Production of Microbial Biopolymer, Poly( γ-glutamic acid) by Bacillus subtilis BS 62
Seong-Hyun Choi*, Jong-Soo Park1, Kyung-Sook Whang, Min-Ho Yoon2 and Woo-Young Choi2 The Institute of Natural Science, Department of Microbiology, Mokwon University,
800, Doan-dong, Seo-gu, Daejeon 302-729, Korea
1Namyang Dairy Products Co., Ltd. Kongju 314-914, Korea
2Department of Agricultural Chemistry, College of Agriculture and Life Sciences, Chungnam National University, 220, Kung-dong, Yusung-gu, Daejeon 305-764, Korea
Received April 29, 2004; Accepted June, 16 2004
Bacterium BS 62, identified as Bacillus subtilis in our previous work, produced poly(γ-glutamic acid) (PGA) in medium composed of glucose, L-glutamic acid and inorganic salts. Cell growth, sporulation and polymer production were monitored during cultivations. Glucose was a better source of carbon than citric acid for PGA production. Both maximum amount of PGA production (19 g per liter culture fluid) and maximum spore formation were achieved at 96 h. HPLC analysis revealed that D- and L- glutamic acid composition ratio of PGA produced by B. subtilis BS 62 was 60/40 from 48 to 120 h cultivation. Weight-average molecular weight of PGA was estimated to be 624 kDa through gel permeation chromatography.
Key words: Poly(γ-glutamic acid), Chungkookjang, Bacillus subtilis
Poly(γ-glutamic acid) (PGA) was found as an extracellular viscous material of Bacillus anthracis in 1937,1) and Fujii2) reported that the main ingredient of natto viscosity was PGA in 1963. PGA is known as a water-soluble polymer comprising the monomer of D- and L-glutamic acids, and hydrolyzed by microorganism in the environment. It is safe for eating as a viscosity element of fermented soybean products such as Chungkookjang and natto.3) PGA is a kind of polypeptide due to the repeating unit of glutamic acid, which connects α-amino and γ-carboxyl residues. However, it is different from most protein in terms of accomplishing with γ- combination as amino acid's polymerism body,4) and its molecular weight varies from ten thousand to several hundred thousand depending on the type of bacilli used.
The medium components vary depending on the microbial source used for PGA production; B. licheniformis and B.
subtilis var. polyglutamicum, among others, produce PGA in the medium consisting of glucose and minerals without L- glutamic acid.5) D- and L-glutamic acid ratio of PGA was influenced by the ingredients such as L-glutamic acid and Mn2+ concentration in the medium.6)
PGA is used as a raw material in food, cosmetics and medicine, and as surface-active agent and coagulant for waste- water treatment. In addition, considerable amount of researches have been conducted using this functional polymer
as drug carrier, functional carrier, and membrane and electric materials.7) PGA benzylester is applicable to fibers with a high quality of dynamics.8) Studies on new material development are more advanced in Japan than in Europe. Park9) reported on the purification and characterization of γ-glutamyl- transpeptidase (γ-GTP). Yoon10) reported that PGA production took place in a fed-batch culture after elevating the cell density of B. licheniformis. Park at al.11) reported that high molecular weight PGA could be used as a therapeutic tool in the treatment of osteoporosis.
In this study, we selected a bacterium, Bacillus subtilis BS 62, on which levan production has previously been reported,12) and investigated its PGA productivity when grown aerobically in the culture medium containing L-glutamic acid and glucose etc. Molecular weight, and D- and L-glutamic acid ratio of the purified PGA were also estimated.
Materials and Methods
Microorganism and culture condition. Home-made Chungkookjang from Chungnam province, Korea, was collected and used for isolating spore-forming bacilli.
Suspended Chungkookjang with nutrient broth in a test tube was heated in boiling water for 10 min, and spread on nutrient agar plate, from which viable colonies were isolated after incubation at 37oC.
Slime-producing colonies were selected from the nutrient agar plate, and cultured in 10 ml of nutrient broth. After overnight cultivation at 37oC, 1.0 ml of the broth culture was transferred into a 500-ml flask contained 170 ml of substrate
*Corresponding author
Phone: +82-42-829-7261; Fax: +82-42-829-7538 E-mail: seonghc@mokwon.ac.kr
Abbreviations: PGA, poly(γ-glutamic acid); γ-GTP, γ-glutamyltranspeptidase
medium containing 70 g L-glutamic acid, 80 g glucose, 5 g yeast extract, 15 g peptone, 3 g urea, and 2 g K2HPO4, pH 7.0 for the production of PGA. Flask cultures were carried out at a 200-rpm rotational mode at 37oC for 6 d.
Viscosity measurement. Ten milliliters of culture fluid was applied to a Cannon-Fenske capillary viscometer, the temperature of the solution was adjusted to 30oC. Flow time was measured as seconds, and the relative viscosity (ηr) was calculated by the following equation:
, density of solution
0, density of water t, flow time (sec) of solution t0, flow time (sec) of water
PGA purification, quantification and molecular weight estimation. After pH of the culture fluid was adjusted to 10.0 by adding 6 N-NaOH, cells were separated by centrifuging for 20 min at 6,000×g and 4oC. The supernatant containing PGA was poured into 3 volumes of ethanol and left overnight.
The resulting precipitate containing crude PGA was collected by centrifugation at 6,000×g for 20 min and 4oC. Precipitated PGA was then dissolved into distilled water, and insoluble contaminants were removed by centrifugation at 6,000×g for 20 min and 4oC. The aqueous PGA solution was adjusted to pH 4.0 by adding 4 N-HCl and poured into 3 volumes of ethanol again, and left overnight. PGA was collected by centrifugation at 6,000×g for 20 min. The purified PGA was dissolved in a small amount of distilled water again, and was finally lyophilized. The dried PGA was weighed for quantification.
Weight- and number average molecular weights (Mw and Mn, respectively) of poly(γ-glutamic acid) were measured by gel permeation chromatography using a GMPWXL column (Viscotek) and a refractive index detector (Viscotek, Model 410). Polyethylene oxide standards of narrow polydispersity were used to construct a calibration curve from which molecular weights of poly(γ-glutamic acid) were calculated.
The mobile phase contained 0.1 M NaNO3, and the flow-rate was 0.8 ml/min.
D-/L-glutamic acid racemization of PGA. To perform complete hydrolysis of the PGA obtained, 100 mg of the sample was hydrolyzed under N2 atmosphere in a sealed glass tube with 6 N HCl for 24 h at 110oC. Subsequently, the sample was evaporated and dried under vacuum, and dissolved in 0.1 N HCl. To derivatize glutamic acid, 20µl of the sample solution was mixed with 40µl of 1% N-(2,4- dinitro-5-fluorophenyl)-L-alanineamide/acetone and 8µl of 1 M NaHCO3, and reacted for 60 min at 40oC. After cooling at room temperature, 4µl of 1 M HCl was added into the sample, which was then dried under vacuum and dissolved in 100µl of methanol. One to four aliquots were injected into a Waters HPLC (Breeze system, 717 Autosampler, 2487 Dual
absorbance detector). The analytical column, a XTerra C18
(3.9 mm I. D.×300 mm) was maintained at 30oC. The eluent for the separation of the derivatives was 50 mM triethylamine phosphate buffer (pH 3.5) with linear gradient from 10% to 40% of acetonitrile. Flow-rate of the eluent was 0.8 ml/min.
Results and Discussion
Isolation and selection of PGA-producing microorganism.
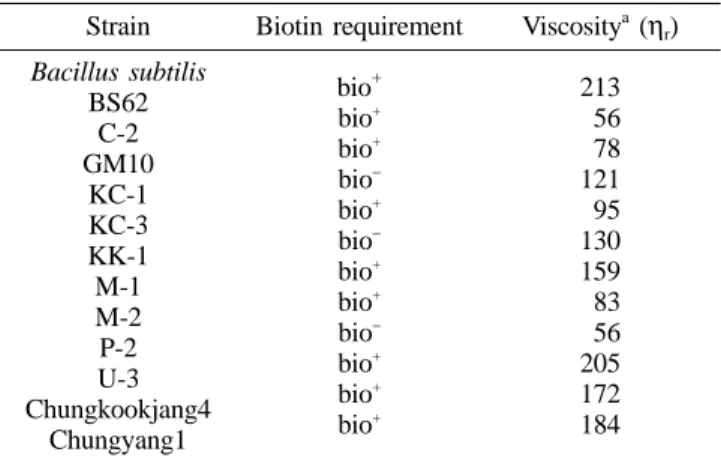
Biotin requirements of the bacilli isolates screened for PGA production on plate and in liquid were also investigated. Using relative viscosity value of a bacterial culture fluid, which was cultured in a slime-producing medium, PGA productivity was estimated (Table 1). Most strains required biotin for growth, except KC-1, KK-1 and P-2 strains, and produced PGA with high viscosity in culture fluid. In particular, B. subitilis BS 62, originally isolated for levan production12), showed the highest production and was, therefore, selected for further study.
Bacterial growth, spore formation and PGA production.
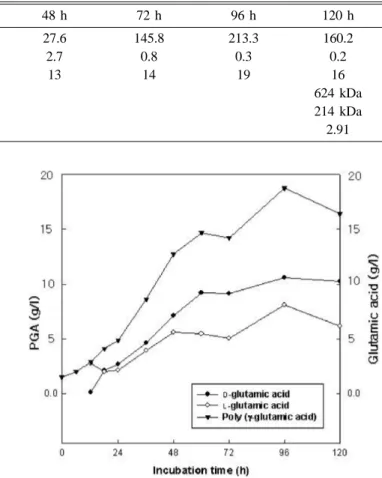
As described in Materials and Methods, cultural characteristics during cultivation, cell growth, spore formation, pH change, production of PGA, and D- and L- type ratio of PGA were investigated. Growth of B. subtilis BS 62 in slime forming medium reached to the end of logarithmic phase within 12 h, and decreased thereafter, showing slow growth rate by 120 h.
The number of spores remained the same as at the beginning of growth curve upto 18 h, and increased slowly thereafter, reaching the maximum level by 96 h, and maintained that level upto 120 h. PGA production also reached maximum level after 96 h, at 19 g per liter of culture fluid. Microbial PGA production was mainly conducted using Bacillus sp., and had relatively high PGA concentration in the culture fluid.
Earlier studies reported that B. subtilis IFO33356) afforded 10~20 g/l of PGA from the culture fluid including 30 g/l of glutamic acid, 20 g/l of citric acid, and 5 g/l of (NH4)2SO4,
whereas B. subtilis F-2-01 afforded 45.5 g/l PGA from a culture fluid composed of 70 and 1 g/l of glutamic acid and ηr ρ t×
ρ0×t0 ---
= ρρ
Table 1. Biotin requirements of the bacili isolates and rela- tive viscosity of their culture fluid
Strain Biotin requirement Viscositya (ηr) Bacillus subtilis
BS62 C-2 GM10
KC-1 KC-3 KK-1 M-1 M-2 P-2 U-3 Chungkookjang4
Chungyang1
bio+ bio+ bio+ bio− bio+ bio− bio+ bio+ bio− bio+ bio+ bio+
213 056 078 121 095 130 159 083 056 205 172 184
aPGA production was roughly compared by the relative viscosity of culture fluid.
glucose, respectively.13) Furthermore, B. subtilis TAM-414) and B. licheniformis A3515) produced 20 and 8~12 g/l of PGA, respectively. PGA-producing microorganisms can be classified into two groups on the basis of the need for glutamic acid as a nitrogen source for PGA production. B. subtilis BS 62 as well as other Bacillus sp. such as B. subtilis ATCC 9945, B. subtilis IFO3335, and B. subtilis F-2-01 required high content of glutamic acid for PGA production, about 2~20 times as much as that of the resulting PGA.16) While PGA producing strains by de novo route like B. sublitilis 5E, B.
licheniformis A 35 and B. subtilis TAM-4 didn't require glutamic acid, therefore their industrial utilities were expected by confirming that substrates used were merely glucose, fructose, urea and NH4Cl.16)
PGA productivity by B. subtilis BS 62 was investigated using citric acid as a carbon source (Table 2). The yield of PGA was 2.0 g/l using a medium of 3 and 2% L-glutamic acid and citric acid, whereas was 19 g/l using a different medium of 7 and 8% L-glutamic acid and glucose, respectively. Citric acid was suggested to be not effective for PGA production using B. subtilis BS 62. Goto and Kunioka17) found that a large
amount of PGA (9.6 g/l), without any by-products such as polysaccharide, was produced by B. subtilis IFO3335 in L- glutamic acid/citric acid medium containing 3% L-glutamic acid, 2% citric acid and 0.5% ammonium sulfate.
Weight-average molecular weight (Mw) value of PGA product harvested from 120 hour-culture fluid was estimated to be 624 kDa, and the polydispersity value (Mw/number average molecular weight) was 2.91 (Table 2). Ko and Gross18) reported that using Bacillus licheniformis ATCC 9945a, Mw
values of PGA products ranged from about 900 to 2,000 kDa, and polydispersity values were between 2.5 and 3.0.
Freeze-dried materials were analyzed by HPLC to determine the levan content (Table 2). The Levan content in culture fluid reached the maximum value of 2.7% at 12 h, after which it declined. The total enzyme activity of levansucrase, a key enzyme for the levan production, reached maximum value at 24 h, and then declined quickly.12) It is suggested that levan produced at the beginning of culture was degraded at the end of culture. According to Choi et al., levan was degraded by invertase releasing fructose as a resulting product.12) Because B. subtilis BS 62 was cultured in PGA- producing medium, the level of levan produced was lower Fig. 1. Cell growth, spore formation and PGA production by
Bacillus subtilis BS 62. Culture was grown as described in the Materials and Methods.
Table 2. Changes in the relative viscosity, levan concentration and PGA amount of the culture fluid during cultivation of Bacil- lus subtilis BS 62, and the PGA molecular weight
Period of cultivation 24 h 48 h 72 h 96 h 120 h
Viscosity (ηr) 7.2 27.6 145.8 213.3 160.2
Levan (%) 2.4 2.7 0.8 0.3 0.2
PGA (g/l) 5 13 14 19 16
Weight average molecular weight (Mw) 624 kDa
Number average molecular weight (Mn) 214 kDa
Polydispersity (Mw/Mn) 2.91
Fig. 2. Composition of D-/L-glutamic acid content in PGA during cultivation of Bacillus subtilis BS 62. Culture was grown on medium containing 7% glutamic acid and 8%
glucose.
than 2.7% of PGA. During cultivation, pH underwent very little change from the initial pH 7.0, keeping almost constant.
Viscosity change. Changes in viscosity of culture fluid with B. subtilis BS 62 is represented in Table 2. The viscosity changes in culture fluid might be influenced by PGA production, and the maximum level also coincided with the maximum PGA production in 96 h.
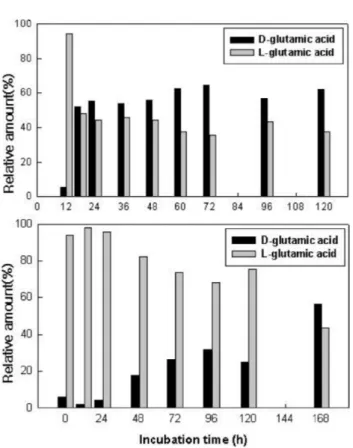
D/L composition ratio and molecular weight of PGA.
Ratios of glutamic acid of PGA produced by B. subtilis BS 62 were analysed by HPLC (Fig. 2 and 3). Up to 12 h culture, glutamic acid composition tended towards a high L-type, then changed towards high a D-type composition ratio between 18 48 h. In addition, the high D ratio further increased with increased culturing, showed average value from 48 to 120 h (D : L = 60 : 40). However, when glucose was replaced with citric acid, high L composition ratio was shown until 120 h. D/
L composition ratio of PGA was changed according to the ingredient of the medium. D/L composition ratios for B.
subtilis IFO333517) and B. subtilis TAM-414) were reported to be 83/17 and 78/22, respectively. The indirect conversion of L-glutamic acid to the D isomer in B. anthracis and B. subtilis has been previously reported.19) By the proposed pathway of PGA synthesis in B. subtilis IFO3335,16) 2-Oxoglutaric acid and L-alanine are synthesized from L-glutamic acid and pyruvic acid. D-Alanine is formed by racemization from L-
alanine, and D-glutamic acid and pyruvic acid are synthesized from D-alanine and 2-oxoglutaric acid.
Acknowledgments. This work was supported by Korean Research Foundation Grant (KRF-2002-050-D00003). We would like to thank to Drs. Moon Hee Sung and Chung Park, Bioleaders Corporation, Daejeon, Korea, for their help in the study of PGA molecular weight determination.
References
1. Ivanovics, G. and V. Bruckner. (1937) Chemische und immunologische Studien uber den Mechanismus der Milzbrandinfektion und Immuni-tat; die chemiseche Struk- tur der Kapselsubstanz des Milzbrand-bazillus und der serologisch identischen spezifischen Substanz des Bacillus mesentericus. Z Immunitatsforsch. 90, 304-318.
2. Fujii, H. (1963) On the formation of mucilage in natto (1). Nippon Nogeikagaku Kaishi. 37, 407-411.
3. Hara, T., Shiraishi, A., Fujii, H. and Ueda, S. (1984) Spe- cific host range of Bacillus subtilis (natto) phages associ- ated with polyglutamic acid production. Agric. Biol. Chem.
48, 2373-2374.
4. Chung, W. S. and Ko, Y. H. (1997) Transformation and mutation of Bacillus licheniformis 9945a producing γ-poly (glutamic acid). Agric. Chem. Biotech. 40, 173-177.
5. Thorne, C. B., Gomez, C. G., Noyes, H. E. and House- wright, R. D. (1954) Production of glutamyl polypeptide by Bacillus subtilis. J. Bacteriol. 68, 307-315.
6. Kunioka, M. and Goto, A. (1994) Biosynthesis of poly(γ-glutamic acid) from L-glutamic acid, citric acid, and ammonium sul- fate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotech- nol. 40, 867-872.
7. Carenza, M. (1992) Recent achievements in the use of radi- ation polymerization and grafting for biomedical applica- tions. Radiat. Phys. Chem. 39, 485-493.
8. Kishi, R., Ichijo, H. and Hirasa, O. (1993) Thermo-respon- sive devices using poly(vinylmethylester) hydrogels. J.
Intell. Mater. Syst. Struct. 4, 533 (Japanese).
9. Park, M. O. (1991) Characterization of the strain Bacillus subtilis BS62 isolated from Chungkookjang and its extracellular γ- glutamyltranspeptidase. MS Thesis, Chungnam National University, Daejon, Korea.
10. Yoon, S. H., Do, J. H., Lee, S. Y. and Chang, H. N. (2000) Production of poly-γ-glutamic acid by fed-batch culture of Bacilluslicheniformis. Biotechnol. letters. 22, 585-588.
11. Park, C., Kim, K. S., Ashiuchi, M., Misono, H., Soda, K.
and Sung, M. H. High molecular weight poly(γ-glutamic acid): synthesis, production and its application. Proc. KMB Annual Meeting, June 24-26, 2003 Muju, Korea.
12. Choi, S. H., Sung, C. and Choi, W. Y. (2001) Levan-pro- ducing Bacillus subtilis BS 62 and its phylogeny based on its 16S rDNA sequence. J. Microbiol. Biotechnol. 11, 428- 434.
13. Kubota, H., Tanaka, T. and Taniguchi, M. (1993) Produc- tion of poly (γ-glutamic acid) by Bacillus subtilis F-2-01.
Fig. 3. Changes in relative amount of D-/L-glutamic acid in PGA produced by Bacillus subtilis BS 62. The culture was grown on the medium: [A], contained 7% L-glutamic acid and 8% glucose; [B], contained 3% L-glutamic acid and 2%
glucose.
Biosci. Biotech. Biochem. 57, 1212-1213.
14. Ito, Y., Tanaka, T., Ohmachi, T. and Asada, Y. (1996) Glutamic acid independent production of poly(γ-glutamic acid) by Bacillus subtilis TAM-4. Biosci. Biotech. Biochem.
60, 1239-1242.
15. Cheng, C., Asada, Y. and Aida, T. (1989) Production of γ- polygluta-mic acid by Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol. Chem. 53, 2369-2375.
16. Kunioka, M. (1997) Biosynthesis and chemical reactions of poly(amino acid)s from microorganisms. Appl. Microbiol.
Biotechnol. 47, 469-475.
17. Goto, A., Kunioka, M. (1992) Biosynthesis and hydrolysis of poly(γ-glutamic acid) from Bacillus subtilis IFO3335.
Biosci. Biotechnol. Biochem. 56, 1031-1035.
18. Ko, Y. and Gross, R. A. (1998) Effect of glucose and glyc- erol on γ-poly(glutamic acid) formation by Bacillus licheni- fomis ATCC 9945a. Biotechnol. Bioeng. 57, 430-437.
19. Hara, T., Fujio, Y., Ueda, S. (1982) Polyglutamate produc- tion by Bacillus subtilis (natto). J. Appl. Biochem., 4, 112- 120.