ABSTRACT
Objective: To evaluate the effectiveness of oral contraceptive pill (OCP) as therapy for endometrial hyperplasia (EH) without atypia in reproductive-aged women compared with oral progestin.
Methods: A retrospective cohort study was carried out in our reproductive center.
Consecutive patients diagnosed with infertility and non-atypical EH identified through electronic database who met inclusion criteria (n=309). Patients were assigned to two treatment groups: OCP (n=216) and oral progestin (n=93); clinical and reproductive outcomes were recorded.
Results: Reversal of EH to normal endometrium, clinical pregnancy, live birth and miscarriage rate. Women in OCP group were younger, had higher prevalence of Polycystic Ovary Syndrome and other uterine pathology and longer duration of infertility than women in progestin group. Reversal of EH was observed in 93.52% women on OCP and in 86.02%
women on progestin (p=0.032; adjusted odds ratio [aOR]= 2.35; 95% confidence interval [CI]=1.06-5.21) after the initial course of treatment for 2 to 6 months. Cyclic OCP (n=184) resulted in better response to treatment compared to continuous OCP (n=32) (95.11% vs.
84.38%; p=0.039; aOR =3.60; 95% CI =1.12-11.55). Clinical pregnancy rate in OCP group was marginally higher than progestin group (87/208, 41.83% vs. 27/90, 30.00%; p=0.054).
Miscarriage (25.29% vs. 29.63%; p=0.654) and live birth rate (31.25% vs. 21.11%; p=0.074) were comparable between the groups.
Conclusion: For the first time we demonstrate that OCP is an effective therapy for non- atypical EH and is associated with higher remission rate compared with oral progestin.
Reproductive outcomes are reassuring and comparable between the two groups.
Keywords: Endometrial Hyperplasia; Contraceptives, Oral, Combined; Progestins;
Drug Therapy; Infertility; Reproductive History
Original Article
Received: Jul 25, 2018 Revised: Dec 3, 2018 Accepted: Jan 1, 2019 Correspondence to Caihong Ma
Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, No. 49, North Garden Road, Haidian District, Beijing 100191, China.
E-mail: macaihong@263.net
*Yang Wang and Victoria Nisenblat contributed equally to this work.
Copyright © 2019. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://
creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ORCID iDs Yang Wang
https://orcid.org/0000-0002-5596-2982 Victoria Nisenblat
https://orcid.org/0000-0003-4553-4631
Yang Wang ,1,2,* Victoria Nisenblat ,1,2,* Liyuan Tao ,3 XinYu Zhang ,1,2 Hongzhen Li ,1,2 Caihong Ma 1,2
1 Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
2Key laboratory of Assisted Reproduction, Ministry of Education, Beijing, China
3Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, China
Combined estrogen-progestin pill
is a safe and effective option for
endometrial hyperplasia without
atypia: a three-year single center
experience
Liyuan Tao
https://orcid.org/0000-0001-7679-2700 XinYu Zhang
https://orcid.org/0000-0001-8562-9489 Hongzhen Li
https://orcid.org/0000-0002-3774-6231 Caihong Ma
https://orcid.org/0000-0002-7270-4379 Funding
This work was supported by the Key Clinical Project of Peking University Third Hospital (grant number: BYSY2015002).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: W.Y., N.V., M.C.; Formal analysis: W.Y., N.V., T.L.; Funding acquisition:
M.C.; Investigation: W.Y., Z.X.; Methodology:
W.Y., N.V., M.C.; Resources: M.C.; Supervision:
M.C.; Validation: Z.X., L.H.; Writing - original draft: W.Y., N.V.; Writing - review & editing:
N.V., W.Y., M.C., L.H.
INTRODUCTION
Endometrial hyperplasia (EH) is a pre-cancerous proliferation of endometrium that results in increased volume and altered architecture of endometrial tissue with endometrial gland to stroma ratio of greater than 1:1 [1]. New 2014 World Health Organization (WHO) classification stratifies EH by the presence of nuclear atypia into atypical and non-atypical forms, which correspond to low and moderate-high risk of developing endometrial cancer [2]. Non-atypical hyperplasia occurs more commonly, and while undergoes spontaneous regression in 80% of cases, 3%–10% progress to atypical forms and 1%–5% to endometrial cancer [3-5].
EH occurs in 6.19–114.36 per 100,000 reproductive-aged women and these estimates are thought to be higher in view of considerable number of asymptomatic undiagnosed women [6]. EH is more common in women presenting with infertility, particularly those of advanced maternal age, and often coexists with polycystic ovary syndrome (PCOS), with a reported prevalence of 23%–36% [7-9].
Most clinical guidelines recommend progestins as a first-line treatment option for non- atypical EH and abstaining from pregnancy until normal endometrium is confirmed on histopathology [10-12]. The disadvantages of progestin therapy include systemic side effects or vaginal bleeding, which are more pronounced with high-dose oral preparations. In addition, progestins can cause thinning of the endometrium and may require considerable time for endometrial recovery after treatment, which may not be acceptable to women who desire immediate pregnancy. There is, however, relative paucity of information about the effect of EH on fertility outcomes and most data are derived from small retrospective analyses in women with atypical EH or early endometrial cancer [13-15].
Since first introduced in early 1960's, combined estrogen-progestin oral contraceptive pills (OCPs) have been used by millions of women worldwide, providing effective contraception and other non-contraception benefits [16,17]. It has been demonstrated that OCPs reduce risk of endometrial cancer with long-lasting post treatment effect [18,19]. Continuous estrogen-progestin replacement in postmenopausal women has been associated with complete regression of pre-existing complex EH and reduced long-term risk of endometrial cancer [20,21]. Serious risks of OCP, such as thromboembolic events, stroke or gallbladder disease are infrequent among healthy women and common side effects are often self-limiting [22]. Thus, it is reasonable to infer that OCP can be effective management option for EH in young women, however, there are no clinical evaluations to support this assumption.
In our institution, OCPs have been initially introduced for managing EH in women who did not respond to progestins and became increasingly utilized as first-line choice due to favorable response. In this study, we aimed to summarize our experience and to evaluate the efficacy of short-term OCP versus oral progestin therapy for non-atypical EH in reproductive- aged women with infertility. We also assessed the reproductive outcomes following
successful reversal of EH with either treatment approach.
MATERIALS AND METHODS
1. Study design and participants
This retrospective cohort study was conducted at the Center for Reproductive Medicine of the Peking University Third Hospital, a teaching university hospital and the center of excellence in reproductive medicine. Consecutive women who presented for evaluation and management of infertility between January 1, 2014 and August 31, 2017 and were diagnosed with non-atypical EH on histopathology were considered for inclusion. The reasons for endometrial sampling were: abnormal uterine bleeding (AUB), previous history of EH or thickened endometrium on ultrasound examination. Only patients who agreed for medical management of EH and for whom repeat histopathology of endometrium was available to ascertain their response to treatment were included in the study. Exclusion criteria were:
atypical EH or malignancy on initial histopathology and concomitant treatment with OCP and progestin. Patients were identified through the hospital electronic billing and pathology databases. Demographic and clinical data, and post-treatment reproductive outcomes were obtained for each participant from the hospital records. We contacted all the patients when the data on reproductive outcomes were not available from the records. Patients were considered as lost to follow-up when they were not contactable after five attempts.
2. Treatment protocol
Following the initial diagnosis, the patients were offered oral progestin or OCP for the period of two to six months. Progestin treatments included Duphaston (dydrogesterone 10 mg;
20–40 mg/day), Fukang (norethisterone 0.625 mg; 5–10 mg/day), Beien/Methypregnone/
Provera (medroxyprogesterone acetate 250 mg/100 mg/10 mg; 40–250 mg/day) or Mi Tuosuo (megestrol acetate 40 mg; 40–120 mg/day). OCP treatment included Diane-35 (ethinyl estradiol 0.035 mg, cyproterone acetate 2 mg; 1 tablet/day) and Marvelon (ethinyl estradiol 0.03 mg, desogestrel 0.15 mg; 1 tablet/day). The treatment regime, including the type and dose of medication, and duration of treatment was at the discretion of the treating clinician in consultation with the patient. Upon completion of the initial treatment, study participants underwent review and endometrial sampling with a Pipelle instrument to ensure regression occurred. Women in whom regression to normal endometrium was confirmed, did not have additional histological evaluation and were recommended annual surveillance with their medical practitioner. Women who did not show histological regression, were given a choice of an additional course of hormonal therapy with OCP or progestin followed by histological surveillance at three–six monthly intervals until regression.
3. Histological diagnosis
Histological assessment of the endometrial samples was performed in the central laboratory of the Peking University Third Hospital by a team of pathologists with 10 to 20 years' experience. EH was diagnosed by using light microscopy according to 2014 WHO classification [2]. Complete remission was considered when post-treatment histopathology demonstrated regular proliferative or secretory endometrium or findings consistent with progestin effect (atrophic glands and pseudo-decidualized stroma) with no evidence of hyperplasia. Each sample was independently evaluated by two pathologists who were aware of previously diagnosed EH but were blinded to other clinical information.
4. Outcome measures
The primary outcome measure was complete post-treatment remission, determined as reversal of EH to normal endometrium.
The secondary outcomes were: 1) AUB during treatment, 2) clinical pregnancy, 3) miscarriage and 4) live birth rate (LBR). AUB was defined as heavy or prolonged menstrual bleeding lasting >8 days, or intermenstrual bleeding of any heaviness or duration [23]. Clinical pregnancy was defined as presence of gestational sac observed on ultrasound. Clinical pregnancy rate (CPR) was calculated as the number of clinical pregnancies divided by the number of women who were trying to achieve pregnancy. Miscarriage was defined as the loss of a clinical pregnancy before 24 weeks gestation and was classified as early miscarriage (pregnancy loss before 12 weeks) and late miscarriage (loss at 12–24 weeks). Miscarriage rate (MR) was calculated as the number of miscarriages divided by the number of clinical pregnancies. Live birth was defined as the birth of at least one living child, irrespective of the duration of gestation. LBR was calculated as the number of women who achieved live birth divided by the number of all patients who pursued childbearing, censoring out the subjects with continuing pregnancy at the time of the data analysis. All outcomes were evaluated on an intention to treat basis.
5. Statistical analysis
Continuous data were presented as mean±standard deviation (SD) or median and interquartile range (IQR) where appropriate. The independent t-test or Mann-Whitney U test were used for comparisons of continuous variables between the groups depending on the distribution of the data. Categorical data were presented as counts and percentages, and comparisons were performed using the Pearson χ2 test or Fisher's exact test as appropriate.
The odds ratio (OR) were adjusted odds ratio (aOR) by using the multivariable binary logistic regression based on the significantly different variables in the univariate analysis. Sensitivity analysis of clinical pregnancy was performed with excluding the patients lost to follow-up or counting them as pregnant. Statistical significance was set at a two-sided p-value <0.05. All statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA).
6. Institutional Review Board approval
All patients treated in our center provide generic consent for use of their personally unidentified data for research and hence no additional study-specific written participation consent was sought. Institutional Review Board approval for this study was obtained from the Ethics Committee of the Peking University Third Hospital (#2017SZ-071).
RESULTS
Overall, 309 patients met the inclusion criteria: 216 women were treated with OCP and 93 women received progestin. Endometrial biopsy at initial diagnosis was obtained at hysteroscopy (233 women) or following in-office endometrial sampling (76 women). The baseline characteristics of the study population are presented in Table 1. Women in OCP group were younger than women in progestin group (31.12±4.45 vs. 33.17±4.29; p<0.001), but this difference was of marginal clinical significance. More women in OCP group were diagnosed with PCOS (43.06% vs. 30.11%; p=0.032) and had other uterine pathology, such as uterine septum, leiomyoma, adenomyosis and unicornuate uterus (55.56% vs. 43.01%;
p=0.043). Duration of infertility was shorter in OCP than in progestin group (median 5, IQR 2–7 years vs. 6, IQR 3–8 years, respectively; p=0.036). Other demographic and clinical characteristics were comparable between the groups. Most participants were nulliparous and hormone treatment-naive. None of the participants reported serious side effects associated with hormone treatments such as venous thromboembolism, stroke or gallbladder disease.
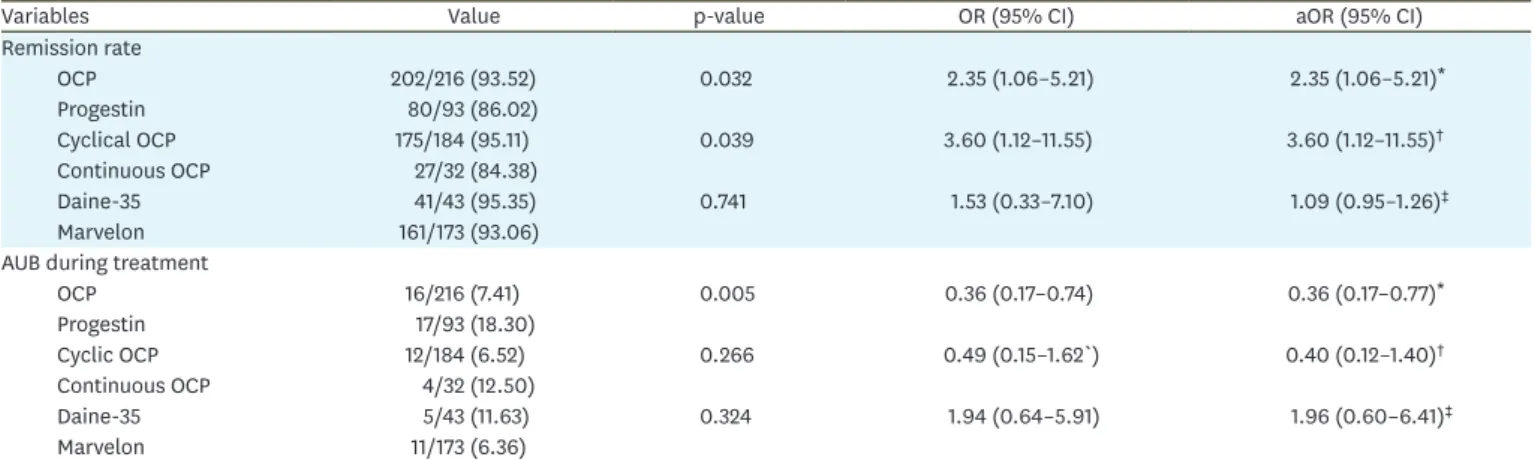
The duration of treatment was similar between OCP and progestin groups (median 3, IQR 2–3 months vs. 3, IQR 3–3 months, respectively; p=0.063). Upon completion of the treatment course, 200/309 (64.73%) women underwent hysteroscopy and 109/309 (35.28%) women had in-office endometrial biopsy. Complete remission was confirmed in 282/309 (91.26%) women. Response to treatment is detailed in Table 2. Women treated with OCP were more likely to achieve complete remission than those treated with progestin (202/216, 93.52% vs.
80/93, 86.02%, respectively; p=0.032; aOR=2.35; 95% confidence interval [CI]=1.06–5.21).
The rate of AUB was significantly lower in OCP (16/216, 7.41%) compared to progestin group (17/93, 18.30%); p=0.005. Cyclic OCP was associated with significantly higher response to treatment (175/184, 95.11%) than continuous administration of active pill (27/32, 84.38%);
p=0.039; aOR 3.60; 95% CI=1.12–11.55. Both Marvelon and Diane-35 led to a comparable reversal rate of EH and had similar bleeding profile. Separate comparison between the three most used progestin regimes, namely dydrogesterone (46 women, 49.46%), medroxyprogesterone (28 women, 30.11%) and megestrol acetate (12 women, 12.90%) Table 1. Baseline characteristics of the study population
Variables OCP group (n=216) Progestin group (n=93) p-value
Demographic characteristics
Age (yr) 31.12±4.45 33.17±4.29 <0.001
BMI (kg/m2) 26.40±4.50 26.27±4.58 0.810
Clinical characteristics
PCOS 93 (43.06) 28 (30.11) 0.032
Oligomenorrhea 136 (62.96) 57 (61.29) 0.781
AUB 59 (27.31) 26 (27.96) 0.908
Other uterine pathology* 120 (55.56) 40 (43.01) 0.043
Endometrial thickness on US before treatment
(cm) 1.1 (0.8–1.3) 1.0 (0.8–1.4) 0.772
Impaired glucose tolerance 51 (23.61) 20 (21.51) 0.678
Treatment with metformin 40 (18.52) 14 (15.22) 0.462
Family history of DM 3 (1.39) 1 (1.08) >0.999
Previous hormone therapy 90 (41.67) 45 (48.39) 0.275
Past history of EH 28 (12.96) 19 (20.43) 0.094
Reproductive characteristics
Gravidity 0 (0–0) 0 (0–1) 0.271
Parity 0 (0–0) 0 (0–0) 0.767
Nulliparity 205 (94.91) 90 (96.77) 0.649
Primary infertility 164 (75.93) 65 (69.89) 0.267
Infertility duration (yr) 5 (2–7) 6 (3–8) 0.036
Infertility cause 0.590
Tubal factor 14 (6.48) 6 (6.45)
Anovulation 93 (43.06) 47 (50.54)
Endometriosis 3 (1.39) 0 (0.00)
Male factor 14 (6.48) 8 (8.60)
Unexplained 33 (15.28) 9 (9.68)
Mixed factors† 59 (27.31) 23 (24.73)
Laboratory investigations
FSH (mIU/mL) 6.10 (4.50–7.40) 6.35 (4.50–7.60) 0.733
LH (mIU/mL) 3.55 (1.90–7.30) 3.60 (1.90–7.10) 0.958
E2 (pmol/L) 192 (151–235) 177.5 (131–295.5) 0.780
Testosterone (nmol/L) 0.70 (0.70–1.10) 0.70 (0.70–0.90) 0.116
Androstenedione (nmol/L) 8.50 (5.00–13.20) 7.30 (4.70–9.90) 0.071
Treatment duration (mo) 3.00 (2.00–3.00) 3.00 (3.00–3.00) 0.063
Values are presented as mean±standard deviation, number (%), or median (IQR).
AUB, abnormal uterine bleeding; BMI, body mass index; DM, diabetes mellitus; E2, estradiol; EH, endometrial hyperplasia; FSH, follicle-stimulating hormone; IQR, interquartile range; LH, luteinizing hormone; OCP, oral contraceptive pill; PCOS, polycystic ovary syndrome; SD, standard deviation; US, ultrasonography.
*Include uterine septum, leiomyoma, adenomyosis and unicornuate uterus; †Include adenomyosis, intrauterine insemination failure, recurrent abortion, male/female chromosome abnormality, etc.
did not reveal significant difference in response rate (Table 3), although the sample size precluded meaningful conclusions.
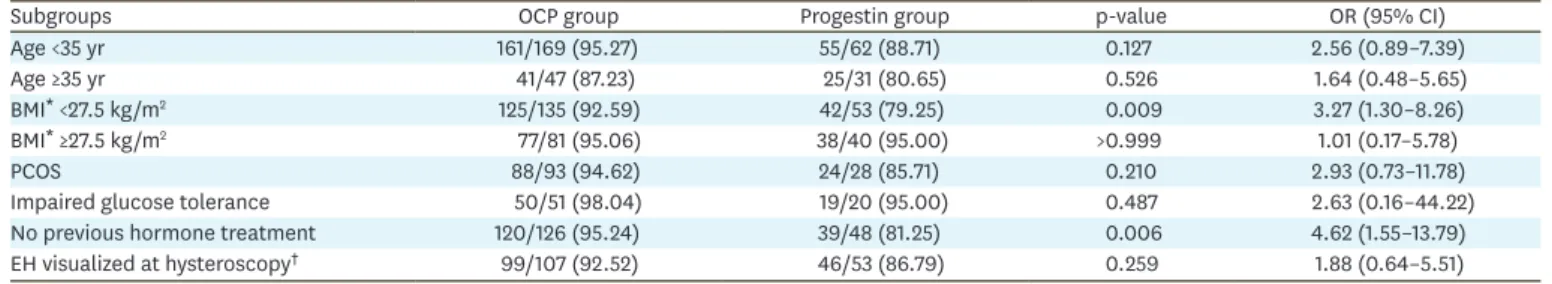
The subgroup analyses revealed that non-obese women with BMI <27.5 kg/m2 (a threshold for obesity in Asian women [24]) and women who were hormone treatment-naive had significantly higher remission rate by OCP than by progestin (Table 4). In contrast, OCP did not provide therapeutic benefit over progestin when women were stratified by age, in obese individuals (BMI ≥27.5 kg/m2), in women with PCOS or glucose intolerance and when gross findings of EH were observed at hysteroscopy (p>0.05).
No study participant was found with progression of non-atypical hyperplasia to more severe forms. Persistent disease was observed in 27 patients, of which 21 accepted an additional course of hormone therapy with either progestin (n=15), OCP (n=4), or combination of both (n=2). Among these patients, regression of EH was observed in 20 women and one woman was lost to follow-up. Six women declined second course of therapy: one woman opted for hysterectomy and five attempted pregnancy.
Overall, 298 study participants ultimately tried to conceive, of whom 114 (38.26%) achieved pregnancy: 19/298 (6.38%) women conceived spontaneously and in 95/298 (31.88%) women pregnancies resulted from assisted conception (seven following ovulation induction and 88 following in vitro fertilization-intracytoplasmic sperm injection [IVF-ICSI]). Eleven patients changed their fertility plans and decided not to pursue fertility any longer.
The reproductive outcomes for each treatment group are presented in Table 5. In OCP group, there were 87 cases of clinical pregnancy, 22 miscarriages (13 early and nine late) and 65 Table 2. Response to treatment: comparison between OCP and progestin and analyses per regime and type of OCP
Variables Value p-value OR (95% CI) aOR (95% CI)
Remission rate
OCP 202/216 (93.52) 0.032 2.35 (1.06–5.21) 2.35 (1.06–5.21)*
Progestin 80/93 (86.02)
Cyclical OCP 175/184 (95.11) 0.039 3.60 (1.12–11.55) 3.60 (1.12–11.55)†
Continuous OCP 27/32 (84.38)
Daine-35 41/43 (95.35) 0.741 1.53 (0.33–7.10) 1.09 (0.95–1.26)‡
Marvelon 161/173 (93.06)
AUB during treatment
OCP 16/216 (7.41) 0.005 0.36 (0.17–0.74) 0.36 (0.17–0.77)*
Progestin 17/93 (18.30)
Cyclic OCP 12/184 (6.52) 0.266 0.49 (0.15–1.62`) 0.40 (0.12–1.40)†
Continuous OCP 4/32 (12.50)
Daine-35 5/43 (11.63) 0.324 1.94 (0.64–5.91) 1.96 (0.60–6.41)‡
Marvelon 11/173 (6.36)
Values are presented as number (%). Adjusted analysis was performed by the multivariable binary logistic regression based on the significantly variables in the univariate analysis.
aOR, adjusted odds ratio; AUB, abnormal uterine bleeding; CI, confidence interval; OCP, oral contraceptive pill; OR, odds ratio; PCOS, Polycystic Ovary Syndrome.
*Adjusted by age, infertility duration, PCOS and other uterine pathology; †Adjusted by impaired glucose tolerance; ‡Adjusted by infertility duration, parity, PCOS and primary infertility.
Table 3. Response to treatment: comparison among different types of progestin
Variables Dydrogesterone
(20–40 mg/day) Medroxyprogesterone
(40–250 mg/day) Megestrol acetate
(40–120 mg/day) χ2 p-value
Remission rate 40/46 (86.96) 24/28 (85.71) 11/12 (91.67) 0.247 1.000
AUB during treatment 6/40 (13.04) 8/28 (28.57) 3/12 (25.00) 3.075 0.225
Values are presented as number (%).
AUB, abnormal uterine bleeding.
live births. In progestin group, there were 27 cases of clinical pregnancy, eight miscarriages (seven early and one late) and 19 live births. Most pregnancies were achieved through fertility treatments in either treatment group. The rate of spontaneous pregnancy was comparable between the groups: 11/208 (5.29%) in women from OCP and 8/90 (8.89%) in women from progestin group (p=0.243). CPR was higher in OCP than in progestin group (41.83% vs. 30.00%, respectively), but the difference only reached borderline significance (p=0.054). No significant difference was demonstrated after adjustment for age, PCOS, presence of uterine pathology and duration of infertility (data not shown). Sensitivity analysis performed with excluding the patients lost to follow-up or counting them as pregnant did not change the obtained results.
MR was somehow lower in OCP than in progestin group (25.29% vs. 29.62%, respectively), although failed to achieve statistical significance (p=0.654). LBR was 31.25% in OCP and 21.11%
in progestin group and was not significantly different between the groups (p=0.074).
DISCUSSION
To the best of our knowledge, this is the first study that evaluates use of OCP as first-line treatment option for EH and is the largest report on fertility outcomes in women with non-atypical EH. Our results demonstrate that short course of OCP is well tolerated and associated with good results. Remission of EH by OCP (93.52%) was significantly higher than by progestin (86.02%) after the initial course of treatment for two to six months. After adjustment for important demographic and clinical confounders, the odds of achieving normal endometrial histology were 2.35 higher than after similar length of treatment with progestin. Non-obese and hormone treatment-naive women had more favorable response to OCP than to progestin. We also showed improved bleeding profile with OCP treatment, which has important implication for patient compliance and satisfaction profile.
Table 4. Remission of EH: comparison between OCP and progestin treatment in different subgroups of the study population
Subgroups OCP group Progestin group p-value OR (95% CI)
Age <35 yr 161/169 (95.27) 55/62 (88.71) 0.127 2.56 (0.89–7.39)
Age ≥35 yr 41/47 (87.23) 25/31 (80.65) 0.526 1.64 (0.48–5.65)
BMI* <27.5 kg/m2 125/135 (92.59) 42/53 (79.25) 0.009 3.27 (1.30–8.26)
BMI* ≥27.5 kg/m2 77/81 (95.06) 38/40 (95.00) >0.999 1.01 (0.17–5.78)
PCOS 88/93 (94.62) 24/28 (85.71) 0.210 2.93 (0.73–11.78)
Impaired glucose tolerance 50/51 (98.04) 19/20 (95.00) 0.487 2.63 (0.16–44.22)
No previous hormone treatment 120/126 (95.24) 39/48 (81.25) 0.006 4.62 (1.55–13.79)
EH visualized at hysteroscopy† 99/107 (92.52) 46/53 (86.79) 0.259 1.88 (0.64–5.51)
Values are presented as number (%).
BMI, body mass index; CI, confidence interval; EH, endometrial hyperplasia; OCP, oral contraceptive pill; OR, odds ratio; PCOS, polycystic ovary syndrome.
*A threshold for obesity in Asian population [24]; †Refers to gross endometrial changes in a form of cystic endometrial thickening or obvious white endometrial elevations visualized at hysteroscopy, which are highly suggestive for EH.
Table 5. Reproductive outcomes in women who completed treatment for EH*
Variables OCP group Progestin group p-value OR (95% CI)
Spontaneous pregnancy 11/208 (5.29) 8/90 (8.89) 0.243 1.75 (0.68–4.50)
Assisted conception† 76/208 (36.54) 19/90 (21.11) 0.009 0.47 (0.26–0.83)
Clinical pregnancy rate 87/208 (41.83) 27/90 (30.00) 0.054 0.60 (0.35–1.01)
Miscarriage rate 22/87 (25.29) 8/27 (29.62) 0.654 0.80 (0.31–2.09)
Early miscarriage rate 13/87 (14.49) 7/27 (25.92) 0.307 1.99 (0.70–5.66)
Live birth rate 65/208 (31.25) 19/90 (21.11) 0.074 1.70 (0.95–3.05)
Values are presented as number (%).
CI, confidence interval; EH, endometrial hyperplasia; IVF-ICSI, in vitro fertilization-intracytoplasmic sperm injection; OCP, oral contraceptive pill; OR, odds ratio.
*A total of 298 women were included in the analysis; 11 women stopped pursuing parenthood and were excluded; †Included ovulation induction (OCP group: n=5, progestin group: n=2 pregnancies) and IVF-ICSI cycles (OCP group: n=71, progestin group: n=17 pregnancies).
The mechanism by which OCP lead to reversal of EH is not entirely clear. It is supposedly related to the reduced exposure to unopposed estrogen and inhibition of estrogen- induced endometrial proliferation [25]. While OCP contain both an estrogen and a progestin component, there is an essentially progestin-dominant suppressive effect on the endometrium mediated via the progesterone receptor [26]. Full progestogenic effect has been observed on endometrium of healthy women on OCP [27]. Similarly, short-term treatment with OCP resulted in endometrial changes consistent with progestin effect in women with Lynch syndrome who are genetically predisposed for developing early- onset endometrial cancer [28]. Endometrial changes included prominent inhibition of proliferation (Ki67), significant decrease in expression of EIG121, IGF-1, sFRP-1, and sFRP4 implicated in endometrial proliferation signaling pathways, and downregulation of apoptosis inhibitor survivin. The authors proposed a molecular mechanism for hormone suppression of endometrial proliferation and showed a concordance between endometrial effect of progesterone and OCP, supporting progestin-dominant effect of the combined pill [28].
The effect of estrogen-progestin treatment on EH has been predominantly evaluated in postmenopausal women on hormone replacement therapy (HRT) who were followed-up with a serial histological assessment of endometrium. Continuous administration of combined HRT resulted in 64%–100% reversal of EH to normal endometrial pattern after at least three months of exposure and has not been associated with new cases of EH during five years of treatment [20,29]. Anecdotal evidence is available on managing EH with OCP. Combined OCP-Metformin regime has been shown to cause remission of complex progestogen-resistant EH in two obese insulin-resistant individuals after three months of treatment, but this effect was partially attributed to metformin and there are no evaluations in large patient groups [30].
Indirect evidence on plausible effect of OCP on endometrium is derived from the
epidemiological studies. Large population studies consistently report protective effect of OCP on endometrial cancer risk with 40%–60% reduced risk of developing endometrial cancer in ever-users of OCP compared with never-users [18,19,31]. There is an apparent association between risk reduction and the duration of OCP treatment, an effect that persisted for more than 44 years [18,19]. The risk-reduction effect of OCP does not seem to depend on personal characteristics such as parity, adiposity or menopausal status or on the dose of estrogen [19,32]. There was no difference in risk reduction for those who used higher estrogen content OCP in the 1960's–1970's [19,33].
In this study, we observed significantly higher response to cyclic OCP regime compared to continuous administration of active pill. This can be explained by the fact that effective sloughing off the endometrial lining during hormone-free interval may play a plausible role in therapeutic effect of OCP.
Our finding of 86.02% EH remission by oral progestin is in line with previous reports. A meta-analysis of 24 observational studies comprising 1,001 women reported that treatment with oral progestins resulted in 66%–89% regression of non-atypical hyperplasia [34]. In this study women received different types of progestins for varying periods of time as there is no consensus on the preferred progestin regime for managing EH with respect to type of progestin, dose and duration of treatment [11]. We did not observe any difference in response to two types of OCP that contained different types of progestin and showed comparable regression rate when the three progestin regimes were compared with each other. No difference between three different types of oral progestins in reversing simple non-atypical
EH has been demonstrated in small randomized study, although direct comparative data in larger cohorts are lacking [35].
We demonstrated that among women who attempted conception, 38.3% (114/298) achieved clinical pregnancy and 28.2% (84/298) had live birth. Most pregnancies resulted from fertility treatments, although 6.38% of pregnancies in our cohort of infertile women were conceived naturally. There was no significant difference in fertility outcomes between women treated with OCP or with progestin, but we observed a trend towards higher pregnancy rate following OCP. It has been proposed that morphometric endometrial changes, altered intra-uterine inflammatory cascade and deranged molecular pathways observed in EH can affect endometrial receptivity, which can lead to implantation defects and decreased chance for pregnancy [36,37]. In PCOS women with simple non-atypical hyperplasia, clinical pregnancy following IVF was 28%–46%
with improved pregnancy rates and decreased odds of miscarriage after complete regression to normal endometrium [38]. Several systematic reviews reported 18%–40% pregnancy rate and 14%–35% LBR after complete remission of atypical complex EH or endometrial cancer [13-15].
Overall, weighing our findings relative to existent literature is difficult, considering that most research focused on women with more advanced disease and unclear baseline fertility status.
It has to be acknowledged that repeat endometrial curettage can increase risk of endometrial adhesions and affect fertility, therefore meticulous attention to endometrial sampling technique to minimize the endometrial trauma is crucial in women willing to retain fertility.
The importance of this study is that for the first time it provides the data on efficacy of OCP as first-line treatment option for EH in a relatively large cohort of 309 young women. We stratified the patients according to the most recent 2014 WHO classification and focused on low-risk non-atypical EH, the most common form of EH. Hence, our data are of relevance for majority of reproductive-aged women diagnosed with this condition. The presented information is important for patient counselling and can be useful for future comparisons of treatment endpoints as new studies using an updated WHO classification emerge. Despite compelling evidence from large epidemiological studies on endometrial protective effect of OCPs, they are rarely prescribed for prevention of endometrial cancer and are not used for managing EH. In addition to their potential therapeutic value in EH, OCP provide effective contraception and present a wide range of non-contraceptive benefits [17] and their role in managing young women with hyperplasia deserves exploration. Furthermore, expanding the range of effective treatment options allows to tailor management to patients' needs and offers personalized approach to patient care. We believe that our report will encourage further research efforts to evaluate the efficacy and safety of OCP for this indication.
The main limitations of this study are retrospective design, single-centre experience, arbitrary allocation of patients to the treatment groups and not uniform length of treatment.
In addition, our results may be confounded by relatively short follow-up and inability to estimate relapse rate of EH. In postmenopausal women with complex non-atypical EH relapse was observed in 28.3% following regression by oral progestin at a median of 13.7 months [39], however the risk of relapse in young low-risk patients is less clear and should be considered in future studies. In addition, while none of the study participants reported serious side effects associated with hormone treatment, we were not able to evaluate the magnitude and prevalence of other side effects including gastro-intestinal symptoms, breast tenderness, mood changes, headaches, weight gain or decreased libido. Each of these symptoms can influence quality of life of women treated for EH and negatively affect their compliance with treatment, hence should be prospectively evaluated in future studies.
Taken together, our data suggest that OCP are effective in managing non-atypical EH in young women and are associated with favorable fertility prognosis. The findings of this study require confirmation in larger cohorts in prospective randomized setting with longer follow- up of gynecological and reproductive outcomes. The questions whether OCP management is associated with shorter time to pregnancy, and what is the optimal type of OCP and duration of treatment remain unanswered. It is important to note that OCP carry risks of adverse effects, thereby may be poorly tolerated or contraindicated for some patients. Treatment decision-making should include consideration of risks, benefits and patient preferences.
Considering lack of observational data on OCP for managing non-atypical EH, we believe that this work will provide valuable evidence in the area where there is little information.
REFERENCES
1. Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer 2004;14:348-53.
PUBMED | CROSSREF
2. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. Lyon: IARC; 2014.
3. Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of
“untreated” hyperplasia in 170 patients. Cancer 1985;56:403-12.
PUBMED | CROSSREF
4. Terakawa N, Kigawa J, Taketani Y, Yoshikawa H, Yajima A, Noda K, et al. The behavior of endometrial hyperplasia: a prospective study. J Obstet Gynaecol Res 1997;23:223-30.
PUBMED | CROSSREF
5. Lacey JV Jr, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas 2009;63:39-44.
PUBMED | CROSSREF
6. Reed SD, Newton KM, Clinton WL, Epplein M, Garcia R, Allison K, et al. Incidence of endometrial hyperplasia. Am J Obstet Gynecol 2009;200:678.e1-6.
PUBMED | CROSSREF
7. Papaioannou S, Tzafettas J. Anovulation with or without PCO, hyperandrogenaemia and
hyperinsulinaemia as promoters of endometrial and breast cancer. Best Pract Res Clin Obstet Gynaecol 2010;24:19-27.
PUBMED | CROSSREF
8. Park JC, Lim SY, Jang TK, Bae JG, Kim JI, Rhee JH. Endometrial histology and predictable clinical factors for endometrial disease in women with polycystic ovary syndrome. Clin Exp Reprod Med 2011;38:42-6.
PUBMED | CROSSREF
9. Farquhar CM, Lethaby A, Sowter M, Verry J, Baranyai J. An evaluation of risk factors for endometrial hyperplasia in premenopausal women with abnormal menstrual bleeding. Am J Obstet Gynecol 1999;181:525-9.
PUBMED | CROSSREF
10. Royal College of Obstetricians and Gynaecologists (RCOG) and the British Society for Gynaecological Endoscopy. (BSGE). Management of endometrial hyperplasia. Green-top guideline No. 67. RCOG/BSGE joint guideline. London: RCOG; 2016 [cited 2017 Feb]. Available from: https://www.rcog.org.uk/en/
guidelines-research-services/guidelines/gtg67/.
11. Committee on Gynecologic Practice, Society of Gynecologic Oncology. The American College of Obstetricians and Gynecologists Committee opinion No. 631. Endometrial intraepithelial neoplasia.
Obstet Gynecol 2015;125:1272-8.
PUBMED | CROSSREF
12. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:170-99.
PUBMED | CROSSREF
13. Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia:
a systematic review and metaanalysis. Am J Obstet Gynecol 2012;207:266.e1-12.
PUBMED | CROSSREF
14. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review.
Gynecol Oncol 2012;125:477-82.
PUBMED | CROSSREF
15. Zhang Q, Qi G, Kanis MJ, Dong R, Cui B, Yang X, et al. Comparison among fertility-sparing therapies for well differentiated early-stage endometrial carcinoma and complex atypical hyperplasia. Oncotarget 2017;8:57642-53.
PUBMED
16. United Nations Department of Economics and Social Affairs Population Division. World contraceptive patterns 2013. New York, NY: United Nations; 2013.
17. Bahamondes L, Valeria Bahamondes M, Shulman LP. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum Reprod Update 2015;21:640-51.
PUBMED | CROSSREF
18. Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners' Oral Contraception Study. Am J Obstet Gynecol 2017;216:580.e1-9.
PUBMED | CROSSREF
19. Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015;16:1061-70.
PUBMED | CROSSREF
20. Wells M, Sturdee DW, Barlow DH, Ulrich LG, O'Brien K, Campbell MJ, et al. Effect on endometrium of long term treatment with continuous combined oestrogen-progestogen replacement therapy: follow up study. BMJ 2002;325:239.
PUBMED | CROSSREF
21. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353-68.
PUBMED | CROSSREF
22. Dragoman MV. The combined oral contraceptive pill -- recent developments, risks and benefits. Best Pract Res Clin Obstet Gynaecol 2014;28:825-34.
PUBMED | CROSSREF
23. Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3-13.
PUBMED | CROSSREF
24. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63.
PUBMED | CROSSREF
25. Lüdicke F, Sullivan H, Spona J, Elstein M. Dose finding in a low-dose 21-day combined oral contraceptive containing gestodene. Contraception 2001;64:243-8.
PUBMED | CROSSREF
26. Cibula D, Gompel A, Mueck AO, La Vecchia C, Hannaford PC, Skouby SO, et al. Hormonal contraception and risk of cancer. Hum Reprod Update 2010;16:631-50.
PUBMED | CROSSREF
27. Coenen CM, Hollanders JM, Rolland R, Spielmann D, Bulten J. The effects of a low-dose gestodene- containing oral contraceptive on endometrial histology in healthy women. Eur J Contracept Reprod Health Care 1996;1:325-9.
PUBMED | CROSSREF
28. Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res (Phila) 2013;6:774-81.
PUBMED | CROSSREF
29. Staland B. Continuous treatment with natural oestrogens and progestogens. A method to avoid endometrial stimulation. Maturitas 1981;3:145-56.
PUBMED | CROSSREF
30. Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol 2008;112:465-7.
PUBMED | CROSSREF
31. Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010;127:442-51.
PUBMED
32. Wentzensen N, de Gonzalez AB. The Pill's gestation: from birth control to cancer prevention. Lancet Oncol 2015;16:1004-6.
PUBMED | CROSSREF
33. Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med 1980;302:551-4.
PUBMED | CROSSREF
34. Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2010;203:547.e1-10.
PUBMED | CROSSREF
35. Ozdegirmenci O, Kayikcioglu F, Bozkurt U, Akgul MA, Haberal A. Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecol Obstet Invest 2011;72:10-4.
PUBMED | CROSSREF
36. Rakha E, Wong SC, Soomro I, Chaudry Z, Sharma A, Deen S, et al. Clinical outcome of atypical endometrial hyperplasia diagnosed on an endometrial biopsy: institutional experience and review of literature. Am J Surg Pathol 2012;36:1683-90.
PUBMED | CROSSREF
37. Ip PP, Irving JA, McCluggage WG, Clement PB, Young RH. Papillary proliferation of the endometrium:
a clinicopathologic study of 59 cases of simple and complex papillae without cytologic atypia. Am J Surg Pathol 2013;37:167-77.
PUBMED | CROSSREF
38. Bian J, Shao H, Liu H, Li H, Fang L, Xing C, et al. Efficacy of the levonorgestrel-releasing intrauterine system on IVF-ET outcomes in PCOS with simple endometrial hyperplasia. Reprod Sci 2015;22:758-66.
PUBMED | CROSSREF
39. Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. Relapse of endometrial hyperplasia after conservative treatment: a cohort study with long-term follow-up. Hum Reprod 2013;28:1231-6.
PUBMED | CROSSREF