Korean Circulation Journal
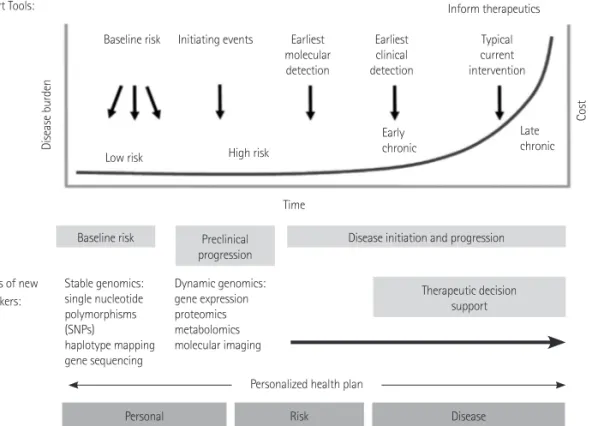
There has been a remarkable progress of personalized medicine in the scientific basis and clinical applications on cardiovascular dis- eases (CVDs) after its introduction in the late 1990s. However, CVDs still remains the leading cause of death in the United States. More- over, there will be needs on the education to doctors and patients for the improvement of knowledge, awareness and attitude on in- corporating such method into clinical applications. Personalized me- dicine will contribute to the evolution of the management practice for CVDs. This article reviews the concepts, the strength, limitations and challenges, and future direction of personalized medicine with a particular focus on CVDs.
Print ISSN 1738-5520 • On-line ISSN 1738-5555
Personalized Medicine in Cardiovascular Diseases
Moo-Sik Lee, MD 1,2 , Andreas J. Flammer, MD 1 , Lilach O. Lerman, MD 3 , and Amir Lerman, MD 1
1
Division of Cardiovascular Diseases and
3Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA
2