~~~2J~N:L!fQ.fQJT\I : "ll32:r:! "1132, 125-131, 2000.
Expression of Folate Receptor, Ovarian Cancer Protein in Cancer Cell Line
Kim, Chong Ho., Park, Young Soon*
De]Xlrtment of Clinicnl Pathology, Wonkwang Health Science College, Iksan Korea Division of Biologicnl Science, Wonkwang University, Iksan, Korea*
The human folate receptor (hFR) is well known as a specific protein binding folic acid and a plasma membrane protein that is anchored to the membrane via a glycosylphopha- tidylinositol (GPI) tail. We investigated the function and expression of hFR by transfecting hFR eDNA constructed from Nasopharyngeal epidermoid carcinoma cell line(KB cell) into HeLa2, Human cervix epitheloid carcimoma cell line, and wild HeLa2 cell line. We isolated and characterized stable clones that expressed detectable levels of hFR protein from the HeLa2 cells transfected with hFR eDNA into wild HeLa2 cells. We discovered that the wild HeLa2 cell line does not express levels of hFR protein as high as KB cell line, but a high level of hFR is expressed in the HeLa2 cells transfected with hFR eDNA. No differences in total folic acid binding or cell surface folic acid binding activity were found between HeLa2 cells transfected with hFR eDNA or KB cell line, but we found that differences of the activities of hFR protein were found between wild HeLa2 cells and HeLa2 transfected with hFR eDNA. We conclude that the function and expression of hFR in HeLa2 cells transfected with hFR eDNA is going well, but low levels of hFR protein are detectable in wild HeLa2 cells.
Key Words: HeLa2, hFR, KB
I .
Introduction
The folates are important sources for DNA synthesis and essential vitamins for cell growth. It has been reported that the uptake of folates through cell membrane into cytoplasm involves membrane protein receptors of a membrane carrier
protein and a high affinity folate-binding protein (Antony, 1991; antony et al., 1982; Antony et al., 1981; Elwood et al., 1986; Waxam et al., 1977).
The membrane-bound folate receptor is anchored to the plasma membrane by a fatty acid linkage, which has been identified in some cells as a GPI tail with a molecular mass of about 40
KD(Luhrs, 1989). Use of immunological method has been identified the hFR in some normal tissues(McHugh et al., 1979; selhub et al., 1979;
Spectra, 1977), KB cells(Elwood, 1989), body fluids(Antony et al., 1982; Waxman, 1975; Waxman et al., 1973), and in some cancer cells(Sheppard et al., 1984; Tomassetti et al., 1993).
eDNA for hFR have been cloned from human KB cells(Elwood, 1989; Sadasivan et al., 1989), human colon carcinoma cells(Lacey et al., 1989), human ovarian carcinoma cells(Coney et al., 1991), human placenta cells(Page et al., 1990;
Ratnam et al., 1989), and L1210 murine leukemia cells(Brigle et al., 1991). However, all the cDNAs derived from human cancer cells have the same coding sequences, although their 5 '-untranslated regions vary from one to another (Coney et al., 1991).
In order to compare the function and expression of hFR in wild HeLa2 cells which normally do not express high levels of hFR, we transfected the recombinant KB eDNA of hFR into wild HeLa2 cells. Here, we demonstrated the fuction, cell surface expression, and folic acid binding activity of the transfected HeLa2 cells and wild HeLa2 cells.
IT. Materials and Methods Materials
( 5
I](histamine derivative of folic acid (#NEX114) was purchased from New England Nuclear (NEN).
3H-folic acid (#MT783) was purchased from Moravek Biochemicals (Brea, CA). Folic acid (#F7876) was purchased from Sigma. Scintillation fluid (#3a70D) was perchased from Research Products International (Mt. Prospect, IL). Acrylamide
and low molecular weight markers were purchased from Bio-Rad (Richmond, CA). Agarose was obtained from Bethesda Research Laboratories (Gaithersburg, MD). All restriction enzymes, ezymes, and reagents for radiolabeling eDNA probes were purchased from Promega (Madison, WI). [ a -32P]- labeled dCTP (#PB10205) and dGTP(#PB10206) (each with a specific activity of >800 Ci/mmol) were obtained from Amersham Corporation (Arlington Heights, IL). Nytran membranes were obtained from Schleicher and & Schuell (Keene, NH). All of other chemicals were of reagent grade or higher, and were purchased from Sigma Chemical Co. The reagents for cells and tissue culture ,were purchased from Gifco Laboratory.
The estabilished and chracterized well HeLa2 cells were purchased from CDC in USA.
Plasmid construction of hFR
The pRc/CMV vector (Invitrogen, San Diego, CA) contains a cytomegalovirus(CMV) promoter and a neomycin resistance gene. The eDNA encoding hFR was isolated from a KB cell library.
The insert-purified hFR eDNA and pRc/CMV expression vector were blunt -ended using the Klenow large fragment (#M220, Promega). The Hind III-linearized pRc/CMV expression vector was ligated with blunt-ended eDNA insert using T4 DNA ligase (#M1802, Promega). Competent E.coli (JM109, #L2001, promega) were transformed with the ligation mixture. Recombinant plasmids were isolated by the Qiagen maxiprep kit (#312162). The sense orientation of the folate receptor eDNA in recombinan pRc/CMV vector was verified by restriction enzyme digests and by DNA sequencing using 35S-dATP (#SJ130, Amersham)
Tissue culture
For our experiments, HeLa2 cells were cultured in Eagle's MEM with non-essential amono acids, 90% Earle's BSS, 10% fetal bovine serum (FBS) and penicillinjstreptomycin/fungizone (PSF). Cells were grown in monolayers in 10 em tissue culture dishes containing 10 ml media at 37
t
under a humidified atmosphere of 5% C02, and were subcultured weekly. To harvest the cells, the medium was decanted and 1 ml of 2.5% trypsin solution was added to the dishes. The cells were incubated at room temperature for 2 min, the excess solution was decanted, and the cells were immediately resuspended in media.Western blot
Each cell lines (2 X 106cells/35mm well) were plated overnight.
Cells were washed twice with 2ml of PBS and scraped into 1 ml PBS and 20 mM EDTA, pH7.4.
The cells were pelleted in a microcentrifuge and solubilized with PBS and 1% TX-100 for 2 hr at room temperture. The samples were spun in a microfuge. The supernatant was assayed for protein and equal amounts of protein (25/lg) were acetone precipitated with ice cold 80% acetone in water overnight at -20 OC. Acetone precipitations were pelleted in a microfufge by spinning for 30min at 4 °C. SDS-PAGE (sodium dodecyl suifate polyacrylamide gel electrophoresis) sample buffer (50/lg of 0.125M Tris, pH6.8, containing 2% SDS, 20% glycerol, 0.08% bromophenol blue and 100 mM DL-dithiothreitol) was added to air dried samples. Samples were vortexed, boiled for 1 Omin, electrophoresed on a 12.5% SDS-PAGE, and transferred to nitrocellulose membrane. Western
blots for hFR were incubated with a 1:200 dilution of mouse antibody to hFR (#Cl3620, Transduction Lab.), incubated with 1: 1000 dilution of goat anti-rabbit IgG(#170-6515, BioRad) conjugated with HRP, and analyzed by the ECL system ( #RPN2106,Amersham).
Folic acid binding assay
The 75% confluent, transfected or not HeLa2 cells and KB cells were washed twice with 3ml of 10mM Na-acetate buffer (pH 4.5) containing 150mM NaCl to dissociate preoccupied folates on the cell surface hFR. The cells were then returned to neutral pH by washing with ice cold PBS (pH 7.4). For assaying cell surface-binding of folic acid, they were incubated in an ice-cold waterbath with 125
1-labeled histamine derivative of folic acid (2 x 104 cpm) in 2 ml of DMEM containing 50 nM cold folic acid and 50 J.Lg/ml BSA, but not FBS. To determine cell surface-specific binding, parallel experiments were performed in the presence of 500-fold excess cold folic acid. After incubation, the cells were washed with the medium, solublized as described above, and counted their radioactivity using a gamma-counter. The same experiments were repeated 5 times, and a variation of the results was less than 10%. In order to determine the total folic acid binding activity, we incubated the cell lysates with 3H-labeled folic acid as described previously(Elwood et al., 1986).
m. Results and Discussion
Expression of hFR
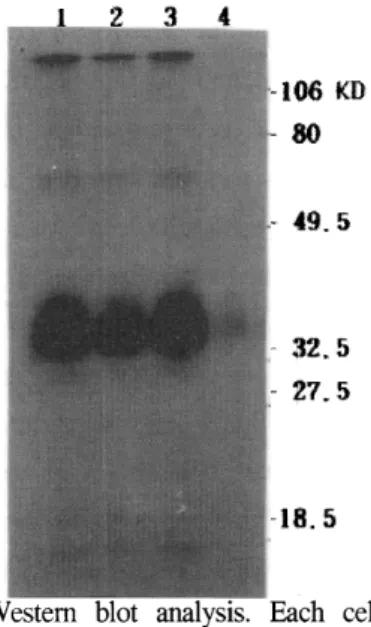
Since HeLa2 cells normally express a low detectable level of hFR mRNA (figure 2, lane 1)
and protein(figure 1, lane 2), we trasfected the cells with pRc/CMV recombinated with human hFR eDNA. To detennine whether the transfectant can express a high level of hFR protein, the cell extracts were prepared and subjected to Western blot analysis using anti-hFR antibody. As shown in Figure 1, the HeLa2 clones transfected with recombinant plasmid (lane 3) overproduced hFR nearly as well as the KB cells (lane 1). On the other hand, much little hFR protein could be detected in the extracts from the wild HeLa2 cells (lane 2). We also did Northern blot to compare the concentration of mRNA in each cell line. The cloned HeLa2 cell also overproduced mRNA nearly as well as KB cell. This data shows that the expression of hFR gene in HeLa2 cell is controled by cis regulation factors on the gene.
Fig. 1.
106 KD 80
49.5
32.5
cell lysate containg 20 f-lg protein were prepared from cloned HeLa2 cell(lane 1), wild HeLa2 cell(lane 2) and KB cell(lane 3), and negative control cell(lane 4) and were subjected to Western blot as described in Materials and Methods.
Folic acid-binding activity
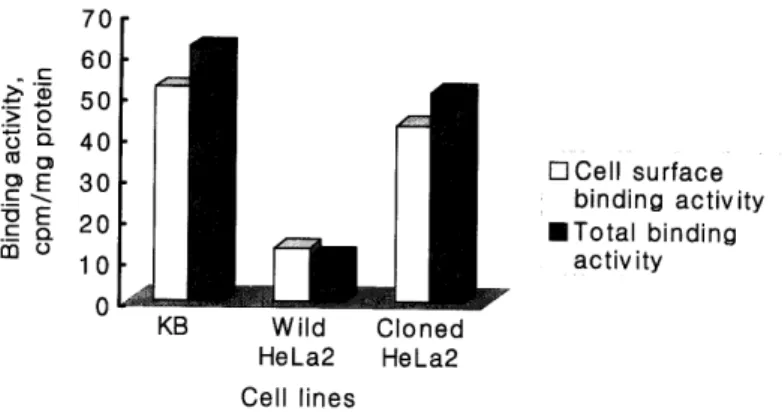
To determine the total folic acid-binding capacityand cell surface binding activity of the wild HeLa2 and HeLa2 transfectant cells, the detergent- solublized extracts were prepared from the cells and subjected to the binding assay using a
1251-labeled histamine derivative of folic acid for cell surface binding activity and 3H-folic acid for total folic acid binding capacity as described under
"Materials and Methods". As shown figure 3, transfected HeLa2 cells and KB cells bind with
1251-labeled folic acid in at least 3-fold higher
amounts on their surface than the wild HeLa2, which produce hFR to be low detectable level.
The figure 2 shows that the cloned HeLa2 cells with hFR eDNA and KB cells producing hFR
1 2 3
28S blt'iR l&'i
Fig. 2. Northern blot analysis. The 10tLg of total RNA isolated from wild HeLa2 cell(lane 1 ), cloned HeLa2 cell (lane 2) and KB cell(lane 3) were hybridized as described in Materials and Methods.
70
-.£ 60
>. Q) 50
~e t3 Q. 40
ctl CJ)
o E 30 uE 20 c - .£ Q.
CD u 1 Q
0 Cell surface binding activity
• Total binding activity
0 KB Wild Cloned
Hela2 Hela2 Cell lines
Fig. 3. The cell surface folic acid binding activity and total binding activity of protein isolated from KB, wild HeLa2 and cloned HeLa2. The cell surface binding activity is calculated by the data multipling 10-2 and the total binding activity is calculated by the data multipling 1
o-
3.were capable of binding to the ligands at least milk., J Bioi Chern 257:10081, 1982
4-fold higher amounts than the total folic acid 3. Antony A.C., Utley C.S., Van Horen K.C., binding capacity of wild HeLa2 cell. Kolhouse J.F. Isolation and characterization of a folate receptor from human placenta., J Bioi Acknowledgment
This work was supported by grants from the Wonkwang University (2000) and Wonkwang Health Science College (2000).
References
1. Antony A.C. Megaroblastic anemia, in Hoffman R., Benz E.J., Shattil S.J., Furie H.J.(eds):
Hematology: Basic principles and practice. NY, Churchill-Livingstone, 392, 1991
2. Antony A.C., Utley C.S., Marcell P.D., Kolhouse J.F. Isolation, characterization and comparision of the solublized particulate and soluble folate binding proteins from human
Chern 256:9684, 1981
4. Brigle, K.E., Westin, E.H., Houghton, M.T., Goldman, I.D. Characterization of two cDNAs encoding folate-binding proteins from L1210 murine leukemia cells. J Bioi Chern 266: 17243, 1991
5. Coney, L.R., Tomassetti, A., Carayannopoulos, L., Frasca, V., Kamen, B.A., Colnaghi, M.I., Zurawski, V.R. Cloning of a tumor-associated antigen: MOV18 and MOV19 antibodies recognize a folate-binding protein. Cancer Res 51:6125, 1991
6. Elwood P.C. Molecular cloning and characterization of human folate-binding protein eDNA from placenta and malignant tissue culture(KB) cells.
J Bioi Chern 264:14393, 1989
7. Elwood P.C., Kane M.A., Portillo R.M., Kolhouse J.F. The isolation, characterization and comparision of the membrane-associated and soluble folatebinding proteins from human KB cells., J Bioi Chem 261:15416, 1986 8. Lacey, S.W., Sanders, J.M., Rothberg,K.G.,
Anderson, R.G., Kamen, B.A. Complemen tary DNA for the folate binding protein correctly predicts anchoring to the membrane by glyco- sylphosphatidylinositol. J Clin Invest 84:715, 1989
9. Luhrs C.A. A human membrane-associated folate binding protein is anchored by a glycosylphosphatidylinositol tail., J Bioi Chem 264:21446, 1989
10. McHugh M., Cheng Y.C. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells., J Bioi Chem 254:11312, 1979 11. Page, S.T., Elwood, P.C. Human folate receptor
"gene family": initial characterization. Blood 76:43, 1990
12. Ratnam, M., Marquardt, H., Duhring, J.L., Freisheim, J.H. Homologous membrane folate binding proteins in human placenta: cloning and sequence of a CDNA. Biochemistry 28:8249, 1989
13. Sadasivan E., and Rothenberg S.P., The complete
amino acid sequence of a human folate binding protein from KB cells determined from the eDNA. J Bioi Chem 264:5806, 1989
14. Selhub J., Gay A.C., Rosenberg I.H. Effect of anions on folate binding by isolated brush border membranes from rat kidney., Biochim Biophys Acta 557:372, 1979
15. Sheppard K., Bradbury D.A., Davies J.M., Ryrie D.R. Cobalamin and folate binding proteins in human tumor tissue., J Clin Pathol 37:1336, 1984
16. Specto R. Identification of folate binding marcromolecule in rabbit choroid plexus., J Bioi Chem 252:3364, 1977
17. Tomassetti A., Coney L.R., Canevari S., Moitti S., Facheris P., Zurawaski V.R., Colnaghi M.I.
Isolation and biochemical characterization of the soluble and membrane forms of folate binding protein expression in the ovarian carcinoma cell line IGROVl., FEBS Lett 317:143, 1993 18. Waxman S. Folate binding proteins., Br J
Haematol 29:23, 1975
19. Waxam S., Schreiber C. Characteristics of folic acid binding protein in folate deficient serum.
Blood 42:291, 1973
20. Waxam S., Schreiber C. Rubinoff M. The significance of folate binding protein in folate metabolism., Adv Nutr Res 1:55, 1977
암세포내에셔 난소암 표지단백절 엽산 수용체 생산
원광보건대학 임상병리과 · 원광대학교 생명과학부*
김종호 · 박영순*
엽산을 특이적으로 결합하여 세포내로 이동하는 human folate receptor(hFR)은 난소
암 marker 단백질로 얄려졌으며 세포의 형질막단백질이다. 우리는 human cervix
epitheloid carcinoma cell line(HeLa2 cell) 에 서 hFR의 발현과 가 능을 연구하기 위 하여 wild HeLa2 cell 에 nasopharyngeal epidermoid carcinoma cell line (KB) 에 서 분 리 한 hFR cDNA를 transfection 시켰다. Transfectants를 분리하여 hFR의 발현 정도를 확인하고 세 포막에 존재하는 hFR와 총 plasma membrane 에 존재하는 hFR의 folic acid 결합능을 분 석하여 wild HeLa2 와 KB 세포뜰과 비교한 결과 cloned HeLa2 의 hFR 발현 정도는 wild
HeLa2 세포 보다 높았고 발현된 hFR 단백질의 folate 결합능도 KB 세포에서 발현되는
뻐R의 기능과 유사하였다.
본 연구는 원광보건대학(2어0년)과 원광대학(2000) 연구비 지원에 의하여 연구되었음.