Introduction Introduction
Asarum sieboldii Miq. (Aristolochiaceae) is a perennial ber- baceous plant and is distributed throughout Republic of Korea, China, and Japan. Traditionally, A. sieboldii has long been used to treat all types of colds, fever, phlegm, allergies, chronic gastirits, and acute toothaches [1,2]. In addition, it has been
scientifically proven that A. sieboldii has various biological activities, such as anti-allergic, anti-inflammatory, anti-noci- ceptive, anti-fungal, anti-cancer, and neuroprotective effect [3-9]. The main physiolgically active substances of A. sieboldii contain asarylketone, safrole, limonene, pellitorine, sesamine and aristolactam, in addition to the essential oil components methyleugenol and xanthoxylol [3,10]. Asiasarum heterotro- Int J Oral Biol 46:85-93, 2021
pISSN: 1226-7155 • eISSN: 2287-6618 https://doi.org/10.11620/IJOB.2021.46.2.85
Methanol extracts of Asarum sieboldii Miq. induces apoptosis via the caspase pathway in human FaDu hypopharynx squamous carcinoma cells
Seul Ah Lee
1†, Bo-Ram Park
2†, and Chun Sung Kim
1*
1
Department of Oral Biochemistry, College of Dentistry, Chosun University, Gwangju 61452, Republic of Korea
2
Department of Dental Hygiene, College of Health and Welfare, Kyungwoon University, Gumi 39160, Republic of Korea
Asarum sieboldii Miq. (Aristolochiaceae) is a perennial herbaceous plant and has been used as traditional medicine for treating diseases, cold, fever, phlegm, allergies, chronic gastritis, and acute toothaches. Also, it has various biological activities, such as antiallergic, antiinflammatory, antinociceptive, and antifungal. However, the anticancer effect of A. sieboldii have been rarely reported, except anticancer effect on lung cancer cell (A549) of water extracts of A. sieboldii. This study investigated the anticancer activity of methanol extracts of A. sieboldii (MeAS) and the underlying mechanism in human FaDu hypopharyngeal squamous carcinoma cells. MeAS inhibited FaDu cells grown dose-dependently without affecting normal cells (L929), as determined by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide and live and dead assay. In addition, concentration of MeAS without cytotoxicity (0.05 and 0.1 mg/mL) inhibited migration and colony formation. Moreover, MeAS treatment significantly induced apoptosis through the proteolytic cleavage of caspase-3, -7, -9, poly (ADP-ribose) polymerase, and downregulation of Bcl-2 and upregulation of Bax in FaDu cells, as determined by fluorescence-activated cell sorting analysis, 4`6-diamidino- 2-phenylindole stain, and western blotting. Altogether, these results suggest that MeAS exhibits strong anticancer effects by suppressing the growth of oral cancer cells and the migration and colony formation via caspase- and mitochondrial-dependent apoptotic pathways in human FaDu hypopharyngeal squamous carcinoma cells. Therefore, MeAS can serve as a natural chemotherapeutic for human oral cancer.
Keywords: Asarum sieboldii Miq., Human FaDu hypopharynx squamous cancer cells, Apoptosis, Oral cancer
Received April 7, 2021; Revised June 10, 2021; Accepted June 15, 2021
*Correspondence to: Chun Sung Kim, E-mail: cskim2@chosun.ac.kr https://orcid.org/0000-0001-8612-3420
†
Seul Ah Lee and Bo-Ram Park contributed equally to this work.
Copyright © The Korean Academy of Oral Biology
CC
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by- nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Original Article IJOB
poides (AR) belonging to the same Asarum genus has been reported to have anti-cancer effects on various cancer cells, such as colon cancer (HCT-116, HT29), ovarian cancer cells (SKOV3 and A2780) and melanoma (G361) cells [7-9,11,12].
However, with the exception of the report that water extract of A. sieboldii induces apoptosis of lung cancer cells (A549), the anti-cancer effect of A. sieboldii has not yet been reported [8].
Oral squamous cell carcinoma (OSCC) is a main type of head and neck squamous cell carcinoma, and according to the World Health Organization, in 2018, the incidence of oral cancer is the fourth highest in the world [13]. OSCC originates from the mucosa of oral organ with the squamous initima and is cha- raterized by high potential invasion and lymph node metastasis [14]. Due to the high metastatic capacity of OSCC, the 5-year survival rate is less than 30% despite the development of comprehensive sequence therapy [13,15,16]. The risk factor for OSCC are related with viral infections and accumulation of genetic mutation as well as chronic exposure to carcinogens such as smoking, alcohol [13,17]. The main treatment of OSCC is surgery combined with radiotherapy or chemotherapy, but many side effects reduce the patient’s quality of life [18,19].
Therefore, the anti-cancer effect is being evaluated in natural products with relatively few side effects, and recently several candidate substances have been reported [20,21]. However, the A. sieboldii for oral cancer has not been clarified. Therefore, to aid understanding of the A. sieboldii anti-cancer activity, we investigated the effect of methanol extraction of A. sieboldii on human FaDu hypopharynx squamous carcinoma cells.
Materials and Methods Materials and Methods
1. Preparation of methanol extracts of Asarum sieboldii (MeAS) Miq.
In order to produce an extract having excellent pharmacologi- cal activity, it was extracted with methanol, which has a broad- er spectrum. A. sieboldii (10 g) of A. sieboldii was extracted in 40 volumes of methanol (v/w) at 37℃ for 24 hours, and then the extract was filtered through filter paper. The supernatant was concentrated in a rotary vacuum evaporator (Eyela, Tokyo, Japan) and lyophilized. The powder was dissolved in dimethyl sulfoxide (DMSO) at 100 mg/mL, and the solution was passed through a 0.45- μM syringe filter. The final extract was stored at –20℃ until use, but after dissolving it was stored at 4℃.
2. Reagents
Dried A. sieboldii purchased from Gangwon-do (Korea).
Crystal violet, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltet- razolium bromide (MTT) and 4`6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Live & Dead viability/cytotoxicity kit was purchased from Molecular Probe (Eugene, OR, USA). Cell scratcher and FITC- Annexin V apoptosis detection kit I were purchased from SPL Life Science (Pocheon, Korea), BD Bioscience (San Diego, CA, USA), respectively. Primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA): cleaved caspase-9 (cat. no. 7237), cleaved caspase-3 (cat. no. 9661), cleaved caspase-7 (cat. no. 9491), poly (ADP-ribose) poly- merase (PARP, cat. no. 9542), Bcl-2 (cat. no. 2872), Bax (cat.
no. 2772) and anti- α-tubulin was purchased from Thermo Fisher Scientific (Rockford, IL, USA). Minimum essential me- dium Eagle (MEM) and a penicillin/streptomycin solution were purchased from Welgene (Daegu, Korea). Fetal bovine serum (FBS) was purchased from Atlas Biologicals (Fort Collins, CO, USA).
3. Cell culture
Human hypopharynx squamous carcinoma FaDu and mouse fibroblast normal L929 cells were purchased from Korean Cell Line Bank (Seoul, Korea). In a humidified 5% CO
2incubator at 37℃, FaDu cells were cultured in MEM medium containing 10% FBS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), and L929 cells were cultured in MEM medium containing 10% FBS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin).
4. Cytotoxicity
FaDu and L929 cells were plated in 12-well plates at a
density 2 × 10
5cells/mL, and incubated for 16 hours. After
incubation, cells were treated with varying concentration of
MeAS (0, 0.05, 0.1, 0.2, and 0.4 mg/mL) for 24 hours. Cyto-
toxicity was determined by measuring formazan generated
by mitochondrial-dependent redox reaction using tetrazolium
salt solution (5 mg/mL). At the endpoint, 100 μL of MTT solu-
tion (5 mg/mL) was added and the formazan formed after 4
hours reaction was dissolved using DMSO, and then measured
absorbance at 590 nm in microplate reader (Epoch; BioTek
Instruments, Winooski, VT, USA). The results was expressed
as the cell viability rate by setting the absorbance of untreated control cells to 100% and the latex-treated cells was calculated as the surviving percentages. The percentage of cell viability was calculated using the following formula:
Cell viability (%) =
Means Abs
•of the sample – Means Abs
•of the blank
× 100 Means Abs
•of the control – Menas Abs
•of the control
5. Live & Dead assay and DAPI staining
FaDu cells were plated in a 4-well chamber slide at a density 1 × 10
5cells/well, and incubated for 16 hours. After incuba- tion, the cells were treated with varying concentration of MeAS (0, 0.1, 0.2, and 0.4 mg/mL) for 24 hours. For Live & Dead assay, the cells were washed with phosphate-buffered saline (PBS), and 100 μL of a working soultion (2 μM calcein-ace- toxymethyl and 4 μM of ethidium homodimer-1 in PBS) was added to each well containing 100 μL PBS, followed by reac- tion in a 37℃ incubator for 20 minutes. For DAPI staining, the cells were washed with PBS, fixed with 4% paraformaldehyde for 10 minutes, and stained with 300 nM DAPI for 20 minutes.
The stained cells were analyzed using a fluorescence micro- scope (Eclipse TE2000; Nikon Instruments, Melville, NY, USA).
6. Flow cytometric analysis
FaDu cells were plated in a 6-well plates at a density 2 × 10
5cells/well, and incubated for 16 hours. After incubation, the cells were treated with varying concentration of MeAS (0 and 0.4 mg/mL) for 24 hours, after which they were harvested using 0.25% trypsin-EDTA and washed with pre-chilled PBS twice. The cells were re-suspended in a 200 μL binding buf- fer, and then stained with FITC-Annexin V/PI (BD Biosciences) according to the manufacturere’s protocol. Subsequently, the stained cells were analyzed using a Gallios flow cytometer (Beckman Coulter Life Science, Indianapolis, IN, USA). The quantitatively data was expressed as density plots using Kaluza analysis software (Beckman Coulter Life Science).
7. Wound healing assay
FaDu cells were plated in a 24-well plates at a density 4 × 10
3cells/well, and incubated for 16 hours. A cell scratcher was used to generate a constant wound in each well. After washing with PBS, non-cytotoxic concentrations of MeAS against FaDu
cells (0.025 and 0.05 mg/mL) and the lowest concentration in- ducing cytotoxicity (0.1 mg/mL) were added to each well, and reacted for two days. EVOS XL Core (Thermo Fisher Scientific, Waltham, MA, USA) was used for cell imaging.
8. Colony formation
FaDu cells were plated in a 6-well plates at a density 2 × 10
3cells/well, and incubated for 16 hours. The non-cytotoxic concentration of MeAS against FaDu cells (0.025 and 0.05 mg/
mL) and the lowest concentration inducing cytotoxicity (0.1 mg/mL) were treated and reacted for 24 hours. After 24 hours, the cells were further cultured in growth medium containin no extracts for 10 days, and then crystal violet staining was performed for colony formation analysis. Culture medium was removed, lightly washed with PBS, and then fixed with 95%
ethanol for 10 minutes. After completely drying, 0.1% crystal violet solution was added and stained for 10 minutes. Rinse lightly with deionized water to remove the unstained areas, the degree of staining was observed with canon G16.
9. Western blot analysis
FaDu cells were plated in a 6-well plates at a density 2 ×
10
5cells/well, and incubated for 16 hours. After incubation,
the cells were treated with varying concentration of MeAS (0,
0.2, and 0.4 mg/mL) for 24 hours, and cells were lysed with
protein extraction reagent (iNtRON Biotechnology, Seongnam,
Korea) for 30 minutes on ice, and then centrifuge at 12,000
rpm for 15 minutes at 4℃. The supernatant was transferred
to a new 1.5 mL tube and protein was quantified using BCA
protein assay (Pierce, Rockford, IL, USA) method. Protein (20
μg) was mixed with 5X sample buffer, denatured at 100℃ for
5 minutes, electrophoresed on 8%, 10% or 15% sodium do-
decyl sulfate polyacrylamide gel electrophoresis gels, and then
transferred to a polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Hercules, CA, USA). Membrans was blcoked with
5% blocking solution (5% bovine serum albumin in Tris-bufferd
saline containing 0.1% Tween-20 [TBS-T] for 30 minutes and
the specific primary antibodies, cleaved caspase-9 (1:1,000),
cleaved caspase-3 (1:500), cleaved caspase-7 (1:1,000),
PARP (1:1,500), Bcl-2 (1:1,000), Bax (1:1,000) and α-tubulin
(1:2,000), were incubated at 4℃ overnight. Thereafter, the
membranes were washed with TBS-T three times for 15 min-
utes each, followed by incubated with a secondary antibody
conjugated with horseredish peroxidase (1:5,000) for 1 hour at
room temperature, and then washed with TBS-T three times for 15 minutes each. Protein were detected by Immobilon Western Chemiluminescent HRP Substrate (ECL; Millipore, Bedford, MA, USA) and visualized on a MicroChemi 4.2 device (DNR Bio Imaging Systems, Jerusalem, Israel).
10. Statistical analysis
All data are expressed as the means ± standard deviation.
All data were derived from at least three independent experi- ments. Statistical significance was determined using one-way ANOVA followed by Turkey’s analyses in GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Statistical sig- nificance was set to *p < 0.05.
Results Results
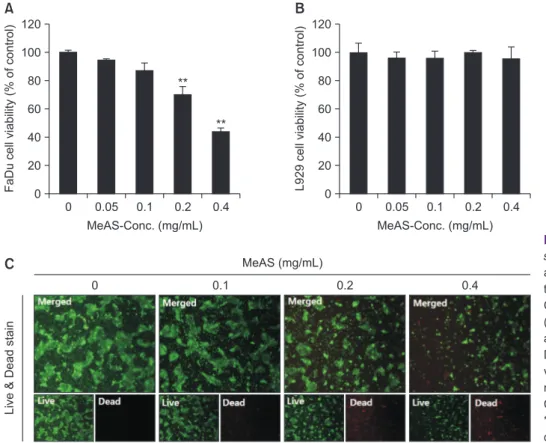
1. Cytotoxic effect of MeAS on FaDu and L929 cells
To verify whether MeAS affects viability of FaDu cancer cells and L292 normal cells, MTT assay and Live & Dead assay were performed. As shown in Fig. 1, FaDu cell viability began to decrease and cell viability rate for each concentration was 95%, 87%, 70%, and 44% at 0.05, 0.1, 0.2, and 0.4 mg/mL,
respectively. However, MeAS did not affect L929 cell viability.
Since MeAS exhibited cytotoxicity at 0.1 mg/mL, further ex- periments were carried out at 0.1, 0.2, and 0.4 mg/mL. Fur- thermore, results of Live & Dead assay shown that in MeAS- treated group, green-fluorescence indicating live cells was gradually reduced, but red-fluorescence indicating dead cells was gradually increased (Fig. 1C).
2. Inhibition effect of MeAS on wound-healing and colony formation of FaDu cells
To investigate whether MeAS affects the unlimited prolifera- tion and wound-healing of cancer cells, colony formation and wound-healing assay were performed. As a result, despite the concentration not showing cytotoxicity, the wound-healing ability was significantly decreased in a concentration-depen- dent manner (Fig. 2A). Furthermore, 0.1 mg/mL, which had a cytotoxic effect about 10%, also showed an effect of signifi- cantly reducing colony formation in FaDu cancer cells (Fig. 2B).
These results suggest that MeAS can delay canceration even at concentrations that do not show cytotoxicity.
0 120
80 60 40
20
FaDu cell viability (% of control)
MeAS-Conc. (mg/mL) 0
0.05 0.1 0.2 0.4 100
0 120
80 60 40
20
L929 cell viability (% of control)
MeAS-Conc. (mg/mL) 0
0.05 0.1 0.2 0.4 100
Liv e & Dead stain
0 0.1 0.2 0.4
MeAS (mg/mL)
A B
C
**
**
Fig. 1. Effect of methanol extracts of Asarum sieboldii (MeAS) on FaDu and L929 cell vi- ability. Cells are treated with various concen- tration of MeAS (0–0.4 mg/mL) for 24 hours.
Cell viability are determined using MTT assay (A, B) and Live & Dead assay (C). Results are expressed as a percentage of the control.
Data are expressed as means ± standard de- viation of three independent experiments. ×20 magnification.
Conc., concentration.
**p < 0.01, ***p < 0.001 compared with the
3. Analysis of nuclear morphology change by MeAS in FaDu cells
To investigate whether the reduction in FaDu cell viability by MeAS was associated with apoptosis, nucleic morphology was analyzed using DAPI stain. As a result, the number of cells with apoptotic nuclear morphology such as chromatin conden- sation and chromatin atrophy significantly was increased in the MeAS-treated group, compared with control group (Fig. 3).
4. Induction of apoptotic cells by MeAS in FaDu cells
When apoptosis is induced, phosphatidylserine (PS) located on the inner cell membrane is turned over and directed outside the cells. Therefore, cells undergoing apoptosis can be ob- served through annexin-V staining, which has a strong affinity with PS. To quantify the apoptotic cell of FaDu by MeAS, flow cytometirc was performed using annexin-V and propidium
iodide. As FACS results (Fig. 4), the early apoptosis rates were 3.3% and 5.1% at 0 and 0.4 mg/mL MeAS, respectively, and late apoptosis rates were 1.0% and 16.9% MeAS, respectively.
The population of total apoptotic cells was increased to 18%
at 0.4 mg/mL, respectively, compared to control group (4.1%) (Fig. 4). These results suggest that inhibition of cell viability by MeAS was associated with apoptosis in FaDu cells.
5. Activation of apoptosis mechanism by MeAS in FaDu cells
Caspases (cystein-aspartic acid-protease) are signaling transduction factors that are very closely related to apopto- sis. Most caspases are cascade-type cystein proteases and cleave the aspartic acid residue of the substrate protein [22].
Therefore, we evaluated whether MeAS-induced apoptosis was related caspase activity using western blot analysis. Fig.
5 shown that cleaved caspase-9, -3 and -7 were dramatically
W ound-healing assay 0 days 2 days
0 0.025 0.05 0.1
MeAS (mg/mL)
Colony formation 5 days
0 0.025 0.05 0.1
MeAS (mg/mL)
A
B Fig. 2. Inhibition of wound-healing and colony
formation by methanol extracts of Asarum sieboldii (MeAS) in FaDu cells. Cells are treated with various concentration of MeAS (0–0.4 mg/mL) for 24 hours. (A) After wound- ing, culture is performed in a medium mixed with extracts for 2-days, and then wound- healing is analyzed. (B) The degree of cancer cells colony formation is measured by colony formation.
DAPI stain
0 0.1 0.2 0.4
MeAS (mg/mL)
Fig. 3. Observation of nuclear change by
methanol extracts of Asarum sieboldii (MeAS)
in FaDu cells. Cells are treated with various
concentration of MeAS (0–0.4 mg/mL) for 24
hours, and then 4`6-diamidino-2-phenylindole
(DAPI, 300 nM) staining are performed. ×20
magnification.
increased by MeAS in a concentration-dependent manner, as a result, PARP, which downstream apoptotic indicator, was cleaved. In addition, MeAS significantly increased the expres-
sion of the anti-apoptosis protein Bcl-2 protein and decreased the expression of the pro-apoptosis protein Bax. These results suggest that MeAS-induced apoptosis can be induced by cas-
MeAS 0 mg/mL MeAS 0.2 mg/mL MeAS 0.4 mg/mL
100 101 102 103 FL1 INT LOG
100 101 102 103 FL3 INT LOG
100 101 102 103 FL1 INT LOG
100 101 102 103 FL3 INT LOG
100 101 102 103 FL1 INT LOG
100 101 102 103 FL3 INT LOG
[A] FL1 INT LOG/FL3 INT LOG
Double stain
[A] FL1 INT LOG/FL3 INT LOG
Double stain
[A] FL1 INT LOG/FL3 INT LOG