i
스 Journal of the Pharmaceutical Society of Korea 13. 80^83(1969)
a-Pieolin 평에 ^ 한 Aspirin 의 별 55웠®
a
(Received June 30, 1969)
Nam Ho Paik, Manki Park: Spectrophotometric Acetylsalicylic Acid with Copper-a-picolin
T etrachloromethane
Determination of Complex in
Acetylsalicylic acid gives a water-insoluble violet complex whh a-Picolin- C u (II) reagent.
The Complex is eztractable well with a mixture of or-Picolin-tetrachlo- romethane solution, the Complex salt dissoWed in the mixed aolution shows a maximum absorption at 620 m/t.
It has a melting point at 171°C-173®C and molar ratio of Acetylsalicylic acid: C u (II) : a-Picolin was estimated as 2:1:2 by continaous variation method and chelate titration method.
Aspirin 외 에관한 로서는 Acid-base Volumetric titration^* 율 버롯 Colorimetry2^
U .V Spectrophotometry지 I.R.시 Fluorometry®* 및 N.M.R®* 과 APC 에서융 호I S 으로도 P.P.
(7* T.L.C®> G.L.C®> Column chromatography^®* 롬 거의 전반에 걸쳐있으나 aspirin
과금속착염울축 ® ? 에 » 한융환S 은없었으므로 * 홈등온 5^ 슴감기약 용 ® 외 효s a 울 aspirin 이 a-picolin월CU) 에^하여 황롭용*^H찮f t 월IS를 람율 5 8 S하고(m.p 171~173°C) 이||텔가 a-picolin CCU 흔합용매에잘 되어품혈fe
울나타내므로이 ^§8^충율 •렸하고 jfcft 620m//에서 몇를 SJ® 하여 aspirin
외효* 나* 을하여좋은 ii콤*를 얻었으며 aspirin 외 a-picolin 떨( I ) 활월외 fe켰율 ig8S행f t
&으로 Jg0•편한바 aspirin : Cu( I ) : a-picolin 의 J t는 2 : 1 : 2 임울알았으므로이에보고함.
SI >gS
Cu.CCHjCOCOa • H jO: G .R. E.M ERCK. IQ-^M a-Picolin: 3$봤 W AKO 33% in CCl4(v/v% ) CCI4:n W i KANTO
College of Pharmacy, Seoul National University
- 8 0 - . «
September 1969 m m # 81
Acetyl Salicylic acid: U.S.P.
Na2S04: Anhydrous Anal. R. Mallinckrodt.
Spectrophotometer: Beckman D.U.
pH meter: Model G Beckman
n s i %
1. 정량* fF
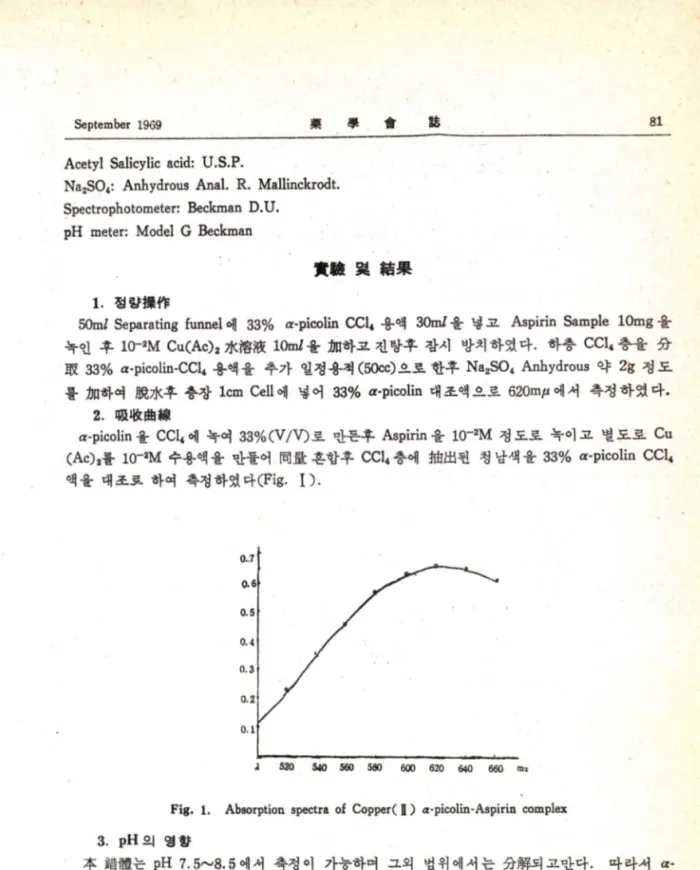
50m/ Separating funnel 에 33% or-picolin CCU 용액 30m/ 을넣고 Aspirin Sample lOmg 울 녹인후 10-2M Cu(Ac)2 lOmZ 율 하고진탕후잠시방처하였다. 하층 CCU 층울 융
M 33% a-picoiin-CCU 용액울추가일정용적 C50cc) 으로한후 NagSO* Anhydrous 약 2g 정도 를:6n하여 aft7jc후충장 Icm Cell 에넣어 33% a-picolin 대조액으로 620m^ 에서측정하였다.
2. 효
a-picolin 율e c u 에녹여 33%CV/V)로만든후 Aspirin 을 lC r2M 정도로녹이고별도로 Cu (Ac)a를 1(T2M 수용액울만돌어 f M :흔합후 CCU 층에 된청남색울 33% a-picolin CCU
액을대조로하여촉정하였다(Fig. I ) .
Fig. 1. Absorption spectra of Copper( H ) a-picolin-Aspirin complex
3. pH 의영향
후황ffi는 pH 7.5~ 8.5 에서측정이가농하며그외범위에서는 되고만다. 따라서 «-
picolin 33% CCU 용액에서 가장좋은걸과를얻었다.
4. 의영향
떨텔의 이실온으로부터 60°C정도까지는 아무영향이 없으나 70°C JiL느으로 하
면약 8(TC 정도에서 같색외딴물질로변화되어 버림.
5. 엘« 의S J t t t
82 Q , : a-Picolin 형에 ^ 한A sp irin외 f흡^ 호1 :쪼
설온에서 33% a-picolinCCl, 용액다HI 후활헬는 과정이끝난후부터는램풍면화를받 지않는다(Fig. 2).
6. IS H의
Aspirin 율 33% a-picolin CCI4 용액 에 10"효M 로 녹어 J i Cu(Ac)2 도 10*■호M 로 33% a-picolin CCI4 용액에녹언후연속적언농도를변화시켜본결과그림과같이 2 : 1 임울알았다(Fig. 3).
0.3
sUndtns I (ram.)120
i
F ig. 2. Effect of standing time F ig. 3. CCu*3/C(CuO + (A S A )J
또한 i g i i를 te S으로얻은후 ether로 세척후 건조 감압 d e sicc ato r에서건조후 fiA c
산 성 에버 수용애적정으로 <r-picolin 의 분석 (0 .1N H C IO 0 및 Cu+"*■불 EDTA 로 측정하여
(indicator PAN Purple-green) Aspirin : Cu : a-picoIin=2 ; 2 임울알았다.
Cu a*picalin
Aspirin
found 11 32 56
Calc 10.44%
30.63%
58-62%
devt 0.56 1.37 -1.62
Remark
«
7. m m m
a-picolin 울 CCl* 에 33%Cv/v) 로녹인액 100 cC 에 Aspirin 을 desiccator cp에서건초풍울 평 량 lOOmg 울 녹이고 0.5, 1, 1.5 2m/등으로
렸하고 33% a-picolin CC1* 액을 채워서 lOmi
로한후 1 0 - ^ Cu(Ac)2 을 5m/ 썩 ;&n태 서 15m/ 로하고추출후 NajSOi Anhydrous 약
Ig 썩 Jn하여 IftTK후 620m/i 에서측정함 (Fig. 4)
m
Aspirin 율가수분해시켜 Fe 과반용시켜비색
범으로하는것보다는간단하개할수었는반 F ig . 4. Calibration CurvQ
September 1969 m m tt 83
면에 a-picolin 의 악취로 인하여 있다고 믿어진다.
조작이 즐겁지 못하나 단일 물질인 경우는 간단히 할 수
References
1. Yaskina. IXS, and Hien N .B. Apteckn Del<r, 13 (1) 69(1964); C A 61, 9361a (1964).
2. Pankratz. R .E . and F.J. Bandelin. J , An, Pharm, Assoc. Sci. Ed. 41, 167 (1952). (T h e Aspirin is hydrolyzed to Salicylic acid and reacted with Ferric titrate to give a violet color 525m/i).
3. Tinker. R.B. and A J , McBay. J . An, Pharm, Assoc. Sci. Ed. 43, 315 (1954).
4. Parke T .V .A .M . Ribley E .E Kennedy and W .W Hiltz. Anal. Chem. 23, 953 (1951).
5. Shibazaki T . Japan, Analyst, 12, (4) 385 (1963).
6. Donald P. Hollis AnaL Chem. 35, 1682 (1963).
7. Higuchi. T .K .P . patel. E.R. Bouow.혁and J, Landsman J , An. Pharm. Assoc, sci Ed. 41 293 (1952), 8. Bailey R .W . Anal Chm . 36(10) m i (1964).
9. NikeUy. J,G. Anal Chem 36. 2248 (1964).
10. J. Levine; J . An. Pharm, Assoc. 46. 687 (1957). R .F , Heuerman and J. Levine; ibid. 47, 276 (1958).
11. Skelley N .E. and Crummett. W .B. And Chem., 35,1680 C1963),