PREVENTION RESEARCH □ REVIEW ARTICLE □
89
INTRODUCTION
During the past two decades, there has been remarkable progress in unraveling the molecular biology of cancer.
Although efforts directed towards developing effective antican- cer therapies are still ongoing, prevention of cancer by dietary phytochemicals is now widely accepted as one of the most practical and promising strategies.
1)Chemoprevention refers to
the use of non-toxic chemical substances to prevent cancer.
1∼3)A large pool of convincing data arising from both laboratory and population-based studies suggests that plant polyphenols are effective in preventing cancer.
1,4,5)One such candidate chemopreventive agent is resveratrol (trans-3,4’,5-trihydroxys- tilbene, structure shown in Fig. 1), which is a phytoalexin formed in various edible plant species including grapes, mul- berries, and peanuts in response to injury, UV irradiation and fungal attack.
6)In a pioneering study by John M. Pezzuto and
Molecular Mechanisms Underlying Chemoprevention with Resveratrol
Joydeb Kumar Kundu and Young-Joon Surh
National Research Laboratory of Molecular Carcinogenesis and Chemoprevention, College of Pharmacy, Seoul National University, Seoul 151-742, Korea
Chemoprevention has been come up as a frontline strategy to fight against cancer. One of the strategies for chemoprevention include the inhibition of phase I xenobiotic metabolizing enzymes responsible for activating procarcinogens and the induction of phase II enzymes facilitating detoxification or antioxidantive defense. Since tumor promotion is a reversible process that involves inappropriate functioning of intracellular signal transduction pathways, controlling deregulated cell signaling network by dietary phytochemicals constitute a rational approach to achieve chemoprevention. Resveratrol, a major constituent of red wine, grapes and other edible plant species, has been documented as an effective chemopreventive agent. While depicting the molecular mechanisms underlying chemopreventive activity of resveratrol, it has been shown that resveratrol can block tumor initiation process by targeting cytochrome P450 enzymes that activate procarcinogens.
Resveratrol also induces several phase II detoxification or antioxidant enzymes by activating a redox-sensitive transcription factor nuclear factor E2-related factor-2, thereby conferring protection against initiation of carcinogenesis. As inflammation is closely linked to tumor promotion, a variety of antiinflammatory phytochemicals exert anti-tumor promotional effects by down-regulating aberrantly expressed cyclooxygenase-2.
Multiple lines of evidence suggest that resveratrol possesses anti-inflammatory and antiproliferative properties.
Of the several intracellular signaling cascades that resveratrol targets, signaling via transcription factors NF-κB and/or AP-1 that regulates transcriptional activation of many proinflammatory and growth promoting genes appears to be the most important. This review will address the molecular basis of chemoprevention by resveratrol, particularly at the level of initiation and promotion stage of carcinogenesis. (Cancer Prev Res 10, 89-98, 2005)
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ Key Words: Chemoprevention, Resveratrol, Nrf2, NF-κB, AP-1, MAPKs
책임저자:서영준, ꂕ 151-742, 서울시 관악구 신림 9동 산 56-1 서울대학교 약학대학 약학과
Tel: 02-880-7845, Fax: 02-874-9775 E-mail: surh@plaza.snu.ac.kr
Correspondence to:Young-Joon Surh
Department of Pharmacy, College of Pharmacy, Seoul National University, San 56-1 Sillim-9 dong, Gwanak-gu, Seoul 151-742, Korea
Tel: +82-2-880-7845, Fax: +82-2-874-9775 E-mail: surh@plaza.snu.ac.kr
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
colleagues,
7)resveratrol was found to interfere with all stages of carcinogenesis. Data from subsequent studies have revealed that resveratrol prevents tumorigenesis induced by diverse chemical carcinogens as well as ultraviolet irradiation.
8∼10)Since dysregulation of intracellular signal transduction pathways have been implicated in pathogenesis of various ailments including cancer, modulation of cellular signaling cascades may represent the molecular target-based chemo- prevention with dietary components.
11)The term “signal transdu- ction therapy” has thus been coined by A. Levitzki in 1994, referring signal transduction events as a potential targets for controlling malignant transformation.
12)Accumulating evidence suggests that resveratrol can modulate diverse signal trans- duction pathways, resulting in the blockade of carcinogen activation and enhancement of detoxification, inhibition of inflammation and cell proliferation, cell cycle arrest, and induction of apoptosis, etc.
10)Several redox-sensitive transcription factors such as NF-κB, AP-1, and Nrf2, and a wide panel of upstream kinases regulating aforementioned transcription factors are targeted by resveratrol. This review will address modulatory effects of resveratrol on multiple molecular events related to tumor initiation and promotion.
MOLECULAR TARGETS FOR ANTI-TUMOR INITIATING EFFECTS OF RESVERATROL
According to the current concept of the chemically-induced carcinogenesis, tumor initiation involves metabolic activation of carcinogens, generally mediated by phase I xenobiotic metab- olizing enzymes. However, there also exist cellular defensive mechanisms of detoxifying or eliminating activated carcinogens from the body. Therefore, effective strategies for blocking tumor initiation would be the suppression of metabolic activation of carcinogens and/or induction of phase II detoxifi- cation enzymes.
1. Inhibition of carcinogen activation and DNA adduct formation by resveratrol
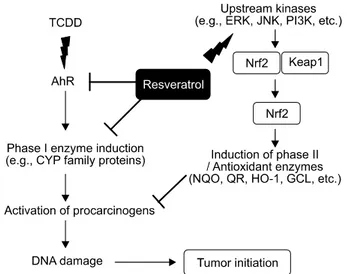
The antioxidant property of resveratrol may contribute to its anti-tumor initiating activity (Fig. 2). Resveratrol was found to inhibit the production of H
2O
2and myeloperoxidase activity, while it restored the cellular glutathione levels and the activity of superoxide dismutase
13)in mouse skin treated with a prototype tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). The activities of O-acetyltransferase and sulfotransferase in human breast cancer (MCF-7) cells treated with the hetero- cyclic amine N-hydroxy-2-amino-1-methyl-6-phenylimidazo- [4,5-b]pyridine (N-hydroxy-PhIP) and subsequent formation of PhIP-DNA adduct were suppressed by resveratrol.
14)The phase I drug metabolizing enzymes belonging to cytochrome P450 (CYP) family appears to be the molecular targets of resveratrol in achieving anti-tumor initiating effects. Recently, Boyce et al.
15)reported that resveratrol inhibited PhIP-induced geno- toxicity in human CYP 1A2 overexpressing Chinese hamster V79 cells possibly by blocking the activity of CYP1A2 responsible for metabolic activation of PhIP. Likewise, resve-
Fig. 1. Chemical structure of resveratrol.Fig. 2. Mechanism of anti-tumor initiating effects of resveratrol.
Resveratrol, as AhR antagonist, blocks carcinogen activation and subsequent DNA damage by suppressing induction of Phase I xenobiotic metabolizing enzymes. Exposure of normal cells to resveratrol results in the activation of a series of upstream kinases, such as ERK, JNK and PI3K, which dis- sociate Nrf2 from its inhibitory counterpart Keap1. Free Nrf2 translocates to the nucleus, where it regulates transcriptional activation of genes encoding phase II detoxification/antioxidant enzymes.
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
ratrol blocked the expression and catalytic activity of CYP1A1 in benzo[α]pyrene ([B[α]P)-treated microsomes from human hepatoma (HepG2) cells and 7,12-dimethylbenzathracene (D- MBA)-treated human breast cancer (MCF-7) cells.
16)Treatment of human bronchial epithelial (BEAS-2B and BEP2D) cells with resveratrol resulted in a dose- and time-dependent inhibition of B[α]P-induced DNA adduct formation as well as attenuation of CYP1A1 and CYP1B1 gene expression
17)The genes encod- ing CYP enzymes are transcriptionally regulated by binding of arylhydrocarbon receptor (AhR) to their promoter regions.
Several investigators have demonstrated that resveratrol sup- presses CYP1A1 transcription by blocking the conversion of the ligand-bound cytosolic AhR into its nuclear DNA-binding form, while others have reported that the CYP1A1 inhibitory activity of resveratrol is rather attributable to its suppression of the interaction between AhR and the transcription initiation complex.
6)We recently reported that resveratrol strongly inhibited 2,3,7,8-tetrachlorodibenzo-p-dioxin-(TCDD)-induced expression of CYP1A1 and CYP1B1 in human mammary epithelial cells
18)by blocking the AhR DNA binding activity.
2. Induction of antioxidant and detoxifying enzymes by resveratrol: Nrf2 as a potential target
While oxidative metabolism of procarcinogens leads to generation of more toxic species capable of causing DNA damage, xenobiotic metabolizing enzymes that catalyze phase II metabolic reactions are involved in carcinogen detoxification.
The enhancement of cellular antioxidative or detoxification capacity appears to be a pragmatic approach for achieving early stage prevention of cancer. The balance between the induction of enzymes responsible for carcinogen activation and those involved in carcinogen detoxification, thus, determines the ultimate risk of chemically-induced carcinogenesis.
19)Detoxi- fying/antioxidant enzymes, such as glutathione-S-transferase (GST), NADPH-quinoneoxidoreductase (NQO)-1, UDP-glucu- ronosyltransferase (UGT), microsomal epoxide hydrolase, γ- glutamylcysteine ligase (γ-GCL), glutathione synthetase, γ- glutamyl transpeptidase, and heme oxygenase-1 (HO-1), can protect cellular components from oncogenic insults. Once induced, these phase II or antioxidant enzymes can facilitate inactivation and subsequent elimination of electrophilic and pro-oxidative carcinogens.
20,21)Many of the genes encoding aforementioned phase II enzymes contain cis-acting elements in
their promoter regions, known as antioxidant response elements (ARE) or electrophile response elements (EpRE) [5’-(G/A) TGA(G/C)nnnGC(G/A)-3’], which are transcriptionally regu- lated by redox-sensitive transcription factors. One of the major transcriptional regulators of ARE-driven genes is Nrf2, which is a member of the cap‘n’collar family of bZIP transcription factor.
22)The molecular mechanisms underlying the activation of Nrf2 in response to electrophiles and other oxidative stress have been reviewed.
21∼23)Nrf2 is sequestered in the cytoplasm as an inactive complex with its cytosolic repressor Kelch-like ECH associated protein 1 (Keap1). Exposure to mild oxidative insult or electrophiles leads to the dissociation of Nrf2 from Keap1 either by direct phosphorylation of Nrf2 or by covalent modification of cysteine thiol residues of Keap-1. Free Nrf2 then translocates to nucleus, where it forms a heterodimer with small Maf protein and binds to cis-acting ARE or EpRE.
24)The dissociation of the Nrf2- Keap1 complex is alternatively facilitated by upstream kinases, such as mitogen-activated protein kinases (MAPKs), phosphoi- nositide-3-kinase (PI3K), protein kinase C (PKC) and protein kinase B (PKB)/Akt-mediated signals (Fig. 2).
The significance of Nrf2 activation in cellular defense against carcinogenic insults was evident from significantly higher burden of B[α]P-induced gastric neoplasia in Nrf2 deficient mice, which were less responsive to the phase II enzyme inducer oltipraz.
25)Mice lacking Nrf2 failed to induce many of the genes responsible for carcinogen detoxification and pro- tection against oxidative stress.
21,23)Moreover, Nrf2-null mice showed a decrease in the basal expression level of genes including epoxide hydrolase, GCL, GST, HO-1, NQO1, and UGT.
26∼28)Therefore, targeted activation of Nrf-2 is considered as a rational approach for chemoprevention, especially at the initiation stage of carcinogenesis.
Resveratrol induced NQO, an Nrf2-regulated detoxifying
enzyme, in mouse hepatoma (Hepa1c1c7) cells.
29)In addition,
several recent studies have demonstrated that the compound
can induce HO-1 expression and activity in human aortic
smooth muscle
24)and rat pheochoromocytoma (PC12) cells
18)via activation of NF-κB and Nrf2, respectively. Recently,
Hsieh et al. demonstrated that the inhibition of human mela-
noma cell proliferation by resveratrol is correlated with the upre-
gualtion of quinone reductase (QR)-2, which is a phase II detoxi-
fying enzyme.
30)Bianco et al. also reported that resveratrol
protected against estrogen-induced oxidative DNA damage in
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
human breast cancer cells by upregulating the expression of QR.
31)RESVERATROL SUPPRESSES TUMOR PROMOTION AND PROGRESSION BY TARGETING SIGNALING THROUGH
NF-κB AND AP-1
Dysfunction of intracellular signal transduction pathways
regulating cellular proliferation and differentiation has been considered as molecular basis of carcinogenesis. Components of intracellular signaling networks include MAPKs, PKC, PI3K, glycogen synthase kinase, PKB/Akt, and tyrosine kinases (e.g., growth receptor and soluble Src kinase). These upstream kinases can be aberrantly turned on by diverse stimuli provoking oxidative and pro-inflammatory stress and often amplified via activation of a battery of redox-sensitive transcription factors
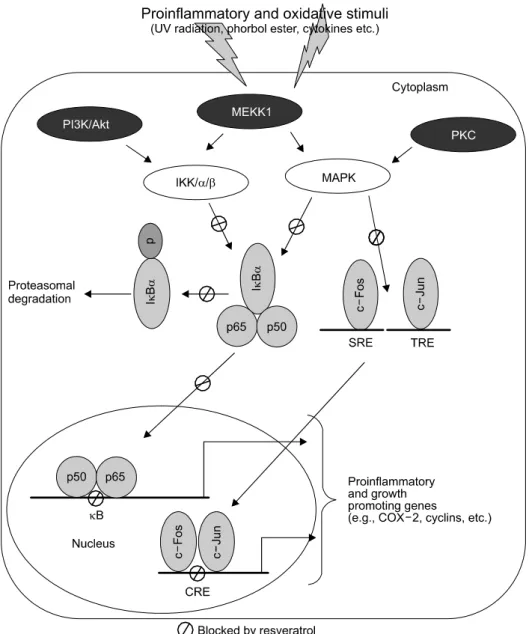
Fig. 3. NF-κB and AP-1 as prime molecular targets of resveratrol. Exposure to proinflammatory and oxidative stimuli cause activation of upstream kinases, such as PI3K/Akt, MEKK1 and PKC. PI3K/Akt and MEKK1 further activate IKK complex, which via phosphorylation-mediated proteasomal degradation of IκBα facilitates release of free NF-κB heterodimer (P65:p50). Free NF-κB then translocates to the nucleus, where it binds to the κB sequences located in the promoter region of target genes. On the other hand, MEKK1 and PKC activate MAPKs, which then targets AP-1 components c-Jun and c-Fos. A heterodimer of c-Jun and c-Fos, thus formed, binds to cyclic AMP response element (CRE) located on the target gene promoters. Resveratrol blocks multiple stages of the signaling via NF-κB and AP-1 as indicated.
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
including NF-κB and AP-1.
1)1. Resveratrol modulation of NF-κB and/or AP-1
The inhibition of cytokine release and pro-inflammatory gene expression and down-regulation of intracellular signal trans- ducing enzymes and transcription factors that regulate expression of pro-inflammatory genes are key molecular mecha- nisms underlying anti-inflammatory and chemopreventive acti- vities of resveratrol.
8,10,32,33)The eukaryotic transcription factors, NF-κB and AP-1, are known to regulate transcriptional activation of various proinflammatory and growth promoting genes in response to diverse oxidative and proinflammatory stimuli. Therefore, targeted inhibition of these redox-regulated transcription factors is considered as the molecular basis of chemoprevention by a wide variety of chemopreventive phyto- chemicals. The anticarcinogenic, anti-inflammatory, and growth- modulaory effects of resveratrol may partially be ascribed to the inhibition of activation of NF-κB and AP-1, and the associated upstream kinases (Fig. 3)
34).
Resveratrol diminished TNF-α- and bacterial lipopoly- sachccaride (LPS)-induced NF-κB DNA binding in human monocyte (THP-1) and myeloid (U-937) cells through inhibi- tion of IκB kinase (IKK) activity.
35)Resveratrol abrogated NF- κB DNA binding in Rat-1 cells, a kind of rat fibroblasts, stimulated with oncogenic H-ras and induced apoptosis as revealed by enhanced DNA fragmentation.
35)In another study, resveratrol suppressed LPS-induced expression of tissue factor in THP-1 cells by inhibiting phosphorylation and transactivation potential of p65, but failed to inhibit the activation or translocation of NF-κB/Rel proteins.
36)Resveratrol inhibited TNF-α-induced DNA binding activity of NF-κB in MCF-7 cells and DMBA-induced rat mammary tumors.
37)In addition, it down-regulated the expression of COX-2 and matrix metalloproteinase (MMP)-9 in mammary tumors, which are NF-κB-regulated gene products, suggesting a protective role of resveratrol in tumor promotion and progression.
37)Moreover, resveratrol inhibited the activation of NF-κB in Cr (VI)-stimulated JB6 cells as assessed by the luciferase reporter gene assay.
38)In normal human epidermal keratinocytes, resveratrol inhibited UVB-induced activation of NF-κB by blocking the activation of IKKα as well as phosphorylation and degradation of IκBα.
39)Alternatively, resveratrol blocked TNF-α-induced activation of NF-κB in a
dose- and time-dependent manner by suppressing phosphory- lation and nuclear translocation of p65 without affecting IκBα degradation in U937 cells.
34)It also inhibited NF-κB activa- tion induced by other stimuli such as TPA, LPS, H
2O
2, okadaic acid, and ceramide in lymphoid (Jurkat-T), HeLa and glioma (H4) cells.
34)Besides suppression of NF-κB, resveratrol inhibited the activation of AP-1.
34)In our recent study, resveratrol pre- treatment inhibited DNA binding of NF-κB by suppressing the activity of an upstream kinase IKKβ in TPA-stimulated mouse skin (J.K. Kundu and Y.-J. Surh, unpublished obser- vation).
A panel of upstream kinases transmits signals to NF-κB and AP-1, thereby regulating transcription of genes encoding effector molecules such as proinflammatory gene products as well as cell cycle regulatory proteins. Resveratrol has been shown to block aberrant activation of many of these upstream kinases, albeit in cell/tissue-and stimuli-specific manner. Res- veratrol inhibited UVC-and TPA-induced transcription of AP-1 reporter gene by blocking the activation of ERK2, JNK1, and p38 MAPK.
34)A recent study from our laboratory also revealed that topically applied resveratrol reduced phorbol ester-induced expression and activity of ERK and p38 MAPKs as well as AP-1 DNA binding in mouse skin in vivo.
40)Similarly, pretreatment of HeLa cells with resveratrol reduced AP-1 DNA binding by suppressing the activation of ERK, JNK and p38 MAPKs.
41)The inhibitory effects of resveratrol on TPA- mediated activation of PKC, c-Jun expression and AP-1 activity in human mammary and oral epithelial cells were also reported.
42,43)Yang et al. demonstrated that resveratrol inhi- bited anchorage-independent growth of human melanoma c83-2c (metastatic) and wm3211 (radial growth phase) cells by diminishing DNA binding and transcriptional activity of AP-1.
44)Treatment of U937 cells with resveratrol inhibited constitutive DNA binding activity of NF-κB.
45)In contrast, resveratrol had little effect on NF-κB DNA binding in TPA-stimulated U937 cells, while it blocked DNA binding activity of AP-1.
46)Resveratrol enhanced the DNA-binding activity of c-Fos, but not of c-Jun, in Caco-2 cells leading to decreased synthesis and increased catabolism of polyamines,
47)thereby preventing cellular proliferation and carcinogenesis.
Resveratrol appears to suppress the activation of NF-κB and/or
AP-1 in a cell/tissue- and stimuli-specific fashion.
10)The
following section of the article will focus on how resveratrol
exerts anti-tumor promoting effects by targeting cellular
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
signaling events.
2. Antitumor-promoting effects of resveratrol
Multiple lines of evidence suggest that resveratrol exerts anti-tumor promoting effects in experimentally-induced carcino- genesis models. An early study by Jang and colleagues demonstrated that topical application of resveratrol suppressed papilloma formation in a two stage mouse skin carcinogenesis model.
7)Since then resveratrol was studied extensively to evaluate its antitumor promotional effect in other organ systems and to explore underlying molecular mechanisms. Bhat et al. demonstrated that resveratrol inhibited N-methyl-N- nitrosourea-induced mammary tumorigenesis in female Sprague- Dawley rats.
48)In another study, pretreatment of female Sprague-Dawley rats with resveratrol reduced the incidence of DMBA-induced mammary tumors as well as suppressed the formation of ductal carcinoma.
37)Moreover, resveratrol inhibited esophageal tumorigenesis in F344 rats treated with N- nitrosomethylbenzylamine (NMBA).
49)Single topical application of resveratrol onto dorsal skin of SKH-1 hairless mice significantly inhibited UVB-induced expression and activities of ornithine decarboxylase (ODC), a biochemical hallmark of tumor promotion.
50)The administration of resveratrol at a dose of 2.5 mg/kg body weight for two weeks diminished N-nitroso- diethylamine-inuced cytosolic ODC levels in mice.
51)Despite its effectiveness as an anti-tumor promotional agent, there have been several conflicting reports that describe the failure of dietary resveratrol in suppressing intestinal tumorigenesis in Apc
Min/+mice
52)as well as inhibiting B[α]P- and 4-(methyl- genesis in female A/J mice.
53)The possible explanation for such failure in achieving chemoprevention in latter studies may be due to conversion of resveratrol to inactive metabolites, such as sulphate- and glucuronide-conjugates.
32)3. Suppression of inappropriately elevated COX-2 expression by resveratrol
Since inflammation is causally linked to tumor promotion, enzymes that mediate the pro-inflammatory process have been implicated in the pathophysiology of many types of human cancers.
54)There is now growing evidence supporting that chronic inflammation may lead to cancers of different organs including stomach, colon, breast, skin, prostate, pancreas, etc.
55~58)Prostaglandins (PGs) play a crucial role in the induction of ODC activity and mouse skin tumor promotion
induced by TPA.
59)It has been suggested that PGE
2and PGF
2αare functionally related to tumor promotion.
60,61)In response to inflammatory stimuli, PGs are produced through metabolic conversion of arachidonic acid by the enzyme cyclooxygenase-2 (COX-2), which is inappropriately up- regulated in various premalignant and malignant tissues.
62,63)Moreover, COX-2 overxepressing transgenic mice
64)are highly susceptible to spon- taneous tumor formation, while COX-2 knock out animals
65)are less prone to experimentallyinduced tumorigenesis. The intestinal adenoma formation in APC mutant mice is strongly reduced in the COX-2
-/-66)as well as in the PG receptor EP
-/-background.
67)Thus, substances with potent anti-inflammatory activities are anticipated to exert chemopreventive effects, particularly in the promotion stage.
Resveratrol has been reported to inhibit COX-2 at both transcriptional
13,49)and post-transcriptional
42,50)levels. An earlier study demonstrated that resveratrol inhibited TPA-induced PG synthesis through suppression of COX-2 activity in human mammary epithelial cells.
42)Similarly, single topical application of resveratrol onto dorsal skin of SKH-1 hairless mice signifi- cantly inhibited COX activity.
50)Resveratrol inhibited TPA- induced PGE
2production by down-regulating COX-2 gene transcription in human mammary and oral epithelial cells.
42,43)Moreover, pretreatment of mouse peritoneal macrophages with resveratrol inhibited LPS-, TPA- or H
2O
2-induced mobilization of arachidonic acid and expression of COX-2, resulting in decreased PGE
2production.
68)Resveratrol down-regulated the expression of both COX-1 and COX-2 mRNA transcripts in NMBA-induced esophageal tumors in F344 rats.
49)According to Murakami et al.,
69)the expression of COX-2 in LPS plus interferon-γ-treated RAW 264.7 macrophages was strongly inhibited by resveratrol. Resveratrol pretreatment inhibited TPA-induced COX-2 expression in female ICR mouse skin, further supporting the anti-tumor promoting potential of resveratrol.
40)4. Resveratrol arrests cell cycle progression
The rapid proliferation of preneoplastic cells needs a faster
rate of cell division. Suppression of abnormal cell cycle
progression may confer protection against clonal expansion of
premalignant cells.
70)Resveratrol caused p21
WAF1/CIP1-mediated
G1-phase arrest and also induced apoptosis in human
epidermoid carcinoma A431 cells.
70)According to this study,
resveratrol inhibited the expression of various cyclins (e.g, D1,
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
D2 and E) as well as the expression and catalytic activities of cyclin dependent kinases (Cdk) -2, -4 and -6, suggesting that resveratrol-induced up-regulation of p21
WAF1/CIP1may inhibit the formation of cyclin-cdk complexes thereby imposing artificial checkpoints at G1/S transition of the cell cycle.
70)Moreover, the antiproliferative effect of resveratrol in A431 cells was associated with a decrease in the expression of E2F transcription factor as well as a reduction in the hyperphos- phorylated form of retinoblastoma protein.
71)Similarly, G1 phase arrest by resveratrol was observed in HepG2 cells.
72)According to a recent in vivo study, resveratrol inhibited the growth of transplantable liver cancer in H22-bearing mice by decreasing the expression of cyclin B1 and cdc2 protein.
73)It was demonstrated that resveratrol inhibited the expression of cyclin B1 in MCF-7, SW480, HCE-7, Seg-1, Bic-1, and HL-60 cells, while it down-regulated the expression of cyclin D1 and A in SW480 cells only, suggesting the cell type-specific effect of resveratrol in blocking the malignant cell cycle progre- ssion.
74)Another study demonstrated that resveratrol inhibited the expression of cyclin D1 and Cdk4, but increased the expression of cyclin E and A in human colon cancer cells Caco2 and HCT-116.
75)Similarly, resveratrol elicited an antipro- liferative effect by targeting cyclin D1 and Cdk4 in DU-145
76)and MCF-7
77)cells, which was associated with the induction of p53 and Cdk inhibitor p21
WAF1/CIP1. Moreover, the kinase activities of the cyclin E and Cdk2 complex in DU-145 cells were inhibited by resveratrol without alteration of their protein levels.
76)In a recent study, resveratrol inhibited the growth of human SK-Mel-28 melanoma cells, whereby it upregulated the expression of cyclins A, E and B1 with subsequent irreversible arrest of these cells in S-phase.
78)CONCLUSION
Emphasis has recently been given to dietary management of cancer. Among numerous dietary phytochemicals that have been identified as potential chemopreventive agents, resveratrol seems to be one of the most fascinating and promising molecule to interfere with each stage of multi-step carcino- genesis. As a polyphenol with antioxidative property, the compound protects against reactive oxygen species-mediated cellular damage, blocks metabolic activation of carcinogens and switch on the transcriptional activation of a battery of detoxi- fication/antioxidant enzymes via activation of redoxregulated
transcription factor Nrf2. The inhibitory effects of resveratrol on tumor promotion and progression have been attributed to its ability to interfere with intracellular signaling via other redox-sensitive transcription factors such as NF-κB and AP-1, which regulate a series of proinflammatory and proliferative genes. With rapid progress in unraveling the mechanistic basis of chemoprevention, the human intervention trials with this compound are anticipated, which will open a new avenue for molecular-target based chemoprevention.
ACKNOWLEDGEMENT
This work was supported by the grant from Research Institute of Pharmaceutical Sciences, Seoul National University.
REFERENCES
1) Surh Y-J. Cancer chemoprevention with dietary phyto- chemicals. Nature Rev Cancer 3, 768-780, 2003.
2) Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res 555, 33-51, 2003.
3) Chun K-S, Surh Y-J. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol 68, 1089-1100, 2004.
4) Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett 215, 129-140, 2004.
5) Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell Biochem Suppl 27, 59-67, 1997.
6) Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 22, 1111-1117, 2001.
7) Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218-220, 1997.
8) Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24, 2783-2840, 2004.
9) Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N.
Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J 19, 1193-1195, 2005.
10) Kundu JK, Surh Y-J. Molecular basis of chemoprevention by resveratrol: NF-κB and AP-1 as potential targets. Mutat Res 555, 65-80, 2004.
11) Kundu JK, Surh Y-J. Breaking the relay in deregulated
cellular signal transduction as a rationale for chemoprevention
with anti-inflammatory phytochemicals. Mutat Res 2005 (in
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
press).
12) Levitzki A. Signal-transduction therapy. A novel approach to disease management. Eur J Biochem 226, 1-13, 1994.
13) Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetrade- canoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett 134, 81-89, 1998.
14) Dubuisson JG, Dyess DL, Gaubatz JW. Resveratrol modulates human mammary epithelial cell O-acetyltransferase, sulfo- transferase, and kinase activation of the heterocyclic amine carcinogen N-hydroxy-PhIP. Cancer Lett 182, 27-32, 2002.
15) Boyce A, Doehmer J, Gooderham NJ. Phytoalexin resveratrol attenuates the mutagenicity of the heterocyclic amines 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 2-ami- no-3, 8-dimethylimidazo[4,5-f]quinoxaline. J Chromatogr B Analyt Technol Biomed Life Sci 802, 217-223, 2004.
16) Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expre- ssion by resveratrol. Mol Pharmacol 56, 760-767, 1999.
17) Berge G, Ovrebo S, Botnen IV, Hewer A, Phillips DH, Haugen A, Mollerup S. Resveratrol inhibits benzo[α]pyrene- DNA adduct formation in human bronchial epithelial cells. Br J Cancer 91, 333-338, 2004.
18) Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh Y-J. Resveratrol inhibits TCDD- induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 25, 2005-2013, 2004.
19) Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect 105(Suppl 4), 965-970, 1997.
20) Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med 36, 1505-1516, 2004.
21) Lee J-S, Surh Y-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 224, 171-184, 2005.
22) Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J 19, 1061-1066, 2005.
23) Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res 555, 133-148, 2004.
24) Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol 69, 41-48, 2005.
25) Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98, 3410-3415, 2001.
26) Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA 96, 12731-12736, 1999.
27) Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236, 313-322, 1997.
28) Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice.
Carcinogenesis 24, 461-467, 2003.
29) Gerhauser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism- based in vitro screening of potential cancer chemopreventive agents. Mutat Res 523-524, 163-172, 2003.
30) Hsieh TC, Wang Z, Hamby CV, Wu JM. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Biochem Biophys Res Commun 334, 223-230, 2005.
31) Bianco NR, Chaplin LJ, Montano MM. Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage. Biochem J 385, 279-287, 2005.
32) Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient:
molecular basis, open questions and promises. J Nutr Biochem 16, 449-466, 2005.
33) Ulrich S, Wolter F, Stein JM. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol Nutr Food Res 49, 452-461, 2005.
34) Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation.
J Immunol 164, 6509-6519, 2000.
35) Holmes-McNary M, Baldwin AS Jr. Chemopreventive pro- perties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 60, 3477-3483, 2000.
36) Pendurthi UR, Meng F, Mackman N, Rao LV. Mechanism of resveratrol-mediated suppression of tissue factor gene expression. Thromb Haemost 87, 155-162, 2002.
37) Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7, 12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-B, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res 62, 4945-4954, 2002.
38) Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun 309, 1017-1026, 2003.
39) Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-B in normal human keratinocytes by resveratrol Neoplasia 5, 74-82, 2003.
40) Kundu JK, Chun K-S, Kim SO, Surh Y-J. Resveratrol inhibits
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets.
Biofactors 21, 33-39, 2004.
41) Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen- activated protein kinase pathways. Mol Pharmacol 60, 217-224, 2001.
42) Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem 273, 21875-21882, 1998.
43) Subbaramaiah K, Michaluart P, Chung WJ, Tanabe T, Telang N, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Ann NY Acad Sci 889, 214-223, 1999.
44) Yang S, Meyskens FL Jr. Alterations in activating protein 1 composition correlate with phenotypic differentiation changes induced by resveratrol in human melanoma. Mol Pharmacol 67, 298-308, 2005.
45) Asou H, Koshizuka K, Kyo T, Takata N, Kamada N, Koeffier HP. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias.
Int J Hematol 75, 528-533, 2002.
46) Shen F, Chen SJ, Dong XJ, Zhong H, Li YT, Cheng GF.
Suppression of IL-8 gene transcription by resveratrol in phorbol ester treated human monocytic cells. J Asian Nal Prod Res 5, 151-157, 2003.
47) Wolter F, Turchanowa L, Stein J. Resveratrol-induced modi- fication of polyamine metabolism is accompanied by induction of c-Fos. Carcinogenesis 24, 469-474, 2003.
48) Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res 61, 7456- 7463, 2001.
49) Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N- nitrosomethylbenzylamine (NMBA)-induced esophageal tumo- rigenesis in F344 rats by resveratrol. Carcinogenesis 23, 1531- 1536, 2002.
50) Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol 186, 28-37, 2003.
51) Khanduja KL, Bhardwaj A, Kaushik G. Resveratrol inhibits N-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J Nutr Sci Vitaminol 50, 61-65, 2004.
52) Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc (Min/+) mice. J Nutr 134, 5-10, 2004.
53) Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as
inhibitors of benzo[α]pyrene plus 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice.
Cancer Lett 137, 123-130, 1999.
54) Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428, 305-327, 1999.
55) Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflam- mation. Am J Physiol Gastrointest Liver Physiol 287, G7-G17, 2004.
56) Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer.
J Urol 172, S6-S11, 2004.
57) O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 85, 473-483, 2001.
58) Whitcomb DC. Inflammation and Cancer V. Chronic pan- creatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 287, G315-G319, 2004
59) Verma AK, Ashendel CL, Boutwell RK. Inhibition by prostaglandin synthesis inhibitors of the induction of epider- mal ornithine decarboxylase activity, the accumulation of pro- staglandins, and tumor promotion caused by 12-O-tetrade- canoylphorbol-13-acetate. Cancer Res 40, 308-315, 1980 60) Furstenberger G, Gross M, Marks F. Eicosanoids and multistage
carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion). Carcinogenesis 10, 91-96, 1989.
61) Narisawa T, Takahashi M, Niwa M, Fukaura Y, Wakizaka A. Involvement of prostaglandin E2 in bile acid-caused promotion of colon carcinogenesis and anti-promotion by the cyclooxygenase inhibitor indomethacin. Jpn J Cancer Res 78, 791-798, 1987.
62) Mohan S, Epstein JB. Carcinogenesis and cyclooxygenase: the potential role of COX-2 inhibition in upper aerodigestive tract cancer. Oral Oncol 39, 537-546, 2003.
63) Williams CS, Mann M, DuBois RN. The role of cyclooxy- genases in inflammation, cancer, and development. Oncogene 18, 7908-7916, 1999.
64) Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA 99, 12483-12488, 2002.
65) Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res 62, 3395-3401, 2002.
66) Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des 8, 1021-1034, 2002.
67) Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F,
Narumiya S, Oshima M, Taketo MM. Acceleration of intestinal
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ