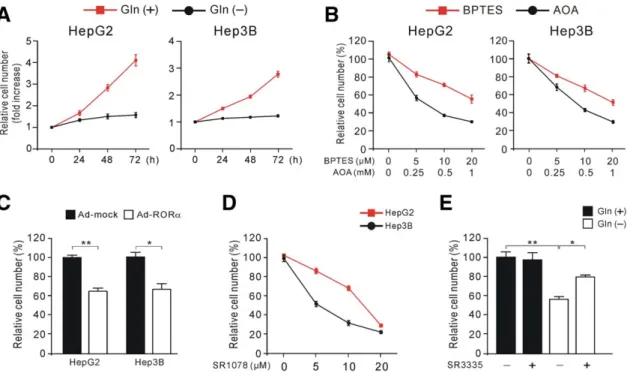
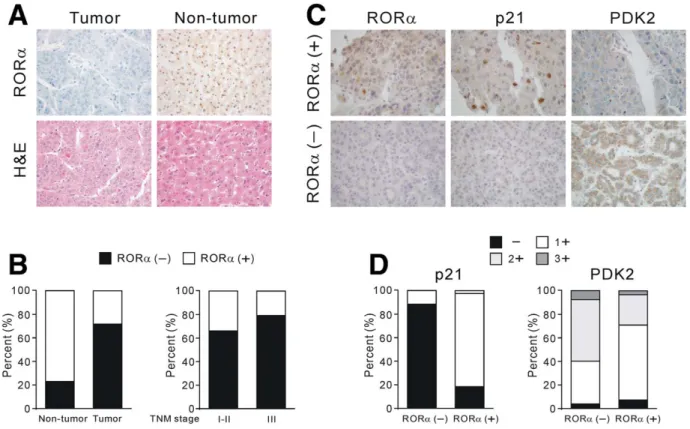
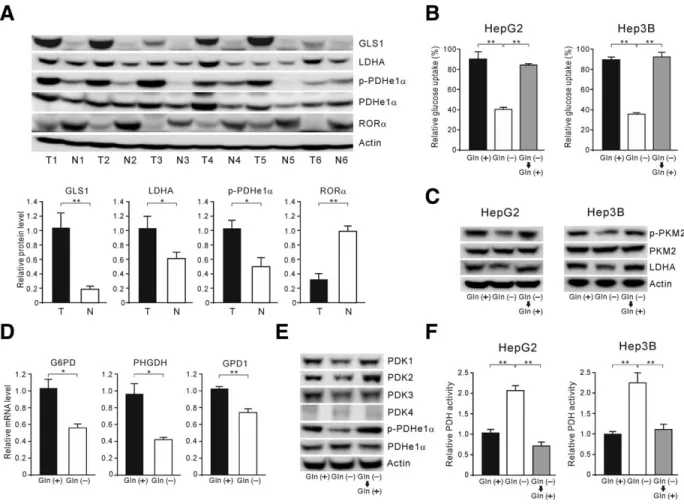
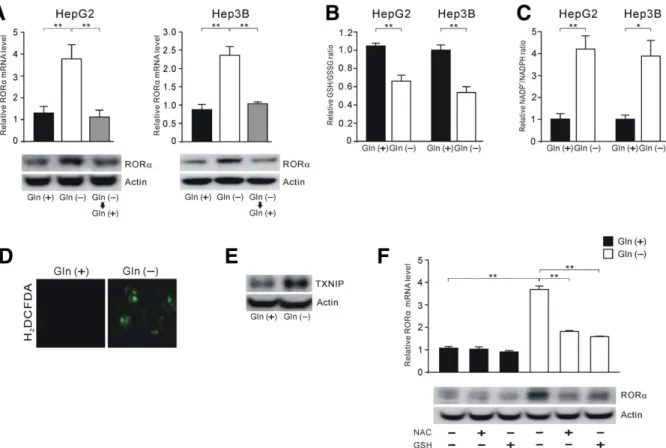
Retinoic Acid-Related Orphan Receptor Alpha Reprograms Glucose Metabolism in Glutamine-Deficient Hepatoma Cells
전체 글
수치
![Fig. 4. Inhibition of PDK2 expression by RORa is p21-dependent. (A) Western blotting analysis of p53, p21, and p-pRb protein levels, in HepG2 cells overexpressing RORa (1, 50 multiplicities of infection [MOI]; 11, 100 MOI)](https://thumb-ap.123doks.com/thumbv2/123dokinfo/4997653.304326/8.918.164.750.109.543/inhibition-expression-dependent-western-blotting-overexpressing-multiplicities-infection.webp)
관련 문서
In the present study, consistent to that report, up-regulation of VEGF mRNA was observed in the colon cancer cell with the acquired resistance to 5-FU and this
whether NME1-depleted cells impairs the Rad51 foci formation in response
Objectives: The present study was conducted to determine the relationship between degree of work performance and job satisfaction in NICU nurses.. Methods: The subjects of
The Study on the Changes of Bacterial Inorganic Phosphate Metabolism in Interactions between Nitric Oxide and Salmonlla enterica serovar Typhimurium..
In this study, two experiments were conducted to understand the effects of additional charge on the detailed growth mechanism of Alq 3 and to determine the effect of
Therefore, in order to help educational instruction in the educational field, this study aims to examine whether in families of middle school students
(1) PCR monitoring of bphC gene of Ralstonia eutropha H850 in soil Amount of bphC gene amplification of H850 cells was not generally proportional to the cell density
In the control group, lifestyle-related factors were significantly different only in blood glucose (p <.01) and there was no significant difference in