Isolated Celiac Artery Dissection and Splenic Infarction in a Patient with Protein S Deficiency:
A Case Report
Protein S (PS) deficiency is a rare blood disorder associated with thrombosis. Only a small number of cases of isolated celiac artery dissection can be found in the literature. We now report a case of isolated celiac artery dissection and splenic infarction in a 44-year old male with PS deficiency. Abdominal computed tomography revealed celiac artery dissection and splenic infarction. The patient’s PS activity was 64% (nl : 70∼140%) upon admission and 52% four weeks later. He was started on a regimen of NPO, antibiotics, and analgesics. He resumed oral intake of food and drugs on hospital day 3 and was discharged to his home on hospital day 8. We report a case of isolated celiac artery dissection with splenic infarction in a patient with PS deficiency that improved with conservative treatment. The patient’s management did not include anti-platelet/thrombotic agents or endovascular/operational procedures.
Min Jeong Kim, M.D., Byung Seup Kim, M.D., In-Gyu Kim, M.D., Jang Yong Jeon, M.D.
Department of Surgery, Hallym Sacred Heart Hospital, Hallym University College of Medicine
Corresponding Author Byung Seup Kim
Department of Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, 896, Pyeongchon-dong, Dongan-gu, Anyang 431-070, Korea
Tel: +82-31-380-3772 Fax: +82-31-385-0157 E-mail: kgiseup@hallym.or.kr
Key Words : Protein S deficiency, Celiac artery, Dissection, Splenic infarction
Received: 2010. 5. 10 Accepted: 2010. 8. 12
Introduction
Protein S (PS) is an extensively studied protein with an important function in the down-regulation of thrombin generation.1 PS deficiency is known as a risk factor for venous thromboembolism (VTE). Arterial thrombosis is very rarely described, and there is no convincing evidence that PS deficiency is a risk factor for arterial thrombosis. Dreyfus et al.2 reported mesenteric arterial thrombosis in PS deficiency for the first time in 1998.
Isolated dissection of visceral artery without associated
aortic dissection is rare,3 and, in most cases, no specific underlying cause is identified.
In this report we describe a case of isolated celiac artery dissection with splenic infarction in a patient with PS deficiency.
Case Report
Our patient was a 44-year-old male who was admitted after presenting with right upper abdominal pain that had lasted for four hours. The pain abruptly developed while he was driving, showed no radiating characteristics, and
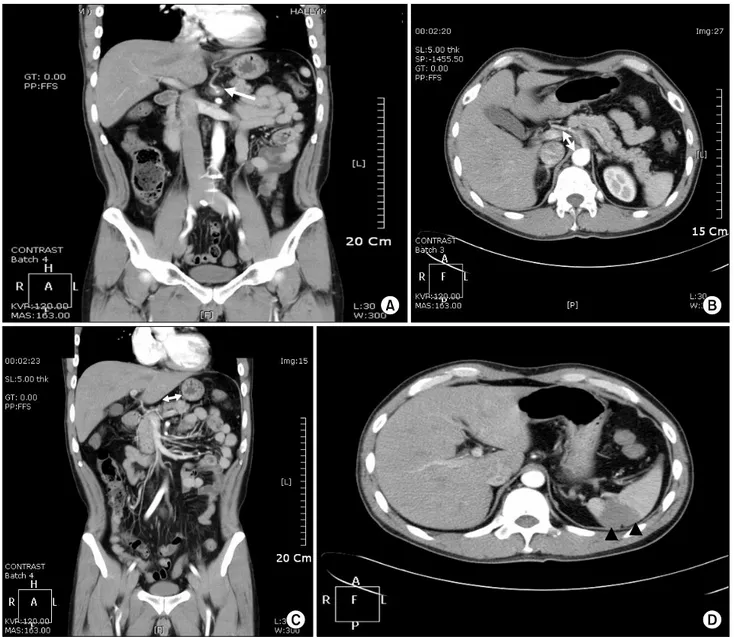
Fig. 1. Abdomino-pelvic CT on admission. An approximately 2.5 cm stretch of low-density and luminal narrowing of the proximal celiac artery are seen (white arrows in A and B). Celiac artery dissection is suggested. Thrombosis in the splenic artery is seen (white arrow in C). The arrowhead (▲) shows the area of splenic infarction (D).
was continuous. He had been diagnosed with pulmonary tuberculosis 30 years prior, and had recovered completely after medication. He had a heavy 10-pack-year smoking history, but had stopped 20 years prior to his admission.
The patient had no history of diabetes mellitus, essential hypertension, hyperlipidemia, heart disease, trauma, vasculitis, or connective tissue disease.
His blood pressure was 120/80 mmHg with a pulse rate
of 80 beats/min. Physical examination demonstrated abdo- minal tenderness in the right upper quadrant without re- bound tenderness. Routine laboratory tests showed leuko- cytosis of 14,400/mm3, but his other values were normal.
An abdominal computerized tomography (CT) scan re- vealed a 2.5 cm low-density stretch with luminal narrowing at the proximal celiac artery and thrombosis in the splenic artery (Fig. 1). Celiac artery dissection was suggested. Atherosclerosis
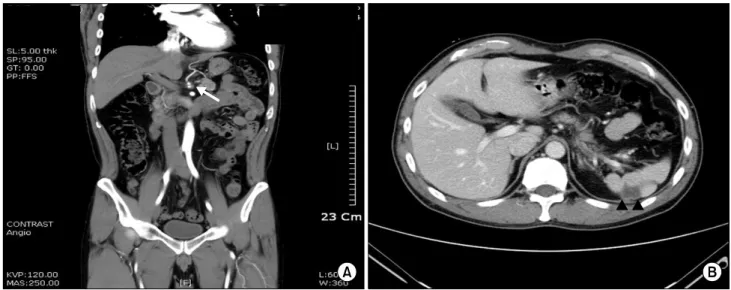
Fig. 2. Follow-up abdomino-pelvic CT on hospital day 7. There is no interval change in the low-density dissection with thrombosis along the celiac artery origin and splenic artery. Contrast flow from the celiac artery to the left gastric and hepatic arteries is improved compared to the first CT scan (A). The patient’s splenic infarction is slightly more aggravated (B). The arrow (←) shows the dissecting celiac artery. The arrowhead (▲) shows the area of splenic infarction.
Fig. 3. Second follow-up abdomino-pelvic CT (5 weeks after admission). There is no significant interval change in the low-density dissection with thrombosis at the origin of the celiac artery and splenic artery. Slightly improved contrast flow in the celiac trunk and splenic artery are demonstrated. There is no significant interval change in contrast flow in the left gastric and hepatic arteries (A). Mild improvement in the state of splenic infarction is observed (B). The arrow (←) shows the dissecting celiac artery. The arrowhead (▲) shows the area of splenic infarction.
or dissections of the abdominal aorta, superior mesenteric artery, and inferior mesenteric artery were not seen. There were no cardiac vegetations upon echocardiography, and the patient’s ejection fraction was 65%.
The test for anti-lupus Ab was negative, anti-thrombin III
was 113% (nl : 75∼125%), anti-nuclear Ab was negative, and anti-dsDNA Ab was negative. Anti-cardiolipin Ab, and anti-phospholipid Ab (IgG, and IgM) were all negative. His protein C activity was 74% (nl: 70∼130%), but his PS activi- ty was decreased to 64% (nl: 70∼140%).
The patient was started on NPO, antibiotics, and anal- gesics. His abdominal pain subsided 24 hours after con- servative treatment. He resumed oral intake on hospital day 3. A repeat CT scan 7 days after admission revealed no interval change in the low density dissection with thrombosis along the celiac artery origin and splenic artery (Fig. 2). Contrast flow from the celiac artery to the left gastric and hepatic arteries was improved, as compared to the first CT scan. His splenic infarction was slightly more aggravated. The patient was discharged home on hospital day 8.
An interval CT scan four weeks later showed no significant interval change in the low-density dissection with thrombosis at the origin of the celiac artery and splenic artery (Fig. 3). Slightly improved contrast flow in the celiac trunk and splenic artery as well as mild improvement in his splenic infarction were also noted. The patient had no specific symptoms. His PS activity was rechecked and was 52% (nl: 70∼140%). Other laboratory tests such as liver function test, complete blood count, and prothrombin time were all normal.
Discussion
PS is a vitamin K-dependent plasma glycoprotein, and possesses important anti-coagulant properties.1 PS serves as a cofactor of antigen presenting cell in the proteolytic degradation of activated coagulation factor V and factor VIII. In the early stages of clot formation, PS is involved in the regulation of fibrinolysis by inhibiting the initial thrombin formation independent of antigen presenting cell and thereby decreasing the rate of thrombin activatable fibrinolysis inhibitor activation. The prevalence of heredi- tary PS deficiency in the general population remains largely unknown, probably because of its rarity and the difficulty of making a correct diagnosis. A study of 3,788 healthy Scottish blood donors suggested that the prevalence of hereditary PS deficiency ranges from 0.03% to 0.13%.
Patients with hereditary PS deficiency typically present with
recurrent deep vein thrombosis, pulmonary embolism, or both. Less common manifestations are superficial, cerebral, visceral, or axillary vein thromboses. The treatment of VTE in patients with PS deficiency only differs from treatment of VTE without thrombophilia in terms of the duration of anti-coagulation. Warfarin treatment should be considered for up to two years, and even for life in the presence of concomitant thrombophilic defects, whereas warfarin treatment is usually continued for 3∼6 months after the first VTE in patients without thrombophilia. Kim and Kim4 reported celiac artery thrombosis and splenic infarction in a patient with protein S deficiency in Korea.
Arterial dissection is defined as cleavage of two layers of the arterial wall caused by intramural hematoma.3 A spon- taneous dissection of a visceral artery without associated aortic dissection is extremely rare. Extra-aortic dissections in order of decreasing frequency include the renal artery, coronary artery, vertebral artery, and visceral arteries. The known causes of arterial dissection are atherosclerosis, trauma, iatrogenic causes, pregnancy, fibromuscular dys- plasia, inflammatory diseases, connective tissue diseases, and other congenital disorders of the vascular wall.
Treatments of celiac artery dissection are diverse and include surgical procedures, transcatheter embolization, and medical anti-coagulation. Woolard and Ammar3 used warfarin for treatment of celiac artery dissection without known cause. Kim et al.5 reported isolated dissection of celiac artery without known cause in a Korean patient.
They used anti-coagulation and anti-hypertensive drugs for treatment of the celiac artery dissection. Takeda et al.6 reported transcatheter embolization for treatment of spontaneous isolated dissection of the celiac and hepatic arteries. Nordanstig et al.7 reported the use of a surgical procedure for spontaneous isolated dissection of the celiac trunk with rupture of the proximal splenic artery. Matsuo et al.8 reported isolated dissection of the celiac artery that was treated conservatively without anti-platelet agents, radiologic intervention, or surgical procedures, as in our case.
Generally, anti-coagulant therapy was seldom used in
artery dissection. The effect of anti-coagulant therapy for isolated splenic artery thrombosis is not well known,too.
We think larger series should be necessary to prove the effect of anti-coagulant therapy in patients with arterial thrombosis and protein S deficiency.
We report a case of isolated celiac artery dissection with splenic infarction in a patient with PS deficiency that improved with conservative treatment, without the use of anti-platelet/thrombotic agents or endovascular/operational procedures.
References
1. ten Kate MK, van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia 2008;14:1222-1228.
2. Dreyfus G, Peroux JL, Hébuterne X, Legoff D, Rampal P.
Mesenteric arterial thrombosis in protein S deficiency.
Gastroenterol Clin Biol 1998;22:955-957.
3. Woolard JD, Ammar AD. Spontaneous dissection of the celiac artery: a case report. J Vasc Surg 2007;45:1256-1258.
4. Kim CW, Kim JW. Celiac artery thrombosis and splenic lnfarction in a patient with protein s deficiency. Korean J Gastroenterol 2007;49:390-394.
5. Kim HK, Choi HH, Huh S. Spontaneous isolated dissection of the celiac artery: a case report. J Korean Soc Vasc Surg 2009;25:53-56.
6. Takeda H, Matsunaga N, Sakamoto I, Obata S, Nakamura S, Hayashi K. Spontaneous dissection of the celiac and hepatic arteries treated by transcatheter embolization. AJR Am J Roentgenol 1995;165:1288-1289.
7. Nordanstig J, Gerdes H, Kocys E. Spontaneous isolated dissection of the celiac trunk with rupture of the proximal splenic artery: a case report. Eur J Vasc Endovasc Surg 2009;37:194-197.
8. Matsuo R, Ohta Y, Ohya Y, et al. Isolated dissection of the celiac artery--a case report. Angiology 2000;51:603-607.