ABSTRACT
Purpose: Clinicians still debate how to manage 2–4 cm papillary thyroid carcinoma (PTC). To understand the characteristics and prognosis of these tumors, we compared clinicopathological prognostic factors and prognosis among 1–2 cm, 2–4 cm, and >4 cm PTC.
Methods: We retrospectively reviewed the medical records of 2,079 patients with primary PTC >1 cm who were diagnosed between 2002 and 2017.
Results: The patients' mean age was 47.9±12.5 years, and 83.2% were women. The follow- up period was 81.1±41.8 months. The tumor recurred in 138 patients (6.6%), and thyroid cancer-related death developed in 3 cases (0.1%). As tumor size increased, so did the proportion of male patients, lymphovascular invasion, resection margin positivity, lymph node (LN) metastasis, metastasis in >5 LNs, T4, N stage, and M stage. Recurrence increased linearly according to tumor size, as did distant metastasis as first recurrence and progression to distant metastasis. Tumor size, N stage, metastasis in >5 LNs, and LN metastasis were significant independent risk factors for PTC recurrence. The recurrence rate of 2–4 cm PTC was 13.4%, while the risk of recurrence was 3 times higher than in 1–2 cm PTC. The 5-year recurrence free survival (RFS) rates of 1–2 cm, 2–4 cm, and >4 cm PTC were 97.0%, 88.0%, and 74.0%, respectively, while the 10-year RFS rates were 95.0%, 84.0%, and 71.0%.
Conclusion: The 2–4 cm PTC may be pathologically distinct from 1–2 cm PTC and should be treated differently.
Keywords: Papillary thyroid carcinoma; Prognosis; Recurrence; Disease free survival
INTRODUCTION
Papillary thyroid carcinoma (PTC) can be treated using thyroidectomy, thyroid stimulating hormone suppression, and radioactive iodine (RAI) therapy. The extent of thyroidectomy to treat PTC is an important consideration for both surgeons and patients. The American Thyroid Association (ATA) guidelines regarding the extent of operation have changed with each revision. In 2006, thyroid lobectomy was recommended for small thyroid cancers involving low-risk, isolated, intrathyroidal PTC without cervical nodal metastases (1). In 2009, thyroid lobectomy alone was deemed sufficient for small (<1 cm), low-risk, unifocal, intrathyroidal papillary carcinomas without prior head and neck irradiation or cervical nodal
Original Article
Received: Nov 5, 2020 Revised: Nov 30, 2020 Accepted: Dec 2, 2020 Correspondence to Yoo Seung Chung
Department of Surgery, Gil Medical Center, Gachon University College of Medicine, 21 Namdong-daero 774 beon-gil, Namdong-gu, Incheon 21565, Korea.
E-mail: dryooseung@gilhospital.com dryooseung@hanmail.net Copyright © 2020. Korean Association of Thyroid and Endocrine Surgeons; KATES This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://
creativecommons.org/licenses/by-nc/4.0/).
ORCID iDs Joon-Hyop Lee
https://orcid.org/0000-0003-0470-7719 Yun Yeong Kim
https://orcid.org/0000-0002-0077-540X Yong Soon Chun
https://orcid.org/0000-0002-7094-677X Heung Kyu Park
https://orcid.org/0000-0002-8284-9221 Sang Tae Choi
https://orcid.org/0000-0002-2074-1733 Jin Mo Kang
https://orcid.org/0000-0002-1477-9778 Yoo Seung Chung
https://orcid.org/0000-0001-9912-051X Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Min Hoi Kim1, Joon-Hyop Lee 1,2, Yun Yeong Kim 1,2, Yong Soon Chun 1,2, Heung Kyu Park 1,2, Sang Tae Choi 1,2, Jin Mo Kang 1,2, Yoo Seung Chung 1,2
1Department of Surgery, Gil Medical Center, Incheon, Korea
2Department of Surgery, Gachon University College of Medicine, Incheon, Korea
Management of 2–4 cm Papillary
Thyroid Carcinoma: Risk of Recurrence
Compared to 1–2 cm and >4 cm
Author Contributions
Conceptualization: Yoo Seung Chung; Data curation: Joon-Hyop Lee, Yun Yeong Kim, Yong Soon Chun, Heung Kyu Park, Sang Tae Choi, Jin Mo Kang; Formal analysis: Joon- Hyop Lee; Investigation: Joon-Hyop Lee;
Methodology: Yoo Seung Chung; Writing - original draft: Min Hoi Kim; Writing - review &
editing: Yoo Seung Chung.
metastases, as determined either radiologically or clinically (2). More recent guidelines recommend applying thyroid lobectomy for both ≤ 1 cm papillary thyroid microcarcinoma (PTMC) and 1–4 cm PTC without extrathyroidal extension (ETE) or clinical evidence of cervical nodal metastases (cN0) (3).
Thyroid lobectomy involves fewer postoperative complications and does not necessitate permanent T4 administration. For this reason, it is considered preferable if the prognosis regarding recurrence and survival is similar to that of total thyroidectomy. Many researchers have investigated whether thyroid lobectomy is sufficient for 1–4 cm PTC, but the answer is still debated.
The extent of thyroidectomy is determined by tumor size, prognosis, and RAI therapy. PTCs of ≤2 cm limited to the thyroid are defined as T1 according to the American Joint Committee on Cancer/tumor, node, metastasis (TNM) cancer staging system. Furthermore, several reports have found that 1–2 cm PTC was not inferior to ≤1 cm PTMC in terms of survival and recurrence (4,5). However, there is currently no consensus regarding how 2–4 cm PTC should be managed. We submit that surgeons should understand the characteristics and prognosis of 2–4 cm PTC more precisely to ensure appropriate treatment and follow-up.
In the present study, we compared clinicopathological prognostic factors and prognosis among 1–2 cm, 2–4 cm, and >4 cm PTC.
MATERIALS AND METHODS
A total of 4,973 primary PTC operations were carried out in our institution between 2002 and 2017. After excluding PTMC, 2,079 patients with PTC were selected and their medical records reviewed retrospectively.
We divided these patients into 3 groups according to tumor size: >1 and ≤2 cm (1–2 cm), >2 and ≤4 cm (2–4 cm), and >4 cm. The following prognostic factors were also identified: age, sex, ETE, lymphovascular invasion (LVI), involvement of resection margin, multiplicity, bilaterality, lymph node (LN) metastasis, TNM stage, and number of metastatic LNs.
During the study period, total thyroidectomy with bilateral central LN dissection was performed in patients with >2 cm PTC. In patients with 1–2 cm PTC, either total thyroidectomy or thyroid lobectomy was performed, depending on the contralateral lobe nodules, patient request, and suspicious LN enlargement. Central LN dissection was performed to harvest the pretracheal, paratracheal, and Delphian LNs during thyroidectomy.
Lateral neck LN dissection including levels II–V was performed in patients with suspected LN metastasis based on preoperative neck ultrasonography or computed tomography (CT). Thyroid lobectomy with ipsilateral central LN dissection was performed when it was unclear from preoperative cytology and intraoperative frozen section whether the lesion was carcinoma despite the large tumor size. Completion thyroidectomy was performed only when the patient agreed to the additional operation after a detailed explanation of why it was medically necessary.
Follow-up studies involved routine annual cervical ultrasonography and semiannual thyroid function tests. If needed, chest CT, bone scan, serum off-Tg, and 131I whole-body scan
(WBS) were performed. Distant metastasis at diagnosis was confirmed based on the first postoperative 131I WBS, postoperative chest CT, or bone scan.
Our Institutional Review Board approved this retrospective study before the patient list was retrieved from the hospital database (GCIRB2020-400).
Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Intergroup differences were assessed using analysis of variance for continuous variables, and Pearson's χ2 test, Fisher's exact test, or linear-by-linear association for categorical variables.
To evaluate recurrence-free survival (RFS), the Kaplan-Meier survival curve with log-rank test and Cox proportional hazard model were used. All P values <0.05 were considered statistically significant.
RESULTS
1. Clinicopathological characteristics of 2,079 patients with PTC
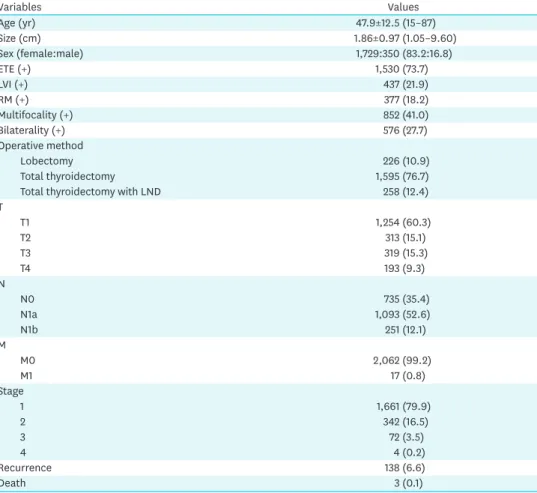
Table 1 shows the clinicopathological characteristics of the 2,079 patients with PTC. The postoperative follow-up period was 81.1±41.8 months. Total thyroidectomy was performed in 89.1% of patients (1,853/2,079), while lateral neck LN dissection was performed
Table 1. Clinicopathologic variables of 2,079 patients with papillary thyroid carcinoma
Variables Values
Age (yr) 47.9±12.5 (15–87)
Size (cm) 1.86±0.97 (1.05–9.60)
Sex (female:male) 1,729:350 (83.2:16.8)
ETE (+) 1,530 (73.7)
LVI (+) 437 (21.9)
RM (+) 377 (18.2)
Multifocality (+) 852 (41.0)
Bilaterality (+) 576 (27.7)
Operative method
Lobectomy 226 (10.9)
Total thyroidectomy 1,595 (76.7)
Total thyroidectomy with LND 258 (12.4)
T
T1 1,254 (60.3)
T2 313 (15.1)
T3 319 (15.3)
T4 193 (9.3)
N
N0 735 (35.4)
N1a 1,093 (52.6)
N1b 251 (12.1)
M
M0 2,062 (99.2)
M1 17 (0.8)
Stage
1 1,661 (79.9)
2 342 (16.5)
3 72 (3.5)
4 4 (0.2)
Recurrence 138 (6.6)
Death 3 (0.1)
Data are shown as mean±standard deviation or number (%).
ETE = extrathyroidal extension; LVI = lymphovascular invasion; RM = resection margin; LND = lateral neck dissection.
simultaneously with total thyroidectomy in 12.4% (258/2,079). Conventional PTC was found in 94.0% of patients. The second most common histological variant was follicular variant PTC (4.9%; 102/2,079). Several other variants of PTC were found, namely tall cell variant (0.4%; 9/2,079), oncocytic variant (0.3%; 7/2,079), solid variant (0.15%; 3/2,079), diffuse sclerosing variant (0.1%; 2/2,079), and encapsulated variant PTC (0.1%; 2/2,079).
Postoperative RAI therapy was performed in 83.6% of patients (1,549/1,853): 81.9% of those with 1–2 cm PTC, 88.2% of those with 2–4 cm PTC, and 90.8% of those with >4 cm PTC.
The tumor recurred in 138 patients (6.6%), and thyroid cancer-related death developed in 3 cases (0.1%). Two of the three patients who died of thyroid cancer had 2–4 cm PTC, while the other had 1–2 cm PTC. Two of these patients had M1 disease at diagnosis (lung/
liver metastasis and lung/bone metastasis, respectively). The other patient underwent total thyroidectomy for 4 cm PTC without LN metastasis and experienced lung metastasis after four local recurrences.
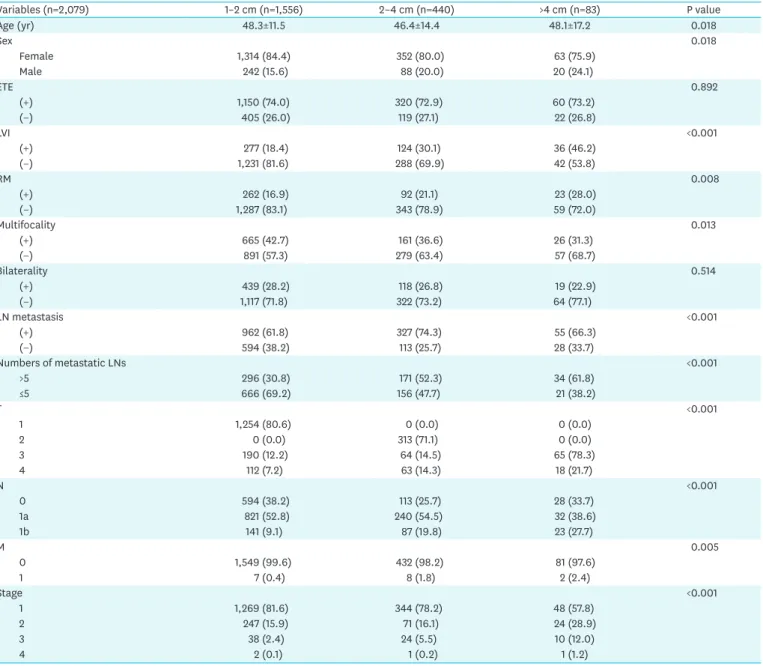
2. Differences in clinicopathological characteristics according to tumor size To evaluate the characteristics of 2–4 cm PTC, we compared the clinicopathological results of 2–4 cm PTC with those of 1–2 cm and >4 cm PTC. We describe the differences in Table 2.
As tumor size increased, so did the proportion of male patients, LVI, and resection margin positivity. Conversely, multiplicity decreased. Metastasis in >5 LNs was more common, and the N stage also increased with increasing tumor size. Distant metastasis at diagnosis was detected more often in larger tumors. Lung metastasis was the most common (16 cases), while bone metastasis was found in 2 cases. There were 2 cases of simultaneous multiple metastases, such as lung with bone or liver. When the prevalence of T4 stage was evaluated separately, it increased according to tumor size (P<0.001).
3. Recurrence in 2,062 patients with PTC
Recurrence status is described in Table 3. To gain an accurate understanding of recurrence, we excluded patients classified as M1 from the recurrence analysis. Recurrence increased according to tumor size, and distant metastasis as the first recurrence increased linearly. After several local recurrences, distant metastasis occurred in 13 cases. The proportion of progression to distant metastasis increased according to tumor size (6.8%, 10.3%, and 14.3%, respectively).
Fourteen cases of distant recurrence involved lung metastasis, while the other involved bone metastasis. One patient experienced brain metastasis after lung metastasis. Progression to distant metastasis after local recurrence involved lung metastasis in all 13 cases. A total of 123 cases of local recurrence were found: 113 in the lateral neck LN, six in the central neck LN, 3 in the contralateral lobe, and one in the mediastinal LN.
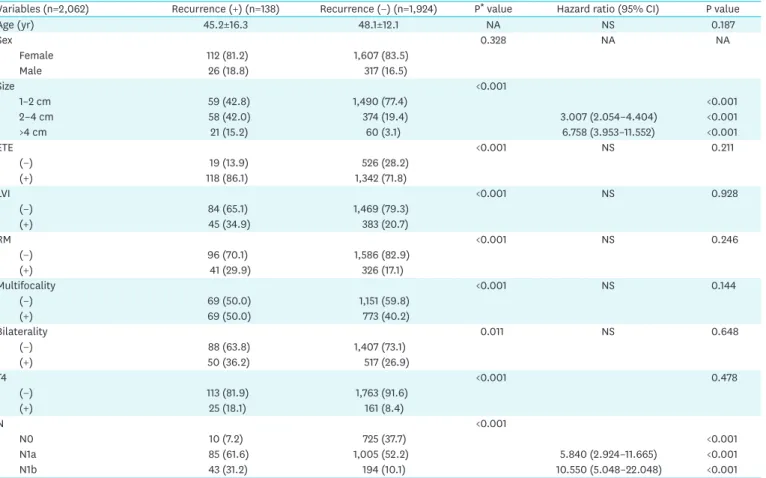
4. Risk factor analysis of recurrences
In the risk factor analysis, we analyzed all variables that were significant in the log-rank test of Kaplan-Meier curve analysis, as well as patient age. Tumor size and N stage were significant risk factors for PTC recurrence (Table 4). When “metastasis in >5 LNs” or “LN metastasis”
were used instead of N stage, both were also significant risk factors for recurrence (hazard ratio [HR], 2.362; 95% confidence interval [CI], 1.608–3.469; P<0.001 and HR, 6.549; 95%
CI, 3.312–12.952; P<0.001, respectively).
Fig. 1 show the RFS curve based on tumor size and N stage of PTC >1 cm. The 5-year RFS was 97.0%, 88.0%, and 74.0% in 1–2 cm PTC, 2–4 cm PTC, and >4 cm PTC, respectively. The
Table 2. Differences of clinicopathologic variables of 2,079 patients with papillary thyroid carcinoma according to tumor size
Variables (n=2,079) 1–2 cm (n=1,556) 2–4 cm (n=440) >4 cm (n=83) P value
Age (yr) 48.3±11.5 46.4±14.4 48.1±17.2 0.018
Sex 0.018
Female 1,314 (84.4) 352 (80.0) 63 (75.9)
Male 242 (15.6) 88 (20.0) 20 (24.1)
ETE 0.892
(+) 1,150 (74.0) 320 (72.9) 60 (73.2)
(−) 405 (26.0) 119 (27.1) 22 (26.8)
LVI <0.001
(+) 277 (18.4) 124 (30.1) 36 (46.2)
(−) 1,231 (81.6) 288 (69.9) 42 (53.8)
RM 0.008
(+) 262 (16.9) 92 (21.1) 23 (28.0)
(−) 1,287 (83.1) 343 (78.9) 59 (72.0)
Multifocality 0.013
(+) 665 (42.7) 161 (36.6) 26 (31.3)
(−) 891 (57.3) 279 (63.4) 57 (68.7)
Bilaterality 0.514
(+) 439 (28.2) 118 (26.8) 19 (22.9)
(−) 1,117 (71.8) 322 (73.2) 64 (77.1)
LN metastasis <0.001
(+) 962 (61.8) 327 (74.3) 55 (66.3)
(−) 594 (38.2) 113 (25.7) 28 (33.7)
Numbers of metastatic LNs <0.001
>5 296 (30.8) 171 (52.3) 34 (61.8)
≤5 666 (69.2) 156 (47.7) 21 (38.2)
T <0.001
1 1,254 (80.6) 0 (0.0) 0 (0.0)
2 0 (0.0) 313 (71.1) 0 (0.0)
3 190 (12.2) 64 (14.5) 65 (78.3)
4 112 (7.2) 63 (14.3) 18 (21.7)
N <0.001
0 594 (38.2) 113 (25.7) 28 (33.7)
1a 821 (52.8) 240 (54.5) 32 (38.6)
1b 141 (9.1) 87 (19.8) 23 (27.7)
M 0.005
0 1,549 (99.6) 432 (98.2) 81 (97.6)
1 7 (0.4) 8 (1.8) 2 (2.4)
Stage <0.001
1 1,269 (81.6) 344 (78.2) 48 (57.8)
2 247 (15.9) 71 (16.1) 24 (28.9)
3 38 (2.4) 24 (5.5) 10 (12.0)
4 2 (0.1) 1 (0.2) 1 (1.2)
Data are shown as mean±standard deviation or number (%).
ETE = extrathyroidal extension; LVI = lymphovascular invasion; RM = resection margin; LN = lymph node.
Table 3. Recurrence status of 2,062 papillary thyroid carcinoma patients except M1 according to tumor size
Variables 1–2 cm (n=1,549) 2–4 cm (n=432) >4 cm (n=81) P value
Recurrence <0.001
(+) 59 (3.8) 58 (13.4) 21 (25.9)
(−) 1,490 (96.2) 374 (86.6) 60 (74.1)
1st Recurrence site* 0.021†
Distant metastasis 3 (5.1) 7 (12.1) 5 (23.8)
Local recurrence 56 (94.9) 51 (87.9) 16 (76.2)
Distant metastasis at last* 0.030
(+) 7 (11.9) 13 (22.4) 8 (38.1)
(−) 52 (88.1) 45 (77.6) 13 (61.9)
Data are shown as number (%).
*Patients with recurrence (n=138); †0.021 in linear by linear association. P=0.055 in χ2 test.
10-year RFS was 95.0%, 84.0%, and 71.0%. According to N stage, the 5-year RFS rates were 99.0%, 93.0%, and 83.0% in N0, N1a, and N1b, respectively. The 10-year RFS rates were 98.0%, 90.0%, and 80.0%, respectively.
Table 4. Risk factors for recurrences of >1 cm papillary thyroid carcinoma patients
Variables (n=2,062) Recurrence (+) (n=138) Recurrence (−) (n=1,924) P* value Hazard ratio (95% CI) P value
Age (yr) 45.2±16.3 48.1±12.1 NA NS 0.187
Sex 0.328 NA NA
Female 112 (81.2) 1,607 (83.5)
Male 26 (18.8) 317 (16.5)
Size <0.001
1–2 cm 59 (42.8) 1,490 (77.4) <0.001
2–4 cm 58 (42.0) 374 (19.4) 3.007 (2.054–4.404) <0.001
>4 cm 21 (15.2) 60 (3.1) 6.758 (3.953–11.552) <0.001
ETE <0.001 NS 0.211
(−) 19 (13.9) 526 (28.2)
(+) 118 (86.1) 1,342 (71.8)
LVI <0.001 NS 0.928
(−) 84 (65.1) 1,469 (79.3)
(+) 45 (34.9) 383 (20.7)
RM <0.001 NS 0.246
(−) 96 (70.1) 1,586 (82.9)
(+) 41 (29.9) 326 (17.1)
Multifocality <0.001 NS 0.144
(−) 69 (50.0) 1,151 (59.8)
(+) 69 (50.0) 773 (40.2)
Bilaterality 0.011 NS 0.648
(−) 88 (63.8) 1,407 (73.1)
(+) 50 (36.2) 517 (26.9)
T4 <0.001 0.478
(−) 113 (81.9) 1,763 (91.6)
(+) 25 (18.1) 161 (8.4)
N <0.001
N0 10 (7.2) 725 (37.7) <0.001
N1a 85 (61.6) 1,005 (52.2) 5.840 (2.924–11.665) <0.001
N1b 43 (31.2) 194 (10.1) 10.550 (5.048–22.048) <0.001
Data are shown as mean±standard deviation or number (%).
NA = not assessed; NS = not specific; ETE = extrathyroidal extension; LVI = lymphovascular invasion; RM = resection margin; CI = confidence interval.
*Log rank test in Kaplan-Meier curve analysis.
RFS
0 1.0
240 180
120 60
0
Follow-up period (mo) 0.8
0.6
0.4
0.2
P<0.001
1–2 cm 2–4 cm
>4 cm
A
RFS
0 1.0
240 180
120 60
0
Follow-up period (mo) 0.8
0.6
0.4
0.2
P<0.001
N0 N1a
N1b
B
Fig. 1. (A) RFS of >1 cm PTC according to tumor size. (B) RFS of >1 cm PTC according to N stage.
RFS = recurrent free survival; PTC = papillary thyroid carcinoma.
DISCUSSION
In the present study, the following prognostic factors increased with tumor size: LVI, resection margin positivity, LN metastasis, N stage, T4, and M stage. The proportion of metastatic LNs >5 was more frequent with increasing tumor size. Recurrence was also significantly more common in the larger size groups, and distant metastasis as the first recurrence and progression to distant metastasis were observed more commonly. In the analysis of risk factors for recurrence of >1 cm PTC, the following were significant variables:
tumor size, N stage, LN metastasis, and metastasis in >5 LNs.
In 2–4 cm PTC, LN metastasis occurred in 74.3% of cases, and ETE was detected in 72.9%.
Distant metastasis at diagnosis occurred in 1.8%. The recurrence rate was 13.4%, and the risk of recurrence was three times higher than that of 1–2 cm PTC. The 5- and 10-year RFS rates in 2–4 cm PTC were significantly lower than in 1–2 cm PTC.
The ATA guidelines recommend lobectomy for PTC measuring >1 cm and ≤4 cm without ETE or LN metastasis. In the present study, 11.4% of 2–4 cm PTCs involved no ETE or LN metastasis (50/440). When we excluded aggressive histology, LVI, and multifocality for consideration of RAI therapy requiring total thyroidectomy, only 8.9% (39/440) of 2–4 cm PTCs remained. Among these 39 patients, 25 underwent total thyroidectomy and 14 underwent thyroid lobectomy. None experienced recurrence. Therefore, low-risk PTCs had good prognosis despite a large tumor size. However, most prognostic factors are confirmed after surgery. Current thyroid cancer management guidelines use many variables related to disease recurrence or patient survival. Unfortunately, most of these variables are only known to surgeons and patients after surgery—many are only confirmed by postoperative pathological reports several days later, such as microscopic ETE, LVI, aggressive histology, resection margin positivity, number and size of metastatic LNs, and multifocality. Surgeons must decide whether to perform total thyroidectomy or lobectomy before the operation is completed. However, they have limited pre- and intraoperative information and are often compelled to perform completion thyroidectomy.
Kluijfhout et al. (6) reported that over half of patients with 1–4 cm, well-differentiated thyroid cancer eligible for lobectomy required completion thyroidectomy based on pathological results. Initially, patients underwent thyroid lobectomy if they had well-differentiated thyroid cancer without gross ETE or LN metastasis before or during surgery, distant metastasis, history of radiation, or family history. Completion thyroidectomy was performed in cases of aggressive histology, LVI, microscopic ETE, positive margins, any positive LN, and multifocality. This constituted 55% of cases (6). Similarly, 57.5% of patients who underwent thyroid lobectomy for 1–4 cm PTC required a second operation based on LN metastasis, ETE, vascular invasion, and aggressive histology (7). Completion thyroidectomy should be avoided if possible, so thyroid lobectomy should not be performed based on tumor size only.
After the ATA guideline was published, several studies investigated the extent of thyroidectomy with 1–4 cm PTCs. One such study evaluated intermediate-risk PTC as defined in the 2015 ATA guidelines—microscopic ETE (T1-2N×M0), clinical N1 stage, or >5 pathological LNs of <3 cm in their largest dimension (T1-2N1M0) and vascular invasion. The results showed that total thyroidectomy and thyroid lobectomy were not statistically different in terms of RFS or disease-specific survival (8). In the matched-pair analysis of the present study, disease-free survival (DFS) in patients with 1–2 and 2–4 cm tumors who underwent
lobectomy did not differ from that in patients who underwent total thyroidectomy after controlling for major prognostic factors (HR, 1.57; 95% CI, 0.75–3.25; P=0.228 and HR, 0.93;
95% CI, 0.30–2.89; P=0.902, respectively). The authors reported that thyroid lobectomy is feasible as an initial surgical approach to these patients, emphasizing that tumor size should not be considered an absolute indication for total thyroidectomy (9).
In contrast, many studies have suggested that 2–4 cm PTC should be treated differently from 1–2 cm PTC. Several reports have described the characteristics and prognosis of 2–4 cm PTC.
Park et al. (10) reported that primary tumor sizes of 2.1–4.0 and >4.0 cm were independent risk factors for disease-specific survival (HR, 2.44; 95% CI, 1.45–4.11; P=0.001 and HR, 7.40; 95%
CI, 4.02–13.63; P<0.001, respectively). The DFS of 2.1–4.0 cm tumors was significantly lower (HR, 1.97; 95% CI, 1.52–2.55; P< 0.001) than that of ≤2.0 cm primary tumors (10). In a large- scale, retrospective study using a National Cancer Data Base of 33,816 PTCs measuring 1.0–3.9 cm and clinically negative LNs, total thyroidectomy to manage 2.0–3.9 cm PTCs improved survival significantly. The authors recommended total thyroidectomy for 2.0–3.9 cm PTC (11).
Primary tumor size >1.95 cm was an independent risk factor for structural recurrence, regardless of the extent of thyroid surgery, in a study of dynamic risk stratification in 1–4 cm PTC (12). Choi et al. (13) reported that total thyroidectomy was beneficial to reduce recurrence in cases of 1–4 cm PTC that was not unifocal, intrathyroidal, or LN metastasis- negative. Furthermore, 2–4 cm PTC conferred a significantly higher risk of recurrence than 1–2 cm PTC (odds ratio [OR], 2.246; 95% CI, 1.769–2.851; P<0.001), as well as lower survival (OR, 2.497; 95% CI, 1.317–4.734; P=0.005) (13).
In a meta-analysis, 1–4 cm PTC conferred a significantly higher recurrence rate after thyroid lobectomy than after total thyroidectomy (14). In other meta-analysis, thyroid lobectomy may have been insufficient in patients with 2–4 cm PTC because it was associated with higher mortality in this group. The overall survival and RFS of total thyroidectomy were significantly higher than those of thyroid lobectomy (HR, 0.88; 95% CI, 0.79–0.99; P=0.03 and HR, 0.56;
95% CI, 0.41–0.77; P<0.0001, respectively) (15).
In the present study, 2–4 cm PTC showed a 13.4% of recurrence rate—three times higher than that of 1–2 cm PTC. The 5- and 10-year RFS of 2–4 cm PTC were significantly worse than those of 1–2 cm PTC, and a 2–4 cm tumor was a significant risk factor for disease recurrence, along with more LN metastases, advanced N stage, and metastasis in >5 LNs. Both T4 stage and M stage were more advanced in 2–4 cm PTC than in 1–2 cm PTC. Both LVI and resection margin positivity were observed more frequently in 2–4 cm PTCs. Therefore, 1–2 cm and 2–4 cm PTC may be pathologically distinct and should be managed differently.
In cases of RAI therapy, total thyroidectomy had to be performed. The significant factors for RAI therapy were tumor size, T3b, T4, aggressive histology, LVI, and metastasis in >5 LNs (3,16). In the present study, 34.3% (151/440) of patients with 2–4 cm PTC were classified as T2M0 without LVI, aggressive histology, or metastasis in >5 LNs. Therefore, 65.7% of patients with 2–4 cm PTC eventually required total thyroidectomy due to RAI.
The present study had several limitations. Firstly, the effect of RAI therapy on disease recurrence was not evaluated because this was a retrospective study. The accurate dose and number of RAI therapy sessions could not be evaluated. Neither could post-treatment Tg
levels. However, the effect of RAI therapy may have been estimated, as RAI was performed more often in patients with larger tumors. Secondly, we did not perform patient matching to evaluate the risk of tumor size. Patient matching would have allowed more precise statistical analysis. Thirdly, molecular biological markers such as BRAF or TERT were not evaluated because our data were retrospective. Future studies should analyze the effect of molecular biological characteristics on the prognosis of disease and its association with tumor size.
CONCLUSION
In the present study, prognostic factors such as LVI, resection margin positivity, LN metastasis, the metastasis in >5 LNs, N stage, T4, and M stage worsened with increasing tumor size. In addition to recurrence, distant metastasis as the first recurrence and progression to distant metastasis also significantly increased with larger tumor size. In the analysis of risk factors for recurrence of >1 cm PTC, tumor size, N stage, LN metastasis, and metastasis in >5 LNs were significant risk factors for disease recurrence. In particular, 2–4 cm PTC had a 13.4% recurrence rate, and the risk of recurrence was three times higher than that of 1–2 cm PTC. The 5-year and 10-year RFS rates of 2–4 cm PTC were significantly lower those of 1–2 cm PTC. Moreover, 2–4 cm PTC showed more LN metastasis, worse N stage, and a higher proportion of patients with >5 metastatic LNs than 1–2 cm PTC, and all of these were significant risk factors for disease recurrence. Therefore, 1–2 and 2–4 cm PTC may be pathologically distinct and should be managed differently.
REFERENCES
1. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16:109-42.
PUBMED | CROSSREF
2. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid CancerCooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer.
Thyroid 2009;19:1167-214.
PUBMED | CROSSREF
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133.
PUBMED | CROSSREF
4. Marques P, Leite V, Bugalho MJ. Retrospective analysis of 255 papillary thyroid carcinomas ≤2 cm:
clinicohistological features and prognostic factors. Eur Thyroid J 2014;3:258-63.
PUBMED | CROSSREF
5. Perrino M, Vannucchi G, Vicentini L, Cantoni G, Dazzi D, Colombo C, et al. Outcome predictors and impact of central node dissection and radiometabolic treatments in papillary thyroid cancers < or =2 cm.
Endocr Relat Cancer 2009;16:201-10.
PUBMED | CROSSREF
6. Kluijfhout WP, Pasternak JD, Lim J, Kwon JS, Vriens MR, Clark OH, et al. Frequency of high-risk characteristics requiring total thyroidectomy for 1-4 cm well-differentiated thyroid cancer. Thyroid 2016;26:820-4.
PUBMED | CROSSREF
7. Anda Apiñániz E, Zafon C, Ruiz Rey I, Perdomo C, Pineda J, Alcalde J, et al. The extent of surgery for low-risk 1-4 cm papillary thyroid carcinoma: a catch-22 situation. A retrospective analysis of 497 patients based on the 2015 ATA guidelines recommendation 35. Endocrine 2020;70:538-43.
PUBMED | CROSSREF
8. Liu J, Zhang Z, Huang H, Xu S, Liu Y, Liu S, et al. Total thyroidectomy versus lobectomy for intermediate- risk papillary thyroid carcinoma: a single-institution matched-pair analysis. Oral Oncol 2019;90:17-22.
PUBMED | CROSSREF
9. Song E, Han M, Oh HS, Kim WW, Jeon MJ, Lee YM, et al. Lobectomy is feasible for 1–4 cm papillary thyroid carcinomas: a 10-year propensity score matched-pair analysis on recurrence. Thyroid 2019;29:64-70.
PUBMED | CROSSREF
10. Park SY, Kim HI, Kim JH, Kim JS, Oh YL, Kim SW, et al. Prognostic significance of gross extrathyroidal extension invading only strap muscles in differentiated thyroid carcinoma. Br J Surg 2018;105:1155-62.
PUBMED | CROSSREF
11. Rajjoub SR, Yan H, Calcatera NA, Kuchta K, Wang CE, Lutfi W, et al. Thyroid lobectomy is not sufficient for T2 papillary thyroid cancers. Surgery 2018;163:1134-43.
PUBMED | CROSSREF
12. Lee YM, Cho JW, Hong SJ, Yoon JH. Dynamic risk stratification in papillary thyroid carcinoma measuring 1 to 4 cm. J Surg Oncol 2018;118:636-43.
PUBMED | CROSSREF
13. Choi JB, Lee SG, Kim MJ, Kim TH, Ban EJ, Lee CR, et al. Oncologic outcomes in patients with 1-cm to 4-cm differentiated thyroid carcinoma according to extent of thyroidectomy. Head Neck 2019;41:56-63.
PUBMED
14. Chan S, Karamali K, Kolodziejczyk A, Oikonomou G, Watkinson J, Paleri V, et al. Systematic review of recurrence rate after hemithyroidectomy for low-risk well-differentiated thyroid cancer. Eur Thyroid J 2020;9:73-84.
PUBMED | CROSSREF
15. Zhang C, Li Y, Li J, Chen X. Total thyroidectomy versus lobectomy for papillary thyroid cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19073.
PUBMED | CROSSREF
16. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Thyroid Carcinoma (Version 2.2020, July 15, 2020). Plymouth Meeting (PA): National Comprehensive Cancer Network; 2020.