1)
Int ro d u c t io n
Treatment options for patients with symptomatic mye- loma range from relatively simple conventional chemo- therapy to high-dose chemotherapy followed by periph- eral stem cell or bone marrow transplantation.1) But treatment of multiple myeloma remains disappointing.
Melphalan based high dose chemotherapy with hemato- poeitic stem cell support increases the response rate and
접수 : 2002년 11월 5일, 수정 : 2002년 11월 15일 승인 : 2002년 11월 20일
Corresponde nce : Soon Na m Lee M.D., Depa rtme nt of Inte rna l Medicine , Ewha Woma ns Unive rs ity
Tel : 82- 2- 760- 5054, Fax : 82- 2- 760- 5382 E- ma il : s nlee @ewha .ac.kr
overall survival.2) However, the relapse rate remains high in all patients, so the disease remains incurable.2 , 3)
Recently, it has been reported that angiogenesis is in- creased in active multiple myeloma, mediated by vascu- lar endothelial growth factor (VEGF) and basic fibro- blast growth factor (bFGF) .4~ 6) It suggested anti- angio- genic drug plays the treatment for myeloma.
Th alidomide is a glutamic acid der ivative that sh ows a potent antiangiogenic activity.7 , 8 ) Also, th is dr ug h as been found as immunomodulatory pr oper- ties and an effective inh ibitor of T NF - alph a.9) Re- cognition of its anti- angiogenic effect led to its evalu- ation in th e treatment of var ious malignancies, wh er e angiogenesis h as been sh own to play an important r ole.
S a lv a g e T h er apy w it h T h a lidom ide in P at ien t s w it h R e lap s e d or R e fr a ct or y M u lt ip le M y e lom a
Do Ye un Kim, M.D.1, Se ock- Ah Im , M. D.1, Chu- Myong Se ong , M. D.1, Soon Na m Le e , M.D.1, Soo- Mee Ba ng , M. D.2, J a e Hoon Lee , M. D.2, S ung- Soo Yoon , M.D.3, Byoung Kook Kim, M. D.3,
Se on Ya ng Pa rk, M. D.3 a nd Myung- J u Ahn , M. D.4
D ep artm ent of I n t ernal M ed icin e1, E wha W om ans Un iv ers ity Colleg e of M ed icin e, D ep artm ent of I n t ernal M ed icin e2, Gachon M ed ical Cent er,
D ep artm ent of I nt ernal M ed icin e3, S eoul N at ional Un iv ers ity Colleg e of M ed icin e, and D ep artm en t of I nt ernal M ed icin e4, H any ang Un iv ers ity Colleg e of M ed icin e, S eoul, K orea
B ac k g roun d : The re a re few the ra pe ut ic o p- tions fo r patie nts with multiple mye loma who re la ps e afte r a uto logous o r a lloge ne ic ste m ce ll tra ns pla ntatio n, o r fo r patie nts who a re re- fracto ry to co nve ntiona l c he mothe ra py a nd not e lig ible for s a lvage hig h- dos e the ra py. Tha lido- mide , a pote nt a nt ia ngioge nic age nt , ha s be e n s ugge ste d as a n e ffective s a lvage the ra py in refra cto ry mult iple mye loma . The a im of this study was to eva luate the effic acy a nd tol- e ra nc e of tha lidomide as a s ingle age nt a s mult ic e nte r tria l in Korea .
Me th od : Fro m Fe brua ry 200 1 to Se pte mbe r 2002, 28 patie nts from 4 institut io ns we re inc luded . At sta rt of treatme nt , a ll patie nts had active d is ea se a nd 17 (6 1%) had rec e ived at le ast o ne a uto logous tra ns pla ntat io n.
Re s ult s : The s e rum or urine leve ls of pa- ra prote in we re red uc ed by at least 90 pe rc e nt in two patie nts , at lea st 50 pe rce nt in three patie nts , a nd at least 25 pe rce nt in two pa- tie nts ; for a tota l res po ns e rate of 25 pe rc e nt . 13 patie nts had sta ble dis eas e a nd 8 patie nts had prog re ss ed . At lea st ha lf of the patie nts had mild or mode rate c onstipation a nd fa- tig ue . More seve re adve rs e e ffects we re in- freque nt .
Conc lu s ion : This study co nfirms t hat tha- lidomide is a n effective a nd safe age nt in pa- tie nts with re la ps ed or refractory multiple mye- loma . (Korean J He matol 2002;38 :259 ~264)
Ke y W ords : Tha lidomide , Multiple mye loma
259
In 1999, Singhal et al. reported the overall response rate of 32% of thalidomide in 84 heavily pre-treated multiple myeloma patient.10 ) The activity of thalidomide in relapsed myeloma has since been confirmed by numer ous studies.1 1~ 13) We report the results of a retr ospective study of the 28 Korean patients fr om 4 centers with refractory or relapsed advanced multiple myeloma wh o treated with thalidomide as a single agent.
P a t ie nt s a nd Me t ho d s
1 . P a t ie nt s
Between February 2001 and September 2002, 28 patients fr om 4 centers were enr olled. Patients ' charac- teristics at the onset of thalidomide treatment are presented in table 1. The median age was 54 years (range ; 34~72) and 61% of patients were males and 93% were stage III. The median time fr om diagnosis to thalidomide therapy was 31 months. Prior to thalido- mide therapy, 10 patients had been treated with con- ventional chemotherapy alone (1~4 lines, median 2
lines) . The previous therapy included either single (N=
15) or double (N=2) autologous transplantation. There was 1 patient wh o received autologous transplant and after then allogeneic transplant due to disease pr ogres- sion. Disease status at study entry was evaluated with respect to the last line of treatment the patient had received before thalidomide therapy ; 23 patients (82%) were classified as relapsed and 5 patients (8%) as re- fractory.
2 . Re s p o ns e a na lys is
Complete response was defined as lack of detectable M- component in serum and/ or urine. Partial and minor responses were defined as 50% and 25% M-component reduction in serum and/ or urine, respectively. Patients with a reduction of less than 25 percent without evi- dence of additional myeloma-related complications were considered to no response. Patients were considered in pr ogression when they did not meet criteria for response or stable disease. In patients with a response, an increase in serum or urine parapr otein levels by more than 25 percent above the nadir value was con- sidered of relapse.
3 . A s s e s s me nt of a dve rs e e ffe ct s
Participating centers were asked to pr ovide detailed information about the occurrence of side effect, their onset date, duration and intensity and about the tha- lidomide dose modifications which were due to these side effect. We confirmed these contents by telephone interviews.
4 . S t a t ic a l a na lys is
Overall survival (OS) was defined as the time elapsed form onset of thalidomide to death whatever the cause of death . Event free survival (EFS) was defined as the time elapsed fr om the onset of thalidomide to pro- gression, stop medication of thalidomide for any rea- son, death fr om any cause, or the last follow- up visit, whichever occurred first. Distributions over time were estimated by Kaplan- Meier analysis.
T a b le 1 . P a t ie nt ' s c ha ra c t e ris t ic s
Characteristics No. of patients (%)
Median age (range) Male/Female Durie- Salmon stage
II III Isotype
IgG IgA Light chains Type of light chain
κ λ
Median tine from diagnosis (range) Chromosomal abnormality (n=10) Disease status at the onset of thalidomide
Relapse Refractory Prior treatment
Standard chemotherapy alone Single autologous transplant Double autologous transplant Autologous+Allogeneic transplant
54 yrs (34~72) 18 (61)/11 (39)
2 ( 7) 26 (93)
23 (82) 3 (11) 2 ( 7)
16 (57) 12 (43) 31 months (1~84)
3 (30)
23 (82) 5 (18)
10 (36) 15 (54) 2 ( 7) 1 ( 3)
Re s u lt s
1 . Re s p o ns e t o t re a t me nt a n d o ut c o me
Response to treatment and patient outcome are summarized in Table 2. Two patients (7%) achieved a complete response. Partial response was observed in 3 patients (11%) and there was minimal response in 2 patients (7%) . Thus, the overall response rate was 25%. The hemoglobin levels and platelet count were improved in 2 patients among responders.
One patient of complete responder was relapsed after withdrawal of thalidomide due to grade 3 skin rash.
One patient wh o sh owed minimal response was relapsed after self withdrawal of thalidomide. The median inter- val between onset of thalidomide and the first decrease of M- pr otein by at least 25% was 1. 7 months (range ; 0. 5~1. 9) . The median response duration was 7. 7
months (range ; 3. 6~15. 4) . Thirteen patients had sta- ble disease. Eight patients pr ogressed.
2 . Tox ic it y
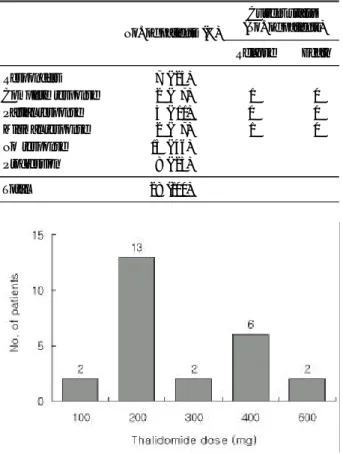
Distribution of the maximum tolerated dose of tha- lidomide is sh own in Fig. 1. Three patients who stop- ped thalidomide medication due to WHO grade ≥3 toxic effects were not considered. Thirteen patients (52%) took the 200mg dose. No patients did not take dose of thalidomide up to 800mg. WHO grade ≥3 toxic effects such as skin rash , paresthesia and constipation, pr ompted dose reduction in 3 patients. As sh own in Table 3, constipation (68%) and lethargy (54%) were the most frequent adverse events. No thalidomide- related mortality was noted and most of toxicities were grade 1 or 2. Neur ologic toxicity such as dizziness, tingling sensation was observed in 4 patients. Nine patients experienced skin rash , in three cases treatment was interrupted.
3 . Eve nt f re e s u rv iva l a nd ove ra ll s u rv iva l
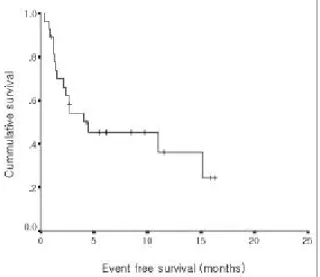
The median follow- up duration from the start of thalidomide treatment was 5. 8 months. Twenty two patients are still alive and followed up as outpatient.
The median EFS was 4 months (Fig. 2) . The median time to OS had not been reached. After 12 months follow- up, 58 percent of patients were alive (Fig. 3) . T a b le 2 . Re s po n s e a nd c u r re nt s t a t u s
No. of patients (%)
Current status (No. of patients) Relapse Death
Responders 7 ( 25)
Complete response 2 ( 7) 1 0
Partial response 3 ( 11) 0 0
Minimal response 2 ( 7) 1 0
No response 13 ( 46)
Progression 8 ( 25)
Total 28 (100)
Fig . 1. Distribution of the maximum tole rate d dose of tha- lidomide .
T a b le 3 . A d v e rs e e ff e c t s of t ha lid o m id e
No. of patients (%) Grade 1~2 Grade 3~4 Constipation
Fatigue Somnolence Lethargy Skin rash Pruritis Liver dysfunction Dry mouth
Tingling or numbness Edema
Dizziness Leukopenia Thrombocytopenia
16 (57) 14 (50) 9 (32) 6 (19) 6 (19) 3 (11) 3 (11) 3 (11) 2 ( 7) 2 ( 7) 1 ( 4) 1 ( 4) 0 ( 0)
3 (11) 1 ( 4) 0 ( 0) 3 (11) 3 (11) 0 ( 0) 0 ( 0) 0 ( 0) 1 ( 4) 0 ( 0) 0 ( 0) 0 ( 0) 1 ( 4)
Dis c us s io n
While conventional therapies have become inef- fective in patients with refractory myeloma, thalidomide has given substantial response. We observed that angiogenesis is increased in myeloma.6) Antiangiogenic therapy represents a novel and possibly less toxic approach to treat malignancies.
This study confirms an antitumor activity of thalido- mide to advanced multiple myeloma. Since almost all available therapy for relapse had failed in most of these patients, the responses observed are impressive.
It is unclear how thalidomide achieve its anti- multiple myeloma cells effect. The median time to response was rapid similar to other study. Even th ough we did not observe bone marr ow angiogenesis after thalidomide, there were no statistically significant differences in posttreatment micr ovessel density.10 ) Fr om these two points of observations, possible other mechanisms of thalidomide except angiogenesis are suggested. Actu- ally, it has known as potent immunomodulary agents such as potent inhibitor of tumor necr osis factor -α,9 , 14) inducers of cytotoxic T -cell pr oliferation15) and secretion of interferon-γ.9) In addition, it may modulates the expression of cell surface adhesion molecules16) that allow myeloma cells to interact with the bone marr ow micr oenvir onment. So it may be action of mechanism to myeloma cells which complex effects on tumor angio- genesis, the immune system, and various cytokines and adhesion molecules. By searching the more detailed mechanisms of action of thalidomide to plasma cells, it will contribute to new insight into tumor biology and development of other novel agents.
The optimal dose of thalidomide remains to be determined. Barlogie et al. concluded that there is a dose-response relationship between tumor cell reduc- tion and a cumulative dose of thalidomide.17) In con- trast, other studies achieved comparable results using much lower dose of the drug.18 , 19) Also, in our study, median dose between responder and nonresponder was not different. This means for some subset of patients, dose de- escalation may be necessary without suffering toxicities. This issue must be solved with the detailed mechanism of action of thalidomide to myeloma.
The overall response rate in most studies so far reported averages appr oximately 30%.1 1~ 13) The re- sponse rate with 25% of this study is slightly below compared to other studies. We can ' t assure that rel- atively low dose of thalidomide in our patients may contribute, th ough the dose-response relation is not clear .
The predictive factors which indicators are associated with response of thalidomide to myeloma patients remains to be unsolved. Barlogie et al have reported that normal cytogenetic, low plasma cell labeling index
≥0. 5% and β2 microglobulin ≥3 mg/ L gave better OS Fig . 2 . Ka pla n- Me ie r estimate of eve nt fre e s urviva l.
Fig . 3 . Ka pla n- Me ie r estimate of ove ra ll s urviva l.
and EFS in myeloma patients who treated with thalidomide.17) Dose response effect was apparent in the high risk subgr oup defined by abnormal cytogenetics, β2 microglobulin, and plasma cell labeling index.
Meanwhile, intergr oupe francoph one du myeloma (IFM) defined poor risk features such as IgA isotype, platelet count < 80×109/ L and serum albumin level <
3g/ dL for myeloma patients with treatment of thalid- omide.20 ) We had limitation for analyze about this concerns because of small sample size and retr ospective study.
As many other reports, constipation was the most common side effect. Almost of the toxicities were mild and easily manageable with out drug -related mortality.
The incidence of skin rash was slightly more common compared to other study. The side effect was dose dependent. Reducing the dose of thalidomide alleviated the effects in 3 patients. One patient, wh o developed grade 3 skin rash during dose up to 600mg, stopped taking thalidomide for 1 month , and then restarted with out skin rash. Side effect such as deep vein thr ombosis was not noted. Hematologic toxicity was not remarkable. Recent report about the adverse effect of thalidomide in advanced myeloma concluded that safety of higher dose (800 mg/ day) of thalidomide given over a long period (> 15 months) .2 1) All the adverse effects did not warrant decrease or termination of therapy, and then tolerance developed to sedation, constipation, and skin lesion later . But the patients in our study tolerated poorly to dose escalation schedule. We assume that there are possible pharmacodynamic differencies of thalidomide between races.
Overally thalidomide was an effective and safe drug.
Recently, thalidomide in combination with other drugs is investigated actively.2 2 , 2 3) Also the role as remission induction or maintenance therapy still need to be deter- mined.
요 약
배 경 : 다발성골수종은 복합화학요법이나 고용량 화학
요법 후 골수이식 등의 치료를 하지만 재발하거나 불응성 인 경우는 효과적인 치료방법이 없다. 최근에 신생혈관 억 제작용을 가진 thalidomide가 효과적인 구제요법으로 제시가 되고 있다. 본 연구는 다기관 연구로 thalidomide 단독 제제의 효과와 독성을 평가하고자 하였다.
방 법 : 2001년 2월부터 2002년 9월까지 4개 기관에서
28례의 환자가 포함이 되었다. 치료 당시 모든 환자는 활동 성이었고 17례 (61%)가 고용량 화학요법 후 자가 골수이식 후에 진행된 경우였다.결 과 : 혈청이나 소변에서 90% 이상의 M단백 감소를
보인 완전관해가 2례, 50%이상의 M단백 감소를 보인 부 분관해가 3례에서 관찰되었고 적어도 25%이상의 M단백 감소를 보인 미세관해가 2례로 전체 반응률은 25%였다.12례가 안전반응을 8례가 진행성 병변을 보였다. 독성 중 가장 흔한 것은 변비나 피곤함이었고 대부분의 독성은 경 미하였다.
결 론 : Thalidomide는 재발성 혹은 불응성 다발성 골수
종 환자에게 효과적인 구제요법으로 부작용은 경미하였 다.Re f e re nc e s
1) Alex anian R, Dim opoulos M : T he treatm ent of m ul- t ip le m y elom a. N E ng l J M ed 330:484 - 489, 1994 2) Bar log ie B : A d vances in the rap y of m ult ip le m y e -
lom a: L es s ons f rom acut e le uk em ia. Clin Cancer R es 3:2605 - 2 613, 199 7
3) At t al M , H ar ou s s eau JL, St oppa AM , S ot t o JJ , F uzibet JG, Ros si JF , Cas as su s P , M ais onneuve H , F acon T , Ifr ah N , P ayen C, Bat aille R : A p ro- sp ect iv e, random iz e d tr ial of a utolog ous bon e m ar- row t ransp lantat ion and chem othe rap y in m ult ip le m y elom a. N E ng l J M e d 335:91 - 99, 1996
4) V acca A , Rib atti D, Roncali L, Serio G, Silvest ris F , Damm acco F : B one m arrow ang iog enes is and p rog ress ion in m ult ip le m y e lom a. B r J H aem atol 8 7:503- 508, 1994
5) Mun shi N , Wils on CS : I ncreas e d bon e m arrow m icrov ess e l dens ity in newly d iag n os e d m ult ip le m y elom a carr ies a p oor p rog nos is . S em in Oncol 28:565 - 569, 2 001
6) P ark SY , Im SA , N am EM , Kim DY, Ch oi YA , Lee KE , Seong CM , Lee SN , Koo H S, Kim S S : E xp res s ion of vas cular en dothe lial g rowth factor and inte rle uk in - 6 in m ult ip le m y e lom a. K orean J H em at ol 36:35 - 42, 2 001
7) D ' Am at o RJ , Loughn an M S , F ly nn E , F olk am an J : T halidom ide is an inhibitor of ang iog en es is . P roc N atl A cad S ci USA 91:4082 - 4085, 1994
8) Bauer KS, Dix on S C, F ig g W D : I nhibitor of ang io- g enes is by thalidom ide re q uires m e tabolic act iva- t ion, which is sp e cies - dep end ent. B iochem P har- m acol 55 :182 7- 1834, 1998
9) H aslett PA , Corr al LG, Albert M , Kapaln G : T halidom ide cos t im ulates p r im ary hum an T ly m - p hocy t e, p ref erent ially ind ucing p rolif erat ion, cy to- k ine p rod uct ion, and cy totox ic resp ons es in the CD 8+ s ubs e t. J E xp M e d 18 7:1885 - 1892 , 1998 10) Sing h al S , Meht a J , Desikan R, Ayer s D, Rober s on
P , E ddlem on P , Mun shi N , An ais sie E , W ils on C, Dh odapk ar M , Zeddis J , Bar log ie B : A nt it um or act i- v ity of thalidom ide in ref ractory m ult ip le m y elom a N E ng l J M e d 341:1565 - 15 71, 1999
11) Rajkum ar SV , W it zig T E : A re v iew of ang io- g enes is and an t iang iog ene t ic the rap y w ith tha- lidom ide in m ult ip le m y e lom a. Cancer T reat R ev 2 6:351 - 362 , 2000
12) Rajkum ar SV , F on sec a R, Dispenzier e A , Lacy M Q, Lu st JA , W itzig T E , Kyle RA , Gert z MA , Gr eipp P R : T halidom ide in the treatm ent of re lap s e d m ul- t ip le m y e lom a. M ay o Clin P roc 75 :897- 902, 2 000 13) Julius s on G, Celsing F , T ur es s on I : F re q uen t g ood
p art ial rem is s ions f rom thalidom id e includ ing bes t resp ons e ev er in p at ients w ith advance d ref ractory and re lap s e d m y e lom a. B r J H em at ol 109:89 - 96, 2 000
14) S am paio EP , S arn o EN, Galilly R, Cohn ZA , Kaplan G : T halid om ide s e le ct ive ly inhibits t um or ne cros is f act or alp ha p rod uct ion by s t im ulate d hum an m ono-
cy t es . J E xp M e d 173:699 - 670, 1991
15) McHug h SM , Rifkin IR, Deig ht on J , W ils on AB, Lachm ann PJ , Lockw ood CM , Ew an PW : T he im m unos upp ress iv e dr ug thalidom ide ind uces T he lp e r ce ll typ e 2 ( T h2 ) an d concom itant ly inhibits T h1 cy t ok ine p rod uct ion in m itog en - and an t ig en - s t im ulat ed hum an p e rip he ral blood m onon uclear cell cult ures. Clin E xp I m m unol 99:160- 167, 1995 16) Geit z H , Han dt S, Zw ing enberg er K : T halidom ide
s e le ct ive ly m od ulates the dens ity of ce ll s urf ace m olecules inv olve d in the adhes ion cas cad e. I m m u-
n op harm acolog y 31:213- 221, 1996
17) Bar log ie B, Desik an R, E ddlem on P , Spencer T , Zeldis J , Mun shi N , Badr os A , Zang ari M , An ais sie E , Epstein J , Sh aug hnes sy J, Ayer s D, Spoon D, T ricot G : E x tende d s urv ival in advance d an d re - f ractory m ult ip le m y e lom a af te r s ing le - ag ent tha- lidom ide : I dent if icat ion of p rog nos t ic factors in a p has e 2 s t udy of 169 p at ients . B lood 98:492 - 494, 2 001
18) P ini M, Bar aldi A , Pietr as ant a D, Allione B, Depaoli L, S alvi F : L ow dos e thalidom ide in the treatm ent of ref ractory m y e lom a. H em at olog ica 85 : 1111 - 1112, 2 000
19) Lar kin M : L ow dos e thalid om ide s eem s t o be eff e ct ive in m ult ip le m y e lom a. L ance t 354 :925, 1999 20) Y akoub - Ag h a I, Att al M , Dum ontet C, Delann oy V ,
Berth ou C, Lam y T , Durv aus V , M oncon duit M , Dug uet C, Duh amel A , F acon T : T halidom ide in p at ients w ith advanced m ult ip le m y e lom a: A s t udy of 83 p at ients - rep ort of the in te rg roup e f rancop hone d u m y e lom a (I F M ) . H em atolog y J 3:185 - 192, 2 002 21) Gr ov er J K, Uppal G, Rain a V : T he adv ers e eff e ct of
thalidom ide in re lap s e d and ref ractory p at ients of m ult ip le m y e lom a. A nn Oncol 13:1636- 1640, 2002 22) Rajkum ar SV, H aym an S, Ger tz MA , Dispenzier i A ,
Lacy M Q, Gr eipp PR, Geyer S , It ur ria N, F on seca R, Lu st JA , Kyle RA , W itzig T E : Com binat ion the rap y w ith thalidom ide p lus dexam ethas one f or newly d iag nos e d m y e lom a. J Clin On col 2 0:4319- 4323, 2 002
23) Garcia - S anz R, Gonzalez - F r aile MI, Sier r a M , Lopez C, Gonzalez M , S an Mig uel JF : T he com - binat ion of thalidom ide, cy clop hosp ham ide and de - xam e thas one ( T ha CyD ex ) is f eas ible an d can be an op t ion f or re lap s ed/ ref ract ory m ult ip le m y e lom a H em atol J 3:43 - 48, 2 002