The ANN model for predicting severe postoperative complication after
gastric cancer surgery is a fair tool with a high level of accuracy and diagnostic value compared to that of linear regression analysis. The ANN model would be a new predictive model in the field of upper gastrointestinal surgery.
Key words: Artificial neuronal network, Gastric carcinoma, Postoperative
complication, Gastrectomy
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FIGURES ··· ⅳ LIST OF TABLES ··· ⅴ ABBREVIATION ··· ⅵ Ⅰ. INTRODUCTION ··· 1 Ⅱ. METHODS ··· 3A. Patients’ inclusion criteria and characteristics ··· 3
B. Data correction ··· 4
C. Prognosis factor selection ··· 6
D. Selection of artificial intelligence algorithms ··· 6
E. Interval validation ··· 8 F. Statistical analysis ··· 8 Ⅲ. RESULTS ··· 9 Ⅳ. DISCUSSION ··· 20 Ⅴ. CONCLUSION ··· 24 REFERENCES ··· 25
iv
LIST OF FIGURES
Fig. 1. Architecture of artificial neural network for predicting postoperative complication in gastric cancer surgery. ··· 7
Fig. 2. The calculated area under the curve according to each method. In addition, as the number of variables included in the model is increasing, the area under the curve also was calculated. The model developed by the artificial neural network with top twenty variables showed the highest score. ··· 13
v
LIST OF TABLES
Table 1. These information were included on a complication record sheet. ··· 5
Table 2. Clinical and surgical characteristics of patients enrolled in modeling data collected for 2010. ··· 10
Table 3. Score of each variable from information gain methods. ··· 12
Table 4. Patients’ characteristics in modeling data and validation data set. ··· 15
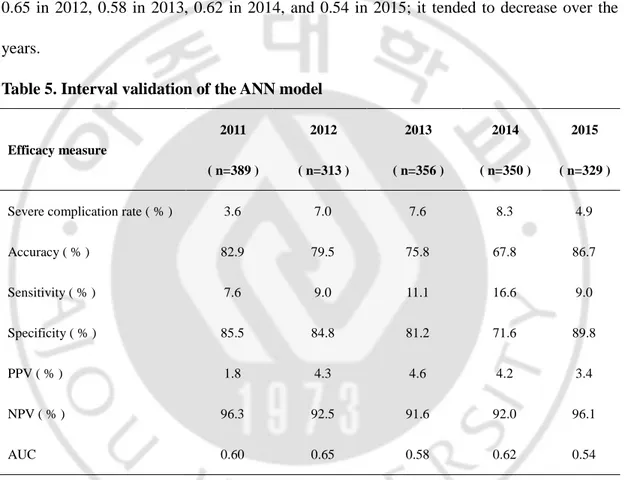
Table 5. Interval validation of the ANN model. ··· 16
Table 6. Interval validation of the remodeling data set. ··· 18
vi
ABBREVIATION
ANN: Artificial neuronal network AUC: Area Under the Cure
POSSUM: Physiological and Operative Severity Score for the enUmeration of
Mortality and morbidity
ASA: American Society of Anesthesiologist
APACHE: Acute Physiology and Chronic Health Evaluation SVM: Support vector machine
C5: Decision tree
ROC: Resection Receiver Operating Characteristic BMI: Body mass index
PPV: Positive predictive value NPV: Negative predictive value
- 1 -
I. INTRODUCTION
Gastric cancer is one of the most common cancers and the third leading cause of cancer-related deaths worldwide, and more than 900,000 cases reported annually(1). Surgical resection remains the only definitive treatment of this malignant disease(2). Gastric cancer surgery is one of the most common procedures for malignant tumor in Asian countries like Korea, China, and Japan where shows the high incidence of gastric cancer. However, Surgery can be associated with meaningful morbidity and mortality(3-5). Therefore, the prediction of postoperative complication in gastric cancer surgery is important in the view of common health or economics. Although postoperative morbidity or mortality from gastric cancer varies across each hospital, there is no objective system to predict them. Development of this system through retrospective data is possible, but complex situations require proper analysis method.
To date, various scoring system has been developed for prediction of postoperative complication, such as Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM)(6), the American Society of Anesthesiologist (ASA) physical status classification, and Acute Physiology and Chronic Health Evaluation (APACHE) II(7). Especially, modified POSSUM score was adapted into a model for the prediction of risk-adjusted postoperative mortality in esophageal and upper gastrointestinal surgery as O-POSSUM(8). Although this system showed the good accuracy for prediction, a half of patients in the previous study were diagnosed with esophageal cancer. Because this predictive system was made based on the data from Western, the results would show the difference with gastric cancer diagnosed with gastric cancer in Asia. In addition, factors
- 2 -
contributing to postoperative complications were very complicated to explain the correlation with a linear regression model using in POSSUM score. Therefore, a new system that could be applied to patients in high prevalence region of gastric cancer may be required.
Artificial neural network (ANN) is a model to predict the future event like ability of the human brain. The human brain is composed of a great many neurons, and each neuron connects to other neurons by communicated network. To predicting the events that were not simply explained, the artificial neural network has been developed. This model can now evaluate noticing trends and patterns in data, finding hidden relationships in data, and “learning” by studying the past. These neural networks can process, analyze, and "learn" relationships in a great deal of data quickly and easily without even being provided any known associations or rules. Therefore, ANN has been increasingly used in medical research, such as resolving the diagnostic or prognostic problems(9-13). However, the use of ANN has not been reported within the field of gastric cancer surgery.
In this study, we collected data related to the postoperative complication, and new specific model for predicting the postoperative complication of gastric cancer surgery was developed as ANN, and then performed the interval validation test using the recent year’ data.
- 3 -
II. Methods
A. Patients’ inclusion criteria and characteristics
From March to September 2010, the data of 186 patients, who were diagnosed with gastric adenocarcinoma and underwent curative resection, were prospectively collected, for the development of an ANN model. From January 2011 to December 2015, we collected the data of 1,737 patients, who were diagnosed with gastric adenocarcinoma and underwent curative resection were retrospectively collected, to assess the prediction accuracy. Surgeries were performed by three surgeons who have an experience of over 80 cases per year.
Resection of the stomach like total or subtotal gastrectomy was decided according to the tumor location. A part of patients was resected by proximal gastrectomy. Lymph node dissection of over D1+β was performed. Reconstruction for total gastrectomy was performed by Roux-en-Y reconstruction and one of Billroth-I, Billroth-II or Roux-en-Y was selected for subtotal gastrectomy. Minimally invasive surgery including laparoscopy or robotic procedures as well as open surgery was applied.
Patients were postoperatively managed with common protocols including diet schedule, discharge plan and so on. The complication was decided when the events occurred within postoperative 30th day abide by the Clavien-Dindo classification of surgical complications(14). Complications that were grade IIIa or higher were considered severe postoperative complications.
The study was reviewed and approved by the Ajou University Hospital Institutional Review Board.
- 4 -
B. Data correction
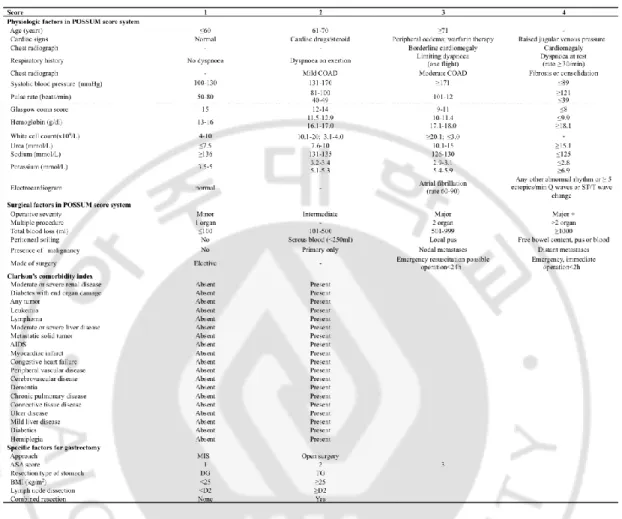
We corrected the clinicopathologic information. Data included already known-parameter associated with the comorbidity and the complications like Charlson’ comorbidity index and POSSUM score. In POSSUM score, 12 variables of physical factor and 6 variables of surgical factor were included. 16 variables in Charlson’s comorbidity index were included. Besides, eight specific factors, which were widely known as the related factor to postoperative complication after gastric cancer surgery from previous studies, were also corrected (Table 1).
- 5 -
Table 1. There information were included on a complication record sheet
POSSUM: Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity; COAD: chronic obstructive airways disease; AIDS: Acquired immune deficiency syndrome; BMI: body mass index; ASA: American Society of Anaesthesiology; MIS: minimal invasive surgery; DG: distal gastrectomy; TG: total gastrectomy.
- 6 -
C. Prognosis factor selection
For development of the new model with ANN, seven variables, which had only one value, were removed. Any patients did not have congestive heart failure, hemiplegia, leukemia and AIDS in Charlson’s index. Tumor, Glasgow coma scale and operative severity in POSSUM score were removed due to one value. Of remained 35 variables, prognosis factors for analysis with ANN were selected by information gain ranking method. Information gain is mostly used for selecting which prognosis factors to use first in a prediction problem. Higher the information gain, higher are the chances of getting pure group in a target group if split on the factor with the highest gain. Information gain ranking method ranked the worth of a prognosis factor by measuring the information gain with respect to the postoperative complication after gastric cancer surgery.
D.
Selection of artificial intelligence algorithms
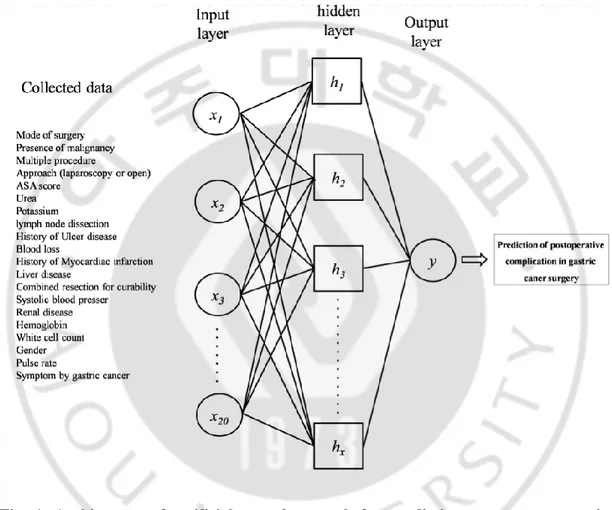
We compared the four algorithms to select algorithm,which had superior performance for complication prediction. The four algorithms for performance comparison are ANN, Support vector machine (SVM)(15), decision tree (C5)(16), and logistic regression. ANN was trained like the way of the brain works with multiple neurons being interconnected to each other through multiple axon junctions. Most ANN used a multilayer neural network with an input layer, one or more hidden layer, and an output layer (Figure 1)(17). The strongness or weakness of the neural connections was determined by repeating training or reinforcement on labeled training data. The weights of the neural connections were adjusted via an optimization method called back-propagation. Back-propagation method was learned by iteratively processing a training data and comparing the ANN prediction with the actual
- 7 -
known target value like complication or non-complication. The weights were adjusted for minimization of the mean squared error between the ANN prediction and the actual target value.
Fig. 1. Architecture of artificial neural network for predicting severe postoperative complication in gastric cancer surgery.
- 8 -
E. Interval validation
To assess the prediction accuracy of 2010 ANN model, we retrospective collected the clinicopathologic information to follow the Table 1. We input the 2011 to 2015 data into the pre-established 2010 ANN model and the validation test for each year was conducted. To identify the role of learning and training for the ANN model, we assume that every year regularly updated date, merged the data such as from 2010 to 2011, from 2010 to 2012, from 2010 to 2013, from 2010 to 2014. For remodeling of additional four new ANN models, we proceed to use the 2010 ANN model as the same manner, prognosis factors were selected by information gain ranking method in 2010 ANN model. Then performed the interval validation test again, the interval validation performed in the lastly merged data and the next year data.
F. Statistical Analysis
First of all, we analyzed the suitability of equation developed with variables included in POSSUM score. Following logistic regression model was applied for analysis.
R/1-R = -5.91 + (0.16 x physiological score) + (0.19 x operative severity score)
The accuracy of each model for predicting the complication after gastric cancer surgery was estimated by the area under the curve (AUC) from the receiver-operating characteristic (ROC) curve. AUC was calculated using MedCalc for Windows, version 9.38 (MedCalc Software, Mariakerke, Belgium).
- 9 -
III. RESULTS
Table 2 shows the demographic and clinical characteristics of the 2010 ANN model. The mean age of 186 patients was 58.8 ± 12.9 years, and the proportion of male patients (67.7%) was higher than that of female. While 20.4% of patients were performed total gastrectomy, most patients underwent distal gastrectomy, and the patients underwent more than D2 lymphadenectomy (31.8%) was less than that the patients underwent less than D2 lymphadenectomy. The mean operation time was 191.9 ± 56.0 minutes other clinical and surgical results were listed.
The logistic regression model developed by variables in POSSUM showed AUC of 0.637. Therefore, previous POSSUM scoring system for prediction the postoperative complication in gastric cancer surgery was not adapted in our data.
- 10 -
Table 2. Clinical and surgical characteristics of patients enrolled in modeling data collected for 2010
Values are presented as number (%) or mean ± standard deviation.
MIS: minimal invasive surgery; BMI: body mass index; ASA: American Society of Anaesthesiology.
- 11 -
In the information gain methods for selection of variables using in various analysis tools (ANN, Logistic regression, SVM and Decision tree), each variable was scored from 0 to 0.026. The highest score was mode of surgery (emergency or elective surgery). The presence of malignancy (primary only, into nodal metastasis or into distant metastasis) (0.026) was second highest score (0.022). Multiple procedure, approach methods (open or minimally invasive surgery) and ASA score were listed according to the score. Other results were listed in Table 3.
- 12 -
Table 3. Score of each variable from information gain methods
- 13 -
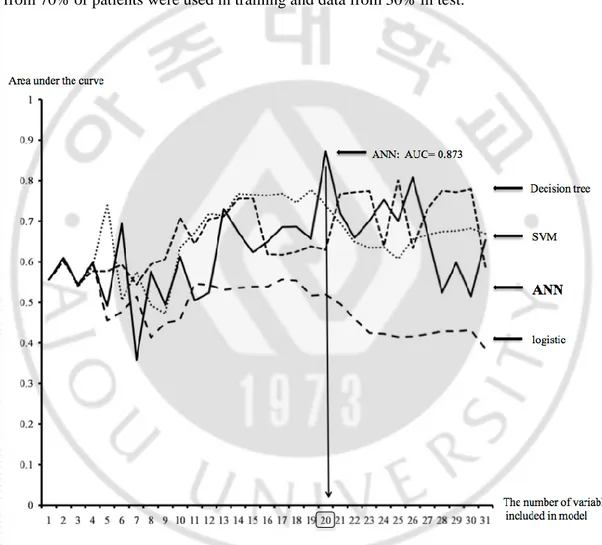
We investigated the AUC increasing the number of variables in the score order for each analytic method (Fig. 2). AUC is highest as 0.873 when the model was developed by ANN with 20 variables of top score comparing with other analytic tools. For this ANN model, data from 70% of patients were used in training and data from 30% in test.
Fig. 2. The calculated area under the curve according to each method. In addition, as the
number of variables included in the model is increasing, the area under the curve also was calculated. The model developed by the artificial neural network with top twenty variables showed the highest score.
- 14 -
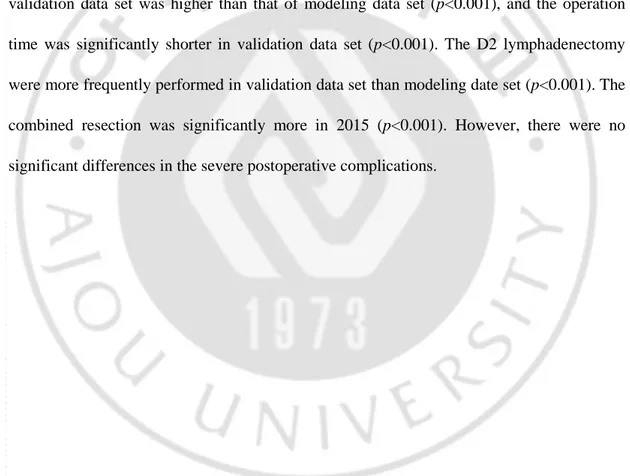
Table 4 shows the patients’ characteristics in modeling data and validation data set. Age, gender, comorbidities, body mass index (BMI), American Society of Anesthesiologists (ASA) score, extension of stomach, pathologic stage, and severe postoperative complication rate were similar in the each year. The most common severe postoperative complications wereanastomotic leakage, intraabdominal bleeding, intraluminal bleeding, wound, intestinal obstruction, fluid collection and pulmonary complication. The proportion of MIS (78.7%) in validation data set was higher than that of modeling data set (p<0.001), and the operation time was significantly shorter in validation data set (p<0.001). The D2 lymphadenectomy were more frequently performed in validation data set than modeling date set (p<0.001). The combined resection was significantly more in 2015 (p<0.001). However, there were no significant differences in the severe postoperative complications.
- 15 -
Table 4. Patients’ characteristics in modeling data and validation data set
Values are presented as number (%) or mean ± standard deviation.
MIS: minimal invasive surgery; BMI: body mass index; ASA: American Society of Anaesthesiology.
- 16 -
We performed interval validation using 2010 ANN model. As the Table 5 shows, the complication rate were 3.6% in 2011, 7.0% in 2012, 7.6% in 2013, 8.3% in 2014, and 4.9% in 2015. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) tended to decrease over the years. The AUC was identified as 0.60 in 2011, 0.65 in 2012, 0.58 in 2013, 0.62 in 2014, and 0.54 in 2015; it tended to decrease over the years.
Table 5. Interval validation of the ANN model
Efficacy measure 2011 ( n=389 ) 2012 ( n=313 ) 2013 ( n=356 ) 2014 ( n=350 ) 2015 ( n=329 )
Severe complication rate ( % ) 3.6 7.0 7.6 8.3 4.9
Accuracy ( % ) 82.9 79.5 75.8 67.8 86.7 Sensitivity ( % ) 7.6 9.0 11.1 16.6 9.0 Specificity ( % ) 85.5 84.8 81.2 71.6 89.8 PPV ( % ) 1.8 4.3 4.6 4.2 3.4 NPV ( % ) 96.3 92.5 91.6 92.0 96.1 AUC 0.60 0.65 0.58 0.62 0.54
AUC: area under the receiver operating characteristic curve; PPV: positive predictive value; NPV: negative predictive value.
- 17 -
Next, we merged the data from 2010 to 2011 and from 2010 to 2012, and so on. As the merged data includes more recent years, the accuracy, sensitivity, specificity of the several years tended to improve slightly. However, they still tended to decrease over the years. This phenomenon is true even in the merged big data set from 2010 to 2014; the accuracy, sensitivity, specificity, and AUC was identified as 94.4%, 95.6%, 94.5% and 0.96 in 2014, and in 2015 the efficacy measure was 86.6%, 12.5%, 90.4%, and 0.64 respectively (Table 6).
- 18 - Table 6. Interval validation of the remodeling data set
AUC: area under the receiver operating characteristic curve; PPV: positive predictive value; NPV: negative predictive value.
- 19 -
We also analyzed which variables were associated with severe postoperative complications. Interestingly, as the merged data was getting bigger, more variables were identified to be significantly associated with the severe postoperative complication. The most common significant factors were ASA score, urea, age, and resection type of stomach and cardiac signs (p<0.01) (Table 7).
Table 7. Clinical factors associated with severe complications
Variable 2010-2011 ( n=575 ) 2010-2012 ( n=888 ) 2010-2013 ( n=1244 ) 2010-2014 ( n=1594 ) ASA score p<0.01 p<0.01 p<0.01 Urea p<0.01 p<0.05 p<0.01 p<0.05 Age p<0.05 p<0.01 p<0.05
Resection type of stomach p<0.05 p<0.01 p<0.01
Diabetes p<0.05 p<0.05
Cardiac signs p<0.05 p<0.05 p<0.05 p<0.05
Extent of lymph node dissection p<0.05 p<0.05 p<0.01
Systolic blood pressure p<0.05 p<0.01
Electrocardiogram p<0.05 p<0.05
Pulse rates p<0.01
- 20 -
Ⅳ. DISCUSSION
To predict the postoperative complication after gastric cancer surgery, multivariate analysis like the logistic regression analysis model has been usually used in previous study(18-20). This model assumes that the several variables selected contribute to postoperative complication as linear pattern. In clinical and biological system, however, nonlinear complex interactions among the variables have been widely known. Because this predictive system was made based on another institute, the results would show the difference with our data. In addition, the incidence of early gastric cancer was increased, the surgical environment has
been changed, and minimally invasive surgery was adopted. Considering this
multi-dimensional relationship, statistical predicting models like ANNs are supposed to be better than the linear model like POSSUM score. In our study, ANN that was developed by the training and test process with data from single center could show that it was a meaningful tool for predicting severe postoperative complication after gastric cancer surgery.
ANN has been used to make a prediction and decision in the various fields such as investments, science, engineering, marketing, and even gambling. Prediction system in these areas requires the consideration the complicated communications and self-learning process that means the development of new model by studying the past. The model using the system like human brain that is composed of a number of neurons, each connected to communicate, is suitable. ANN can process, analyze and learn the relationships in a lot of data quickly like human brain. In biologic and clinical system, the correlations between the variables are complicated as much as other fields. Therefore, several studies in the medical field have
- 21 -
demonstrated the success of the ANN for prediction the outcomes(12,21). The major limitation of ANN is no satisfactory explanation of their behavior has been offered. This is a limitation if ANN model interpretability is important to clinician. However, medical variables have non-linear relationships in real world. Much of multivariate analysis models (like regression model) were assuming linear relationships between the independent and dependent variables. ANN has capability of approximation non-linear function of their variables.
Gastric cancer surgery is one of the most common surgeries in the worldwide. The multicenter randomized controlled clinical trial about standard surgical procedure including D2 lymphadenectomy for gastric cancer progressed in Western society has been showed morbidity and mortality rates of 43 - 46 percent and 10 - 13 percent, respectively(22,23). However, result of large scale in Korea showed the lower morbidity and mortality rate(19,24), findings similar to the results of our study. Several reports described that it was caused by the difference of incidence, experience of surgeons, cancer characteristics including tumor location, and so on(25,26). We supposed that predicting system developed in Western countries would be inappropriate to apply to the patients in Eastern countries. In present study, only 6 (systolic blood presser, pulse rate, white cell count, urea, potassium, and hemoglobin) of 12 physiologic factors in POSSUM system were included in developing the 2010 ANN model using information gain ranking method (Table 3). As the merged data ANN model shown, there were small numbers of physiologic factors in POSSUM system identified to be significantly associated with the severe postoperative complication, such as systolic blood presser and pulse rate (p<0.001) (Table 7). As the Table 7 shown, the patients occurred diabetes, hypertension, kidney disease could increase the severe postoperative
- 22 -
complications, the patients are going to perform D2 lymphadenectomy or total gastrectomy. Therefore, we suggest that to prevent severe postoperative complications, it must pay more attention to these patients.
To assess the prediction accuracy of 2010 ANN model, we input the 2011 to 2015 data into the pre-established 2010 ANN model and the validation test for each year was conducted. As the results shown in Table 5, we identified the diagnostic value were decreased. Year by year, this could be caused by the initial modeling sample size was small, and collected data for a particular period of time. We assume that every year regularly updated date could improve diagnostic value. Therefore, we merged the data and remodeling the ANN. After training and learning performed the interval validation test again, as the merged data includes more recent years, the accuracy, sensitivity, specificity of the several years tended to improve slightly. Therefore, the ANN model requires on-going learning is necessary. As the Table 7 shown, the merged data was getting bigger, more factors were identified to be significantly associated with the severe postoperative complication. Therefore, it suggests that to propose a high predicted rate, it must be large data set(27).
This study had several limitations. Firstly, in spite of there were no significant differences in severe postoperative complications between the modeling data set and validation data set, several clinical characteristics were different each year. For instance, the proportion of open surgery (48.4%) in modeling data was higher than that of validation data set (p<0.001), and the operation time was significantly longer in modeling data set (p<0.001). The D2 lymphadenectomy was more frequently performed in validation data set (p<0.001). The reason mainly due to the surgical environment such has been changed year by year. The laparoscopic technique has been evolved from laparoscopy-assisted to totally laparoscopic,
- 23 -
and the surgical instruments such as three-dimensional laparoscope, robotic surgery system, ultrasonically activated shears, and endo-staplers have been also improved as well. Because of there were many clinical studies providing the feasibility of D2 lymphadenectomy with minimally invasive surgery(28,29), we adopted and experienced minimally invasive surgery in our hospital. Secondly, the ANN model needs to perform external validation, to assess the prediction accuracy. However, the ANN is a fair tool to predict severe postoperative complication after gastric cancer surgery. Therefore, prospective big data research is needed to prove advantage of ANN in gastric cancer surgery.
- 24 -
Ⅴ. CONCLUSION
The ANN model for predicting of severe postoperative complication after gastric cancer surgery is a fair tool with a high level of accuracy and diagnostic value compared to that of linear regression analysis. However, its diagnostic value was not reproduced in the interval validation using the recent year data. Therefore, continuous learning and training of the ANN model to reflect surgical variance seem to be needed for the application in the clinical practice.
- 25 -
REFERENCES
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Kim JG, et al. Long-term
outcomes and survival after laparoscopy-assisted distal gastrectomy for gastric cancer: three-year survival analysis of a single-center experience in Korea. J Surg Oncol. 2011;104(5):511-5.
3. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al.
Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2016.
4. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34(12):1350-7.
5. Information Committee of Korean Gastric Cancer A. Korean Gastric Cancer
Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16(3):131-40.
6. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit.
Br J Surg. 1991;78(3):355-60.
7. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of
- 26 -
8. Tekkis PP, McCulloch P, Poloniecki DR, Prytherch DR, Kessaris N, Steger AC.
Risk-adjusted prediction of operative mortality in oesophagoastric surgery with O-POSSUM. Br J Surg. 2004;91(3):288-95.
9. Eftekhar B, Mohammad K, Ardebili HE, Ghodsi M, Ketabchi E. Comparison of
artificial neural network and logistic regression models for prediction of mortality in head trauma based on initial clinical data. BMC Med Inform Decis Mak. 2005;5:3.
10. Vukicevic AM, Stojadinovic M, Radovic M, Djordjevic M, Cirkovic BA, Pejovic T,
et al. Automated development of artificial neural networks for clinical purposes: Application for predicting the outcome of choledocholithiasis surgery. Comput Biol Med. 2016;75:80-9.
11. Aleksic M, Luebke T, Heckenkamp J, Gawenda M, Reichert V, Brunkwall J.
Implementation of an artificial neuronal network to predict shunt necessity in carotid surgery. Ann Vasc Surg. 2008;22(5):635-42.
12. Baxt WG, Skora J. Prospective validation of artificial neural network trained to identify acute myocardial infarction. Lancet. 1996;347(8993):12-5.
13. Francis NK, Luther A, Salib E, Allanby L, Messenger D, Allison AS, et al. The use
of artificial neural networks to predict delayed discharge and readmission in enhanced
recovery following laparoscopic colorectal cancer surgery. Tech Coloproctol.
2015;19(7):419-28.
14. Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications. Annals of Surgery. 2004;240(2):205-13.
15. N. Cristianini, J. Shawe-Taylor, An Introduction to support Vector Machines: and
- 27 -
16. G. Stiglic, S. Kocbek, I. Pernek, P. Kokol, Comprehensive decision tree models in bioinformatics, PLoS One 2012;7 (3):e33812.
17. JV T. advantages and Disadvantages of Using Artificial Neural Networks versus Logistic Regression for Predicting Medical Outcomes. J clin epideminol. 1996;1996(49):6. 18. Kunisaki C, Makino H, Takagawa R, Sato K, Kawamata M, Kanazawa A, et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23(9):2085-93.
19. Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92(9):1099-102.
20. Persiani R, Antonacci V, Biondi A, Rausei S, La Greca A, Zoccali M, et al. Determinants of surgical morbidity in gastric cancer treatment. J Am Coll Surg. 2008;207(1):13-9.
21. Izenberg SD, Williams MD, Luterman A. Prediction of trauma mortality using a neural network. Am Surg. 1997;63(3):275-81.
22. Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345(8952):745-8.
23. Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347(9007):995-9.
24. Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased
- 28 -
Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2015.
25. Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of
gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251(4):640-6.
26. Sano T. Gastric cancer: Asia and the world. Gastric Cancer. 2017;20(Suppl 1):1-2.
27. Beleites C, Neugebauer U, Bocklitz T, Krafft C, Popp J. Sample size planning for
classification models. Anal Chim Acta. 2013;760:25-33.
28. Hong SS, Son SY, Shin HJ, Cui LH, Hur H, Han SU. Can Robotic Gastrectomy Surpass Laparoscopic Gastrectomy by Acquiring Long-Term Experience? A Propensity Score Analysis of a 7-Year Experience at a Single Institution. J Gastric Cancer. 2016;16(4):240-6.
29. Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, et al. Efficacy of
laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355.