표면적이 증가된 분별침전에서 친수성 고분자물질을 이용한 paclitaxel의 입자 크기 감소
임용규, 김진현*
공주대학교화학공학부
Received: October 12, 2015 / Revised: October 30, 2015 / Accepted: November 6, 2015
서 론
Paclitaxel
은현재난소암,
유방암,
카포시종양(Kaposi’s sarcoma)
및비소세포성 폐암(non-small cell lung cancer)
치료에가장효과있는항암물질이며적응증(
류마티스성관 절염및알츠하이머치료)
과처방방식의확대로향후그수 요는계속늘어날전망이다[16, 19]. Paclitaxel
의주요생산방법에는주목나무
(yew tree)
에서직접추출하는방법,
주목나무에서전구체
(baccatin III, 10-deacetylpaclitaxel
등)
를 얻어반합성하는방법,
주목나무에서유도된식물세포를배 양하는방법이있다[1, 3, 14].
식물세포배양법은기후,
환경 등에의한영향을받지않고생물반응기내에서안정적으로생산이가능하기때문에일정한품질의
paclitaxel
을대량생산할수있다는장점이있다
.
일반적으로 물에잘녹지않는 원료의약품
(active phar- maceutical ingredient, API)
의경우입자크기를작게하여그활용도를높이고자한다
.
입자크기가작아질수록제형 시용해속도,
약물분산의균일성,
경구생체이용률등을향상시킬수있다
[2, 7].
또한정제후건조단계에서잔류수분및잔류용매제거에도상당히도움이된다
[12]. Paclitaxel
은Biopharmaceutics classification system(BCS) class IV
의약품으로분류되어물에대한용해도가매우낮아그활용도에많은제한이있다
[7, 8].
입자크기를줄이기위하여,
“top-down(breaking-down)”
과“bottom-up(building-up)”
기술이이용되고있다
[2].
이미제조된큰입자의의약품을다양한방식의
milling
이나고압호모게나이저에의해작은입자로분쇄하는
top-down
기술은조업과정에서많은에너지가요구되고의약품의오염이나분해가발생할수있다
.
반면 분자수준의의약품을분무건조,
증발,
초임계안티솔벤트등 이접목된침전기법에의해작은입자의의약품을제조하는
bottom-up
기술은낮은수율과높은장비비용이발생할수있다
.
본연구에서시도되는분별침전은bottom-up
기술 에포함된다.
기존문헌에의하면분별침전시다양한종류의이온교환 수지를사용하여반응액부피당표면적을증가시킴으로써
paclitaxel
의입자크기를감소시킬뿐만아니라침전효율Decrease in the Particle Size of Paclitaxel by Increased Surface Area Fractional Precipitation Process Using Hydrophilic Polymer
Yong Kyu Lim and Jin-Hyun Kim*
Department of Chemical Engineering, Kongju National University, Cheonan 331-717, Republic of Korea
In this study, we applied an increased surface area fractional precipitation with hydrophilic polymer for decreasing the particle size of the anticancer agent paclitaxel from plant cell cultures. The addition of polymer resulted in a considerable decrease in the size of the paclitaxel precipitate. Among the polymers (HPMC 2910, PVP-K90, PVA) used, PVP-K90 was the most effective for the inhibition of precipitate growth. When PVP-K90 (0.2%, w/v) was used, the paclitaxel particles were four to five times smaller, having less than a 20 µm radius, than those obtained in the absence of the polymer. In addition, the precipitate size was inversely correlated with the absolute value of zeta potential.
Keywords: Paclitaxel, increased surface area fractional precipitation, polymer, particle size, decrease
*Corresponding author
Tel: +82-41-521-9361, Fax: +82-41-554-2640 E-mail: jinhyun@kongju.ac.kr
© 2015, The Korean Society for Microbiology and Biotechnology
(
침전시간단축)
을향상시킬수있었다[11].
또한침전공정 에고분자물질을첨가해줌으로써megestrol acetate
의입 자크기를감소시킬수있는연구결과가보고되었다[2].
이 러한연구결과로부터이온교환수지와고분자물질을접목하 여분별침전을수행할경우시너지효과에의해좀더효과적으로작은입자의
paclitaxel
을제조할수있을것으로예상된다
.
즉,
이온교환수지를이용한표면적이증가된분별침 전공정에고분자물질을첨가함으로써paclitaxel
의입자크 기를효율적으로감소시킬수있을것으로판단된다.
따라서 본연구에서는이온교환수지(Amberlite IRA 400 OH)
을이 용한표면적이증가된분별침전공정에세종류의고분자물 질[PVA(polyvinyl alcohol, MW 31,000-50,000), HPMC 2910(hydroxypropyl methylcellulose 2910), PVP-K 90 (polyvinylpyrrolidone-K 90)]
를각각첨가하여분별침전시간에따른
paclitaxel
입자성장양상을조사하고궁극적으로입자크기를효과적으로감소시킬수있는지를평가하 였다
.
재료 및 방법
식물재료
본실험에사용된식물세포배양액은
Taxus chinensis
의잎으로부터 얻은 세포주
(cell line)
를 이용하였으며,
수정된Gamborg’s B5
배지[5]
에서24
oC,
암조건으로교반(150 rpm)
하 여배양하였다.
식물세포배양후배양액으로부터decanter (Westfalia, CA150 Claritying Decanter)
와고속원심분리기(
α-Laval, BTPX205GD-35CDEEP)
를이용하여식물세포와 세포조각(cell debris)
을회수하였다.
회수한식물세포와세 포조각을합하여바이오매스라하였다.
본연구에사용된바 이오매스는(
주)
삼양제넥스로부터제공받았다.
Paclitaxel 분석
Paclitaxel
함량 분석을 위해HPLC system(Waters, USA)
과Capell Pak C
18(250
×4.6 mm, Shiseido, Japan)
칼럼을사용하였다.
이동상은아세토니트릴과증류수혼합 용액(35/65-65/35, v/v, gradient mode)
을 유속1.0 ml/min
으로흘려주었다.
시료주입양은20 ml
이며227 nm
에서UV
에의해검출하였다. HPLC
분석은표준정량곡선을이용하 였으며표준시료는Sigma-Aldrich
제품(
순도: 95%)
을사용 하였다[15].
분별침전(fractional precipitation)을 위한 시료준비 식물세포배양액으로부터회수한바이오매스와메탄올의
비율을
1/1(w/v)
로하여실온에서4
회반복추출한후농축(
원액의30%)
하였다.
액-
액추출을위해농축된메탄올용액에메틸렌클로라이드를첨가
(
메탄올농축액의25%)
하고,
30 min
동안교반후정체시켜상분리를유도하였다.
액-
액추출을수행하여
paclitaxel
이포함된하층인메틸렌클로라이드층으로
paclitaxel
을회수하여농축/
건조하였다.
건 조된추출물(crude extract)
을메틸렌클로라이드에20% (v/w)
비율로녹이고흡착제sylopute(Fuji Silysia Chemical Ltd.,
Japan)
를건조된추출물대비50% (w/w)
비율로첨가하여40
oC
항온조에서30 min
동안교반시킨후여과하였다.
여 과액은30
oC,
감압상태에서건조하고,
건조된시료를메틸렌 클로라이드에녹인후헥산에떨어뜨려헥산침전을수행하 였다(
메틸렌클로라이드/
헥산= 1/10, v/v).
헥산침전후여 과를통하여paclitaxel
침전물을얻고35
oC
에서24
시간동 안진공건조하고,
건조된시료를메탄올에용해하고(
순수 한paclitaxel
의함량: 0.5%, w/v)
메탄올이61.5%
가될때까 지한방울씩천천히증류수를떨어뜨린후4
oC
에서보관하 여paclitaxel
침전물을얻었다.
침전후침전물을여과하고35
oC
에서24
시간 동안 진공 건조하여ODS C
18 컬럼(50 mm
×500 mm, Shiseido, Japan)
과Silica
컬럼(50 mm
×500 mm, Shiseido, Japan)
이장착된HPLC
공정으로추가 정제하였다[13].
최종정제된paclitaxel
순도는98.0%
이었 으며시료준비과정을Fig. 1
에나타내었다.
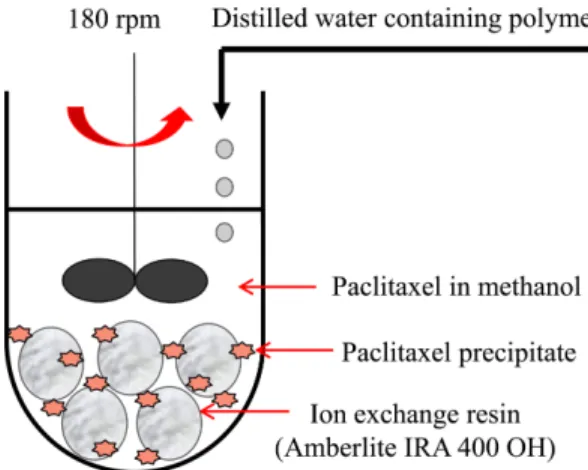
표면적이 증가된 분별침전(increased surface area fractional precipitation)
표면적이증가된분별침전공정의개략도를
Fig. 2
에나타내었다
.
기존문헌[2, 4]
에서원료의약품의입자크기감소에효과적인세종류의 고분자물질
(HPMC 2910, PVP-K 90, PVA 31,000
−50,000)
를각각농도(0.1, 0.2, 0.3%, w/v)
를달 리하여증류수에녹인후첨가하여분별침전을수행하였다.
시료를메탄올에녹이고(
순수한paclitaxel
함량기준: 0.3%,
w/v),
메탄올이61.5%
가될때까지고분자물질이포함된증류수를교반
(~180 rpm)
하에한방울씩떨어뜨렸다.
분별침전공정의반응액부피당표면적
(S/V)
증가를위해이온교환수지
(Amberlite IRA 400 OH)
를첨가(S/V = 0.428 mm
-1)
한 후4
oC
로항온항습기(KCL-2000W, EYELA, Japan)
에보관 하여paclitaxel
침전물을얻었다[11].
이온교환수지는35
oC
에서하루동안건조한후실험에사용하였다.
분별침전시간변화에따른
paclitaxel
침전양상을전자현미경을이용하여관찰하였다
.
침전물 형태 및 크기 확인
분별침전동안
paclitaxel
침전물형태확인및크기측정을 위하여 전자현미경
(SV-35 Video Microscope System,
Some Tech., Korea)
을사용하였다[6].
분별침전을통해얻은paclitaxel
침전물을고배율(
×100)
에서관찰하였다.
관찰된paclitaxel
침전물은IT-Plus software(Some Tech., Korea)
에서동화상으로확인하였으며이를통해paclitaxel
침전물 의형태와크기를확인하였다.
제타전위(zeta potential) 측정
표면적이증가된분별침전공정에서고분자물질을첨가하
지않은경우와동일농도
(0.2% w/v)
로세종류의고분자물질
(PVA, HPMC 2910, PVP-K90)
을각각첨가한경우분별 침전(24
시간)
용액에서의 제타전위를 측정하였다. ELS-Z
(Photal, Japan)
를이용하여제타전위를측정하였으며측정된제타전위는
ELS-Z software
를사용해확인하였다.
결과 및 고찰
고분자물질이 입자 형태에 미치는 영향
본연구에서는
paclitaxel
의입자크기를획기적으로감 소시키기위하여,
이온교환수지로표면적이증가된분별침Fig. 1. Preparation of sample for increased surface area fractional precipitation with hydrophilic polymer. The process consists of biomass extraction, liquid-liquid extraction, adsorbent treatment, hexane precipitation, fractional precipitation, ODS-HPLC, and silica- HPLC.
Fig. 2. Schematic diagram of increased surface area frac-
tional precipitation with hydrophilic polymer for decreasing
the particle size of paclitaxel precipitate. The surface area per
working volume (S/V: 0.428 mm
-1) of the reactor was increased by
addition of ion an exchange resin (Amberlite IRA 400 OH). The
methanol composition in water, pure paclitaxel content, and tem-
perature were 61.5% (v/v), 0.3% (w/v), and 4
oC, respectively.
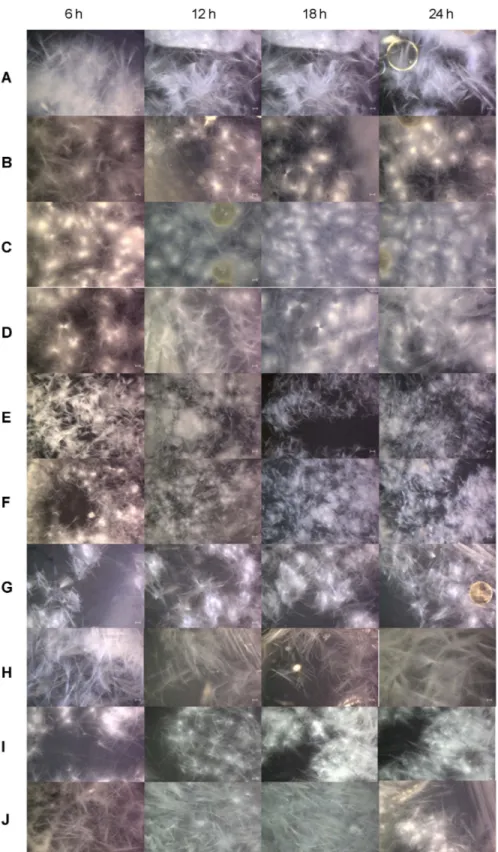
Fig. 3. Electron micrograph of paclitaxel precipitates formed by increased surface area fractional precipitation at 6 −24 h:
(A) Control, (B) 0.1% (w/v) HPMC 2910, (C) 0.2% (w/v) HPMC 2910, (D) 0.3% (w/v) HPMC 2910, (E) 0.1% (w/v) PVP-K90, (F) 0.2%
(w/v) PVP-K90, (G) 0.3% (w/v) PVP-K90, (H) 0.1% (w/v) PVA, (I) 0.2% (w/v) PVA, (J) 0.3% (w/v) PVA. The methanol composition in
water, pure paclitaxel content, and temperature were 61.5% (v/v), 0.3% (w/v), and 4
oC, respectively. Scale bar indicates 10 µm.
전공정에고분자물질을첨가하여분별침전을수행하였다
.
표면적이증가된분별침전공정에서고분자물질첨가유무 에따른paclitaxel
침전양상(
입자형태)
을조사하였다.
고분자물질첨가유무에따른입자형태를
Fig. 3
에나타내었다
.
고분자물질이첨가되지않은경우(control, Fig. 3A),
입 자모양은부채꼴형태로대칭을이루며길게뻗어나가는모 양을 보였다.
고분자물질HPMC 2910
의 농도(0.1, 0.2, 0.3%, w/v)
를달리하여첨가한경우(Fig. 3B, C, D),
고분자물질이첨가되지않은경우
(Fig. 3A)
보다전반적으로원형형태
(
둥근형태)
의입자모양을보였으며입자크기는좀더 작아지면서 가늘어짐을알수있었다.
농도가 증가할수록paclitaxel
침전물입자간좀더많이뭉쳐있는경향을보였다
.
고분자물질PVP-K90
의농도(0.1, 0.2, 0.3%, w/v)
를달 리하여첨가한경우(Fig. 3E, F, G),
입자모양은일정하고균 일한크기의부채꼴형태를나타내었으며HPMC 2910
의경 우보다전반적으로좀더뭉쳐있으며작은크기를보였다.
고분자물질PVA
의농도(0.1, 0.2, 0.3%, w/v)
를달리하여첨 가한경우(Fig. 3H, I, J),
입자모양은부채꼴형태로바늘과 같이가늘고길게형성되었으며높은농도(0.3%, w/v)
에서는 부채꼴형태라기보다는불규칙한바늘형태를나타내었다.
고분자물질이 입자 크기에 미치는 영향고분자물질첨가유무에따른입자크기를
Fig. 4
에나타 내었다.
고분자물질이첨가되지않은경우(control),
분별침 전6, 12, 18, 24
시간일 때 입자 크기는 각각48.4, 47.2,
45.8, 45.3
μm
로침전시간이경과함에따라입자크기가미미하게감소하는경향을보였다
.
선행연구에의하면표면적 증가물질(
이온교환수지)
이첨가되지않은분별침전의경우 침전시간이경과함에따라paclitaxel
침전물의입자크기가커지는경향을보였다
[11].
하지만이온교환수지첨가에의해표면적이증가된분별침전의경우침전시간이경과함에 따라입자크기가오히려감소하였는데
(Fig. 4, control),
이 는분별침전과정에서이온교환수지와핵의충돌횟수가증 가되어많은핵이생성됨에따라침전물크기가작아지는것 으로판단된다[9]. HPMC 2910
이첨가된경우(Fig. 4A),
분 별침전6, 12, 18, 24
시간에서입자크기는각각농도0.1%
(w/v)
일때46.3, 37.4, 35.4, 34.8
μm,
농도0.2%(w/v)
일때26.5, 23.0, 21.2, 21.0
μm,
농도0.3%(w/v)
일때41.0, 33.3, 32.8, 32.3
μm
이었다. HPMC 2910
농도가0.1%(w/v)
에서0.2%(w/v)
로증가할경우에는paclitaxel
입자크기가전반 적으로감소하였으나0.2%(w/v)
에서0.3%(w/v)
로농도가증가할경우에는
paclitaxel
입자크기가오히려다시증가하였다
.
즉, HPMC 2910
의경우0.2%(w/v)
농도에서가장작 은paclitaxel
침전물을얻을수있었다. PVP-K90
이첨가된 경우(Fig. 4B),
분별침전6, 12, 18, 24
시간에서입자크기는각각 농도
0.1%(w/v)
일 때27.5, 27.8, 27.6, 25.7
μm,
농도0.2%(w/v)
일때17.2, 17.1, 17.0, 16.8
μm,
농도0.3%(w/v) Fig. 4. Effect of polymer concentration on the particle size of paclitaxel during increased surface area fractional precipita- tion: (A) HPMC 2910, (B) PVP-K90, (C) PVA. The methanol composition in water, pure paclitaxel content, and precipitation temperature were 61.5% (v/v), 0.4% (w/v), and 4
oC, respectively.
●: Control; ○: 0.1% (w/v) polymer; ▼: 0.2% (w/v) polymer;△:
0.3% (w/v) polymer.
일때
22.8, 22.4, 22.4, 22.0
μm
이었다. HPMC 2910
의경 우와마찬가지로PVP-K90 0.2%(w/v)
에서가장작은입자의 침전물을얻었다. PVA
이첨가된경우(Fig. 4C),
분별침전6, 12, 18, 24
시간에서입자크기는각각농도0.1%(w/v)
일때55.2, 44.9, 40.0, 39.6
μm,
농도0.2%(w/v)
일때36.6, 34.4, 30.9, 30.5
μm,
농도0.3%(w/v)
일때48.0, 36.3, 35.1, 31.0
μm
이었다. HPMC 2910
와PVP-K90
의경우와마찬가지로PVA 0.2%(w/v)
에서가장작은입자의침전물을얻었다.
결과적으로표면적이증가된분별침전공정에고분자물질첨 가는
paclitaxel
입자크기감소에효과가있었으며,
그효과 는PVP-K90, HPMC 2910, PVA
순으로나타났다. Paclitaxel
순도(~98.0%)
및수율(>85%)
은분별침전동안거의변화가 없었으며이러한현상은기존의연구결과와도유사함을알 수있었다[11].
이상의결과로부터paclitaxel
의입자크기를 획기적으로줄이기위하여,
표면적이증가된분별침전공정 에고분자물질(HPMC 2910, PVP-K90, PVA)
을첨가하는방 식이매우효과적임을알수있었다.
첨가된고분자물질이 고체-
액체계면의표면장력을낮추고paclitaxel
핵생성속 도를증가시켜(
핵성장저해)
입자크기가작아짐을알수있었다
[4].
더나아가고체-
액체계에고분자물질흡착은새로생성되는 소수성 표면의 계면장력을 감소시키고 공간적
(steric)
또는정전기적(electrostatic)
안정화를제공하여핵성장을저해하게된다
[4].
특히PVP-K90
의경우가장작은입자의
paclitaxel
을얻을수있어침전과정에서paclitaxel
입자성장저해에가장효과적임을알수있었다.
이러한결 과는PVP-K90
와paclitaxel
입자와의높은친화력(affinity)
에기인하는것으로판단된다[2].
즉,
고분자물질과paclitaxel
입자간의친화력이증가할수록paclitaxel
입자성장을저 해하는데더효과적인공간적저해요소(steric barrier)
역할 을하기때문이다.
또한paclitaxel
입자크기감소를위한최 적의고분자물질농도(0.2%, w/v)
가존재하였는데,
이는첨 가된고분자물질의농도가일정량이상이되면용액의높은 점도로인한antisolvent(nonsolvent)
인증류수를향한용매(
메탄올
)
의적절한확산을억제하기때문으로판단된다[2].
높은고분자물질농도에따른입자크기증가현상은다른원 료의약품의경우에도보고되었다
[2, 10, 17, 18].
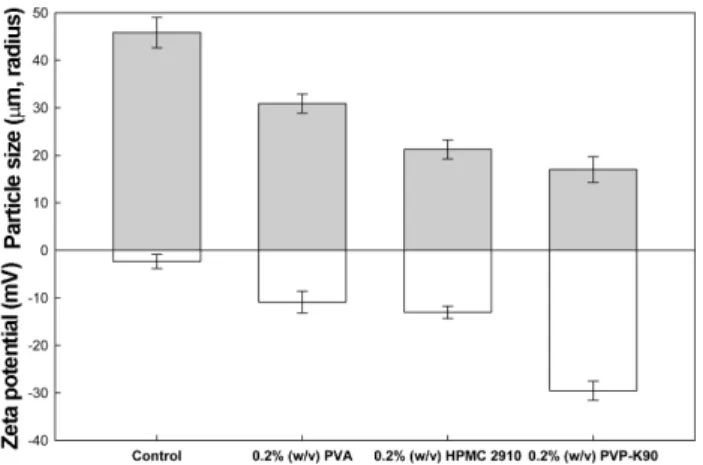
입자크기와 제타전위의 관계
표면적이증가된분별침전공정에서고분자물질을첨가하
지않은경우와동일농도
(0.2% w/v)
로세종류의고분자물질
(PVA, HPMC 2910, PVP-K90)
을각각첨가한경우분별 침전용액에서의제타전위를측정하여제타전위와입자크기 와의상관관계를조사하였다. Fig. 5
에서보는바와같이고 분자물질을첨가하지않은경우(control)
분별침전용액의제 타전위값은-2.35 mV(
입자반지름: 45.3
μm)
이었으며,
고분자물질
PVA, HPMC 2910, PVP-K90
을각각첨가하였을경우분별침전용액의제타전위값은
-10.90 mV(
입자반지름
: 30.5
μm), -13.05 mV(
입자반지름: 21.0
μm), -29.54 mV (
입자반지름: 16.8
μm)
로나타났다.
분별침전시paclitaxel
침 전물의입자크기와분별침전용액의제타전위값을비교해 보면,
제타전위절대값이증가할수록입자크기가감소하였 다.
즉,
침전물의입자크기는제타전위절대값에반비례함 을알수있었다.
표면적이증가된분별침전공정에고분자 물질을첨가할경우분별침전용액의제타전위을증가시켜 침전물을더욱안정화시키고,
이로인해침전물들이분별침 전용액속에서서로뭉치지않고안정되게유지되어작은입자의침전물이얻어지는것으로판단된다
[2, 20].
이러한결과는기존문헌에보고된
paclitaxel
침전물의입자크기와 분별침전용액의제차전위와의상관관계와도일치함을알 수있었다[15].
요 약
본연구에서는표면적이증가된분별침전공정에친수성 고분자물질을첨가하여식물세포유래항암물질
paclitaxel
의입자크기를감소시키고자하였다.
표면적이증가된분별 침전공정에고분자물질(PVA, HPMC 2910, PVP-K90)
을첨 가할경우침전물의입자크기는획기적으로감소하였다.
첨 가된고분자물질중PVP-K90
가가장효과적이었으며, PVP-
K90
농도0.2%(w/v)
로첨가한경우고분자물질을첨가하지않은 경우보다
4
−5
배 정도 가장 작은 입자 크기(<20
μm
radius)
의침전물을얻었다.
또한분별침전을통해얻은침Fig. 5. Particle size of paclitaxel precipitate and zeta potential
of reacting solution using polymer during increased surface
area fractional precipitation with an ion exchange resin
(Amberlite IRA 400 OH). The methanol composition in water,
pure paclitaxel content, precipitation temperature, and precipita-
tion time were 61.5% (v/v), 0.3% (w/v), and 4
oC, 24 h, respec-
tively.
전물의입자크기는분별침전용액의제타전위절대값에반 비례함을알수있었다
.
Acknowledgments
This research was supported by Basic Science Research Pro- gram through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant Number: 2015016271).
References
1. Baloglu E, Kingston DG. 1999. A new semisynthesis of pacli- taxel from baccatin?. J. Nat. Prod. 62: 1068−1071.
2. Cho EB, Cho WK, Cha KH, Park JS. 2010. Enhanced dissolu- tion of megestrol acetate microcrystals prepared by antisol- vent precipitation process using hydrophilic additive. Int. J.
Pharm. 396: 91−98.
3. Choi HK, Son SJ, Na GH, Hong SS, Park YS, Song JY. 2002.
Mass production of paclitaxel by plant cell culture. Korean J.
Plant Biotechnol. 29: 59−62.
4. Dalvi SV, Dave RN. 2009. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisol- vent precipitation. Ind. Eng. Chem. Res. 48: 7581−7593.
5. Gamborg OL, Miller RA, Ojima K. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res.
50: 151−158.
6. Jeon KY, Kim JH. 2009. Improvement of fractional precipita- tion process for pre-purification of paclitaxel. Process Bio- chem. 44: 736−741.
7. Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S.
2011. Formulation design for poorly water-soluble drugs based on biopharmaceytics classification system: Basic approaches and practical applications. Int. J. Pharm. 420: 1−
10.
8. Khadka P, Ro J, Kim H, Kim I, Kim TJ, Kim H, Cho JM, Yun G, Lee J. 2014. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavail- ability. Asian J. Pharm. Sci. 9: 304−316.
9. Kim SY, Kim KJ, Ryu SK. 2002. In situ analysis of growth mechanism in batch crystallization of phosphoric acid. Theo-
ries and Applications of Chem. Eng. 8: 3445−3448.
10. Labouret AD, Thioune O, Fessi H, Devissaguet JP, Puisieux F. 1995. Application of an original process for obtaining colloi- dal dispersions of some coating polymers: preparation, char- acterization, industrial scale-up. Drug Dev. Ind. Pharm. 21:
229−241.
11. Lee JY, Kim JH. 2012. Decease in the particle size of pacli- taxel by increased surface area fractional precipitation.
Korean J. Microbiol. Biotechnol. 40: 169−174.
12. Pyo SH, Kim MS, Cho JS, Song BK, Han BH, Choi HJ. 2005.
Efficient purification and morphology characterization of pacli- taxel from cell cultures of Taxus chinensis. J. Chem. Techol.
Biotechnol. 79: 1162−1168.
13. Pyo SH, Park HB, Song BK, Han HB, Kim JH. 2004. A large- scale purification of paclitaxel from cell cultures of Taxus chin- ensis. Process Biochem. 39: 1985−1991.
14. Rao KV, Hanuman JB, Alvarez C, Stoy M, Juchum J, Davies RM, et al. 1995. A new large-scale process for taxol and related taxanes from Taxus brevifolia. Pharm. Res. 12: 1003−
1010.
15. Ryu JK, Kim JH. 2012. Effect of zeta potential on fractional precipitation for the purification of paclitaxel from plant cell cul- tures of Taxus chinensis. Korean J. Microbiol. Biotechnol. 42:
114−120.
16. Schiff PB, Fant J, Horwitz SB. 1979. Promotion of microtubule assembly in vitro by taxol. Nature 277: 655−667.
17. Stainmesse S, Orecchioni AM, Nakache E, Puisieux F, Fessi H. 1995. Formation and stabilization of a biodegradable poly- meric colloidal suspension of nanoparticles. Colloid Polym.
Sci. 273: 505−511.
18. Thioune O, Fessi H, Devissaguet JP, Puisieux F. 1997. Prepa- ration of pseudolatex by nanoprecipitation: influence of the solvent nature on intrinsic viscosity and interaction constant.
Int. J. Pharm. 146: 233−238.
19. Wani MC, Talyor, HL, Wall, ME, Coggon P, McPhail AT. 1971.
Plant antitumor agent. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifo- lia. J. Am. Chem. Soc. 93: 2325−2327.
20. Zhang HX, Wang JX, Zhang ZB, Le Y, Shen ZG, Chen JF.
2009. Micronization of atorvastatin calcium by antisolvent pre- cipitation process. Int. J. Pharm. 374: 106−113.