Copyrightⓒ 2011, The Korean Academy of Oral Biology
149
Journal of Oral Biology
Protective Effects of Alpinia katsumadai Extract Against Oxidative Stress
Eul Jae Lee and Jeong Hee Kim
Department of Oral Biochemistry and Institute of Oral Biology, College of Dentistry, Kyung Hee University, 130-701, Korea (received July 5, 2011 ; revised August 24, 2011 ; accepted September 2, 2011)
In the present study, total methanol extracts prepared from Alpinia katsumadai showed significant protective effects against the oxidative stress induced by hydrogen peroxide, UV-C or γ-ray irradiation. These protective effects were substantially increased by treatment with 20~100µg/ml of the extract. The A. katsumadai total methanol preparation was further fractionated into n-hexane, dichloromethane, ethylacetate, n-butanol and water fractions. Among these five fractions, the ethylacetate and butanol fractions of A.
katsumadai showed the strongest protective effects against oxidative stress induced by UV-C and γ-ray irradiation.
These fractions also showed high DPPH radical scavenging and lipid peroxidation inhibitory activities. In addition, both fractions displayed cell proliferation activation effects, as evidenced by significant increases in colony formation. Our current data thus suggest that the mechanisms underlying the protective effects of A. katsumadai against oxidative damage may include radical scavenging, protection against cell membrane damage and stimulation of cell proliferation.
Key words: medicinal plant extract, protection, reactive oxygen species
Introduction
Reactive oxygen species (ROS) are an entire class of highly reactive molecules derived from the metabolism of oxygen.
Moreover, ROS can cause extensive damage to cells and tissues, during infections and various degenerative disorders such as cardiovascular disease, aging, and neurodegenerative
diseases like Alzheimer’s disease, mutations and cancer (Harman, 1994; Cox and Cohen, 1996; Finkel and Holbrook, 2000).
Mammalian cells possess elaborate defense mechanisms for radical detoxification. Key metabolic steps are the superoxide dismutase catalysis of the dismutation of superoxide to hydrogen peroxide and oxygen, and the conversion of H2O2 into water and oxygen by catalase and glutathione peroxidase, which destroys toxic peroxides. In addition to antioxidant enzymes, several small-molecule antioxidants play important roles in the antioxidant defense systems. These can be divided into com- pounds made in vivo, and compounds obtained from diet.
Glutathione, bilirubin, and melatonin are examples of the former, and vitamins such as α-tocopherol, β-carotene, and ascorbic acid and micronutrient elements such as zinc and selenium examples of the latter (Halliwell and Gutteridge, 1998).
In recent years, there has been a worldwide trend towards the use of the natural phytochemicals present in berry crops, teas, herbs, oilseeds, beans, fruits and vegetables (Velioglu et al., 1998; Deiana et al., 1999; Kitts et al., 2000; Lee and Shibamoto, 2000; Wang and Jiao, 2000; Lie and Xie, 2000).
Natural antioxidants have a wide range of biochemical activities, including inhibition of ROS generation, direct or indirect scavenging of free radicals, and alteration of intra- cellular redox potential (Finkel and Holbrook, 1991). One of our laboratory’s interests is on the investigation of the mech- anism of cellular response to various oxidative stress and other cell damaging agents. In addition, we are trying to develop a potential cellular damage-protective agent which can reduce cellular damages induced by various chemical and physical damages (Kim et al., 1998; Lee et al. 2003; Han et al., 2006).
The plant used in this study, Alpinia katsumadai (Zingiberaceae), was selected based on its traditional use in Chinese medicine and results reported earlier (Kim et al., 1998, 2000). This plant has been used as traditional medicine to treat a variety of gastric disorders and emesis. Recent reports have shown antiviral activity against rotavirus and
*Corresponding author: Prof. Jeong Hee Kim, Department of Biochemistry and Institute of Oral Biology, College of Dentistry, Kyung Hee University, 1 Hoeki-Dong, DongDaeMoon-Ku, Seoul, 130-701, Korea. Tel: +82-2-961-0915, Fax: +82-2-960-1457 E-mail: jhkimh@khu.ac.kr
influenza virus (Kim et al., 2010; Kwon et al., 2010). It is also reported that neuro-protective effect of A. katsumadai after ischemic damage through anti-oxidative effect (Jeong et al., 2007; Li et al., 2011). Previous investigations of A.
katsumadai have reported a variety of diarylheptanoids (Kuroyanagi et al., 1983), chalcones and flavonoids (Yushiro et al., 1968), monoterpenes, sesquiterpenoids (Saiki et al., 1978; Lawrence et al., 1972), stilbenes and labdanes (Brown et al., 1998). Yang et al. (1999), also, isolated two novel diarylheptanoids from the seeds of A. katsumadai and their structures were determined by spectroscopic analysis. Al- though it is known that these components have antioxidant activity, very little research has been done on antioxidant or biological activity in vivo of A. katsumadai. In this study, we would like to report our findings of protective effect of A.
katsumadai extract against cell damages caused by UV-C and γ-ray irradiation. In order to investigate the underlying mech- anism of the protective effect, DPPH radical scavenging, the lipid peroxidation inhibitory and colony forming activities are tested and reported.
Materials and Methods
Preparation of total methanol extract and fraction samples
A. katsumadai (100 g) was extracted at 80oC in 70%
methanol for 3 hr. The extract was then filtered and the filtrate was concentrated under low pressure using a vacuum rotary evaporator (Eyela, Japan). The remaining residue was lyophi- lized in a freezing-dryer (Ilsin, Korea) and stored at −70oC.
Approximately 10 g of powdered extract was recovered. The powder was dissolved in dimethyl sulfoxide (DMSO) and diluted with phosphate buffered saline (PBS, pH 7.4) to give final concentrations used in the experiments.
Fraction samples were prepared as the followings. Frozen- dried methanol extract sample was dissolved in d-H2O and the equal volume of n-hexane was added and extracted twice. Then the equal volume of dichloromethane (CH2Cl2), ethylacetate (EtOAc) and n-butanol (BuOH) were added to the water fraction one by one and the extraction procedure was performed twice. Each fraction samples were dried in a vacuum rotary evaporator (Eyela, Japan) and the water fraction was frozen- dried using a freezing-drier (Ilsin, Korea).
Chemicals
The following chemicals were obtained from Sigma Chem- ical Co. (St Louis, MO, USA): dimethyl sulfoxide (DMSO), [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide]
(MTT), 1,1-diphenyl-2-picrylhydrazyl (DPPH). Hydrogen per- oxide was purchased from Fluka Chemical Co. (Buchs, Swiss).
All other chemicals were of the highest analytical grade and purchased from common sources.
Cell culture, treatment and cell viability assay
Chinese hamster lung cell line V79-4 (ATCC CCL-93) was grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS), 2 mM glutamine, and antibiotics. Cultures were maintained at 37oC in a humidified atmosphere of 95% air and 5% CO2. For hydrogen peroxide treatment, V79-4 cells were seeded in a 96-well plate and incubated for 16 hr. Then 100µM H2O2 was added to the culture and cells were incubated for 24 hr at 37oC. For γ-ray treatment, cells were irradiated with indicated doses of 60Co γ-rays (Picker, USA) at a dose rate of 0.9 Gy/min at room temperature. For UV-irradiation, V79-4 cells were plated in a 96 well plate as described below and irradiated with 300 J/m2 of UV-C (254 nm) using a UV cross- linker (Stratagene, USA).
Cell viability was estimated by the MTT assay, which is based on the cleavage of a tetrazolium salt by mitochondrial dehydrogenases in viable cells (Hansen et al., 1989). V79-4 cells were treated with various concentrations of extract or fraction samples (4, 20, or 100µg/ml, respectively) for 1 hr where stated. Cells were incubated for an additional 24 hr at 37oC. During the last 4 hr, cells were incubated with 20µl of MTT stock solution (5 mg/ml) in 200µl medium at 37oC.
Samples were then extracted with acidic isopropanol and the absorbance was measured with the ELISA reader (Bio-Rad, USA) at 570 nm. The relative cell viability was determined by the amount of MTT converted to the insoluble formazan salt.
The optical density of formazan formed in control cells was taken as 100% of viability. The data are expressed as mean percentage of viable cells as compared to the respective control cultures.
DPPH free radical scavenging activity.
In order to measure antioxidant activity, the DPPH free radical scavenging assay was carried out as described else where (Lee et al., 2003).
Lipid peroxidation inhibitory activity.
Lipid peroxidation was assayed by the measurement of malondialdehyde (MDA) according to the method described elsewhere (Lee et al., 2003).
Clonogenic assay
Cells were seeded in 90 mm plates, incubated for 7 days in a CO2 incubator. The cells were fixed in methanol:acetic acid (3:1) and stained with trypan blue. Colonies of more than 50 cells were scored. Plating efficiencies were about 55~60% for the cell line used.
Statistics
All data represent means ± S.E. Statistical analysis was per- formed using analysis of variance followed by the Student’s t- test.
Results
Protective effect of total extract on various celluar damages
In order to study cell damage protective effect of a medi- cinal plant, A. katsumadai total methanol extract, we treated cells with chemical and physical stress which cause cellular damage. Cell viability after H2O2, UV-C and γ-ray treatment was reported in elsewhere (Ji and Kim 2009, Lee and Kim, 2008) and it was confirmed again in this study. First of all, cells were pre-treated with total extract before 50µM of H2O2 treatment at which cells showed approximately 50% of cell viability. At 4µg/ml concentration, there was no protective effect. However at higher concentration, the cell viability of H2O2 treated cells was increased as dose-dependent manner
of total extract treated (Fig. 1A); 109.6% and 172.1% of cell viability at 20 and 100µg/ml of total extract, respectively.
In order to study UV-C protective effect of a medicinal plant, A. katsumadai total methanol extract, we pre-treated cells with the total extract 1 hr prior to the 300 J/m2 of UV-irradiation.
When cells were pre-incubated with total extract, cell viability was significantly increased (Fig. 1B). At 4, 20 and 100µg/ml of total extract treatment cell viability was increased to 111.1%, 147.3% and 152.4%, respectively.
From cell viability assay after γ-ray irradiation, we observed that approximately 65.9% of cells were survived after 10 Gy of γ-ray irradiation as shown in Fig. 1C. In order to investigate the effect of A. katsumadai total extract, cells were pretreated with total extract 1 hr prior to 10 Gy of γ-ray irradiation. The relative cell viability was measured and plotted as shown in Fig. 3. At 4, 20 and 100µg/ml of total extract treatment cell viability was increased to 108.2%, 144.9% and 137.2%, respectively.
Protective effect of fraction samples against UV and γ -ray-induced cell damages
In order to narrow down the fractions which contain the protective activity against cell damaging stress, we prepared
Fig. 1. Effect on cell viability of A. katsumadai total methanol extract against H2O2 (A) UV (B) and γ-ray (C) induced oxidative damages. Cells were treated with various concentrations of total extract 1 hr before H2O2 or UV or γ-ray treatment, then the relative cell viability was measured. Each experiment was performed at least 3 times and data are expressed as average percent change from control ± S.D.
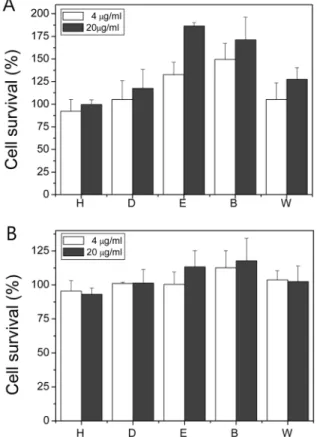
Fig. 2. Effect of fraction samples on the cell viability against UV (A) or γ-ray (B) induced oxidative stress. Cells were treated with fraction samples 1 hr prior to 300 J/m2 of UV or 10 Gy of γ-ray irra- diation. Each experiment was performed at least 3 times and data are expressed as average percent change from control ± S.D. White and gray bars indicate 4 and 20µg/ml of samples, respectively.
five fraction samples as described in materials and methods;
hexane, dichloromethane, ethylacetate, butanol and water frac- tions. Fraction samples were tested for the protective affect against UV-induced cell damage. Among 5 fraction samples, ethylacetate, and butanol fractions revealed significant pro- tective effect against UV-induced cellular damage (Fig. 2A).
At 20µg/ml, ethylacetate fraction revealed 186.4% cell via- bility compared to no-treatment control group. Butanol fraction showed 171.2% cell viability at the same concentration as that of ethylacetate fraction.
Fraction samples were tested for the protective affect against γ-ray irradiation. Among 5 fraction samples, ethylacetate and butanol fractions showed significant increased the relative cell viability of γ-ray irradiated cells. At a concentration of 20 µg/
ml of ethylacetate and butanol fraction samples, the relative cell viability was similar, 113.3% and 117.7%, respectively (Fig. 2B).
DPPH radical scavenging and Lipid peroxidation inhibition activity
In order to investigate the possible mechanism involved in the protection activity against UV-induced cellular damage, DPPH radical scavenging and lipid peroxidation inhibition activity of the fraction samples were measured. Among fraction samples, ethylacetate and butanol fractions which showed significant UV and γ-ray induced cellular damage protective effect in previous experiment were tested for DPPH radical scavenging activity. Both fractions revealed similar scavenging activities and the response was dose-dependent (Fig. 3); for ethylacetate fraction, 69% at 4µg/ml and 90% at 20 µg/ml; for butanol fraction, 69% at 4µg/ml and 88% at 20 µg/ml.
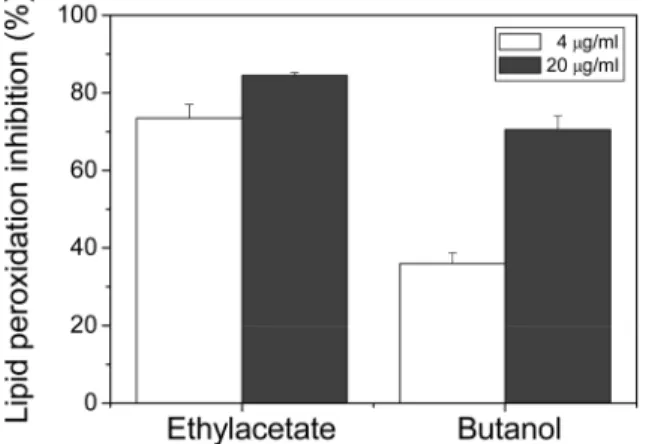
We further tested the lipid peroxidation inhibition activity of ethylacetate and butanol fraction samples. As shown in Fig. 4, Ethylacetate fraction showed slightly higher lipid peroxidation inhibitory effect; at concentration of 4µg/ml, approximately
73.5% and at 20µg/ml, approximately 84.5% of inhibition was observed. For butanol fraction 36% (at 4µg/ml) and 70.5% (at 20µg/ml) of inhibitory activity were observed.
Colony formation of ethylacetate and butanol fraction samples
The cytotoxicity of the ethylacetate and butanol fraction samples was measured using clonogenic assay (Fig. 5). Cells were seeded in multiple culture dishes incubated with 20µg/
ml of fraction samples for 7days. After 7 days of incubation, colonies with more than 50 cells were counted and the relative colony formation was plotted. As shown in Fig. 5, colony formation was slightly increased compared to no-treatment control; for ethylacetate fraction, 119.4% and for butanol fraction 110.8%.
Discussion
In recent years, there has been a worldwide trend towards the use of the phytochemicals present in natural plants.
Fig. 4. Lipid peroxidation inhibition activity of ethylacetate and butanol fraction samples. Each experiment was performed at least 3 times and data are expressed as average percent change from control ± S.D.
Fig. 5. Effect of ethylacetate and butanol fraction samples to clono- genic activity of Chinese hamster lung (V79-4) cells. Cells were treated with 20µg/ml of fraction samples and incubated for 7 days.
The relative number of colonies formed were observed and plotted.
Fig. 3. DPPH radical scavenging activity of fraction samples. Frac- tion samples were added to DPPH solution and radical scavenging activity was measured at 520 nm. Each experiment was performed at least 3 times and data are expressed as average percent change from control ± S.D. White and gray bars indicate 4 and 20µg/ml of samples, respectively.
Efforts to find oxidative cell damage attenuators including radioprotectors among plants products are also made (Arora et al., 2005). Earlier studies showed that extracts from Areca catechu var. dulcissima showed strong scavenging activity against the superoxide anion radical (Ohsugi et al., 1999). A cyclic phenylacetamide of Solvia miltiorrhiza was found to be a scavenger of the DPPH radical (Choi et al., 2001). Methyl gallate from Toona sienesis showed protective against H2O2- induced stress (Hsieh et al., 2004). The active fraction of Pilea microphylla (L.) ethanol extract showed anti-oxidant and radioprotective effect (Prabhakar et al., 2007). The radiopro- tective effect of Coleus aromaticus on Chinese hamster fibroblast cells was reported (Rao et al., 2006).
In addition, the protective activity of sulforaphane-con- taining broccoli sprout extract against UV-B-induced skin cancer was reported (Dinkova-Kostova et al., 2006). The animal received high dose of extract showed 50% reduction in tumor burden, incidence and multiplicity. Treatment of Green tea polyphenol (GTP) resulted in inhibition of UV-B-induced protein oxidation in vitro and in vivo. GTP also inhibited UV- induced expression of matrix metalloproteinases (Vayalil et al, 2004). The protective effect of soybean oil and its methanolic extract against UV-C irradiation by using comet assay was reported. It was concluded that the potential antioxidants found in the extract may be responsible for the protective effect (Wang et al., 2003).
In this study we reported that the protective effect of A.
katsumadai extract against various oxidative damage includ- ing H2O2 treatment, UV-C and γ-ray irradiation. Methanolic total extract showed significantly increased cell viability against oxidative cell damaging treatment used in this study.
Among fraction samples, ethylacetate, and butanol fractions increased the cell viability significantly. Among two fractions ethylacetate fraction showed the highest relative cell viability ratio even though both samples showed significant increase in cell viability. In order to investigate the mechanism behind the cell protection activities, we measured DPPH radical scavenging activity and lipid peroxidation inhibitory activity.
Among fraction samples, ethylacetate fraction revealed the highest DPPH radical scavenging and lipid membrane perox- idation inhibition activity. Fraction samples showed higher protective activity also revealed higher colony forming activity measured by clonogenic assay. Taken together, it seems that the mechanism behind the protective effect against oxidative cellular damaging treatment is free radical scavenging and cell membrane protection against peroxidation. In addition, increase in cell proliferation is involved in the protection mechanism. Further studies are needed to be confirmed by in vivo study and to identify the active protective molecule in fraction sample.
References
Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R,
Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: present status and future prospects.
Phytother Res. 2005;19:1-22.
Brown JE, Rice-Evans CA. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998;29:247-55.
Choi JS, Kang HS, Jung HA, Jung JH, Kang SS. A new cyclic phenyllactamide from Salvia miltiorrhiza. Fitoterapia. 2001;
72:30-4.
Cox DA, Cohen ML. Effects of oxidized low density lipoproteins on vascular contraction and relaxation. Pharmacol. Rev. 1996;
48:3-9.
Deiana M, Aruoma OI, Bianchi M, Halliwell B, Aeschbach R, Corongiu FP. Inhibition of peroxinitride-dependent DNA base modification and tyrosine nitration by the extra virgin olive oil-derived antioxidant hydroxytyrosol. Free Radic.
Biol. Med. 1999;26:762-9.
Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stepheson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high- risk mice by sulforaphane-containing broccoli sprout extracts.
Cancer Lett. 2006;240:243-52.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239-47.
Food Chem. Toxicol. 2004;42:843-50.
Halliwell B, Gutteridge JMC, Free radicals in biology and medicine, third ed. Oxford University Press, London. 1998.
Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-induction effect of ellagic acid. Anticancer Res. 2006;26:3601-6.
Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Method. 1989;119:203-10.
Harman D. Free radical theory of aging, increasing the functional life span. Ann. NY Acad. Sci. 1994;717:1-15.
Hsieh TJ, Liu TZ, Chia YC, Chern CL, Lu FJ, Chuang M, Mau SY, Chen SH, Syu YH, Chen CH. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem Toxicol. 2004;42:843-850.
Jeong GS, Li B, Lee DS, Byun E, Kang DG, Lee HS, Kim YC.
Cytoprotective constituents of Alpinia katsumadai seeds against glutamate-induced oxidative injury in HT22 cells.
Natural Product Sciences. 2007;13:268-71.
Ji SJ, Kim JH. Protective effects of Betula platyphylla var.
japonica extracts against the cellular damage induced by reactive oxygen species. Int. J. Oral Biol. 2009;34:15-20.
Kim HH, Kwon HJ, Ryu YB, Chang JS, Cho KO, Hosmillo MDT, Rho MC, Park SJ, Lee WS. Antiviral activity of Alpinia katsumadai extracts against rotaviruses. Res. Vet. Sci.
2011, in press.
Kim JH, Lee EJ, Hyun JW, Kim SH, Mar WC, Kim JK. Re- duction of radiation-induced chromosome aberration and apoptosis by dithiothreitol. Arch. Pharm. Res. 1998;21:683-87.
Kim JH, Lee EJ, Shin DO, Hong SE, Kim JK. Protective effect against oxidative stress in medicinal plant extract. J. Kor.
Asso. Radiat. Prot. 2000;25:37-43.
Kitts DD, Yuan YV, Wijewickreme AN, Hu C. Antioxidant properties of a North American gingseng extract. Mol. Cell.
Biochem. 2000;203:1-10.
Kuroyanagi M, Noro T, Fukushima S, Aiyama R, Ikuta A, Itokawa H, Morita M. Studies on the Constituents of the Seeds of Alpinia katsumadai Hayata. Chem. Pharm. Bull.
1983;31:1544-50.
Kwon HJ, Kim HH, Yoon SY, Ryu YB, Chang JS, Cho KO, Rho MC, Park SJ, Lee WS. In Vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol. J. 2010;7:307-15.
Lawrence BM, Hogg JW, Terhune SJ, Pichitakul N. Terpenoids of two Amomum species from Thailand. Phytochem.
1972;11:1534.
Lee KG, Shibamoto T. Antioxidant properties of aroma com- pounds isolated from soybeans and mung beans. J. Agric.
Food Chem. 2000;48:4290-93.
Lee MK, Kim JH. Betula platyphylla var. japonica extract prevent ultraviolet C light-induced cell damage in Chinese hamster fibroblast (V79-4) cells. Int. J. Oral Biol. 2008;33: 137-141.
Lee SE, Shin HT, Hwang HJ, Kim JH. Antioxidant activity of extract from Alpinia Katsumadai seed. Phytother. Res. 2003;
17:1041-47.
Li H, Park JH, Yan B, Yoo KY, Lee CH, Choi JH, Hwang IK, Won MH. Neuroprotection of Alpinia katsumadai seed extract against neuronal damage in the ischemic gerbil hippocampus is linked to altered brain-derived neurotrophic factor. Lab.
Anim. Res. 2011;27:67-71.
Lie C, Xie B. Evaluation of the antioxidant and pro-oxidant effects of tea catechin oxypolymers. J. Agric. Food Chem.
2000;48:6362-66.
Ohsugi M, Fan W, Hase k, Xiong Q, Tezuka Y, Komatsu K.
Active-oxygen scavenging activity of traditional nourishing- tonic herbal medicines and active constituents of Rhodiola sacra. J Ethnopharmacol. 1999;67:111-19.
Prabhakar KR, Veerapur VP, Bansal P, Parihar VK, Kandadi MR, Kumar PB, Priyadarsini KI, Unnikrishnan MK. Antioxidant
and radioprotective effect of the active fraction of Pilea microphylla (L.) ethanolic extract. Chembioint. 2007;165:22- 32.
Rao BS, Shanbhoge R, Upadhya D, Jagetia GC, Adiga SK, Kumar P, Guruprasad K, Gayathri P. Antioxidant, anticlasto- genic and radioprotective effect of Coleus aromaticus on Chinese hamster fibroblast cells (V79) exposed to gamma radiation. Mutagenesis. 2006;21:237-42.
Saiki Y, Ishikawa Y, Uchida M, Fukushima S. Essential oil from chinese drug ‘caodoukou’, the seeds of Alpinia katsumadai.
Phytochem. 1978;17:808.
Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Invest. Dermatol. 2004;122:1480-87.
Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998;46:4113-17.
Wang H, Li Q, Ying J, Luo Z, Wang Y. The protective effect and mechanism of soybean oil and its extracts on DNA damage in human ECV304 cells exposed to UV-C. Biochim. Biophys.
Acta, 2003;1626:19-24.
Wang SY, Jiao H. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J. Agric.
Food Chem. 2000;48:5672-76.
Yang Y, Kinoshita K, Koyama K, Takahashi K, Tai T, Nunoura Y, Watanabe K. Two novel anti-emetic principles of Alpinia katsumadai. J. Nat. Prod. 1999;62:1672-74.
YH, Chen CH. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxi- dative stress and DNA damage in MDCK cells.
Yushiro K, Shuhiti T, Ikuo Y. Studies on the constituents of the seeds of Alpinia Katsumadai.Yakugaku Zasshi 1968;88:239- 41.