91 책임저자:정주호, 130-701, 서울특별시 동대문구 회기동 1
경희대학교 고황의학연구소 Tel: 02-961-0298, Fax: 02-968-0560 E-mail: jhchung@khu.ac.kr
접수일:2008년 6월 3일, 게재승인일:2008년 6월 18일
Correspondence to:Joo-Ho Chung
Kohwang Medical Research Institute, School of Medicine, Kyung Hee University, 1, Hoegi-dong, Dongdaemun-gu, Seoul 130-701, Korea Tel: +82-2-961-0298, Fax: +82-2-968-0560
E-mail: jhchung@khu.ac.kr
Genetic Difference of Angiotensin Converting Enzyme (I/D) Polymorphism in Korean Lung Cancer
and Schizophrenia Patients
Jee Yeong Yun1, Yong Ho Kim2, Bum Shik Kim3, Keon Sik Kim4 and Joo-Ho Chung1
1Kohwang Medical Research Institute, 2Department of Surgery, 3Department of Thoracic and Cardiovascular Surgery,
4Department of Anesthesiology and Pain Medicine, School of Medicine, Kyung Hee University, Seoul 130-701, Korea
The reduced incidence of cancer observed in schizophrenia patients is one of interesting medical puzzles in research field of cancer development over decades. It has been reported that genetic factor towards schizophrenia is associated with reduced vulnerability to lung cancer. Genetic difference between lung cancer and schizophrenia might provide us with possible clues to understand molecular mechanisms of the pathophysiology of cancer or schizophrenia. We tested the genetic association between lung cancer and schizophrenia by analyzing well-known in/del (I/D) polymorphic site in the angiotensin converting enzyme (ACE) gene. ACE I/D polymorphism was studied in 94 lung cancer, 89 schizophrenia, and 1,267 healthy controls in Korean population. Genotype and allele frequencies of ACE I/D polymorphism showed significant differences between lung cancer and schizophrenia [genotype, p=0.0252; allele, p=0.0325; odds ratio (OR)=0.6341; 95% confidence interval (CI)=0.4173−0.9636]. Compared with controls, schizophrenia showed no significant differences in genotype and allele frequencies (genotype, p=0.2382; allele, p=0.5882;
OR=1.0877; 95% CI=0.8022−1.4747). Lung cancer showed significant differences in genotype and allele frequencies (genotype, p=0.0017; allele, p=0.0005; OR=1.7153; 95% CI=1.2612−2.3328). The results suggest that ACE I/D polymorphism may be associated with the development of lung cancer, and may, in part, be explained a medical puzzle about low incidence of lung cancer in schizophrenia patients.
(Cancer Prev Res 13, 91-95, 2008)
Key Words: Association, Angiotensin converting enzyme, Lung cancer, Polymorphism, Schizophrenia
INTRODUCTION
Reduced cancer risk in schizophrenia patients has been one of interesting issues in epidemiological perspectives over decade.1∼3) Several studies have been reported the lower incidence of cancers, in particular, lung cancer in patients with schizophrenia compared with normal population.4,5) The mechanism underlying this lower risk for cancer in schizo- phrenia has not been fully elucidated. Recently, genetic back- ground which regulate the neuropsychiatric processes are suggested as a possible cause of this phenomenon.6,7) However,
it is little known whether patents with schizophrenia have the genetic characteristics of tumor resistance. Also, it is little known that the genetic make-up of schizophrenia and lung cancer is different from normal population.
Angiotensin converting enzyme (ACE), or kininase II, is a dipeptidyl carboxypeptidase that plays an important role in blood pressure regulation by hydrolyzing angiotensin I into angiotensin II. Although ACE has been studied primarily in the context of its role in blood pressure regulation, this widely distributed enzyme has many other physiologic functions. The ACE gene is located on 17q23. The ACE has exons (25), transcript length (4,195 bp), and protein length (1,306 re-
sidues). Many studies have associated the presence or absence of a 287 bp Alu repeat element in this gene with the levels of circulating enzyme or cardiovascular pathophysiology.8,9) Two most abundant alternatively spliced variants of this gene encode two isozymes - the somatic and testicular forms are equally active. Multiple additional alternatively spliced variants have been identified but their full length natures have not been determined. Tiret et al.10) demonstrated that the individual variability of plasma ACE was associated with an insertion (I)/deletion (D) polymorphism involving about 250 bp situated in intron 16 of the ACE gene, so-called ACE/ID polymorphism.
Rigat et al.11) found that the ACE/ID polymorphism was strongly associated with the level of circulating enzyme. The mean plasma ACE level of DD subjects was about twice that of II subjects, with ID subjects having intermediate levels.
Rigat et al.12) determined that the ACE insertion corresponds to an Alu repetitive sequence and is 287 bp long.
In this study, we analyzed I/D polymorphism of the ACE gene in the context of comparison of genetic make-up between lung cancer and schizophrenia. Genotype distributions of ACE/ID polymorphism between patients with schizophrenia/
lung cancer and healthy controls were also analyzed.
MATERIALS AND METHODS 1. Subjects
A total 94 patients who diagnosed for lung cancer were included in this study. Eighty nine patients who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia were included. For comparison to normal population, 1267 normal controls were also included. Subjects were recruited at Kyung Hee University Medical Center, Keimyung University Dongsan Medical Center, and Korea Cancer Center Hospital. However, patients with hypertension, diabetes, hyperlipidemia, stoke, and cardiac diseases were excluded. Written informed consent was obtained from all subjects. This study was approved by the Ethics Review Committee of the Medical Research Institute, Kyung Hee University Medical Center, Seoul, Korea. The study was in accordance with the Declaration of Helsinki.
2. Genotyping
Blood samples from each subject were obtained in EDTA tubes and kept at −70oC. Genomic DNA was extracted from
1 ml whole blood using a DNA Isolation Kit for Mammalian Blood (Boehringer Mannheim, USA) and it was kept at −20oC.
Polymerase chain reaction (PCR) was performed in 30μl reaction volume containing 10 mM Tris, pH 8.3, 50 mM KCl, 2.5 mM each dNTP, 0.5 U Taq polymerase (Biotool, Korea), optimal amount of MgCl2 and 0.1μg genomic DNA, 10 pmole of primer pairs. The amplification was then performed in thermo-cycler, a Perkin Elmer GeneAmp PCR system 2700 (Roche Diagnostics corporation, USA). The sense oligonu- cleotide primer was 5'-CTGGAGACCACTCCCATCCTTTCT-3', and the antisense primer was 5'-GATGTGGCCATCACATT- CGTCAGAT-3'. PCR conditions: 94oC for 30 s; 60oC for 30 s; 72oC for 1 min; and 72oC for 7 min to terminate the reac- tion. PCR products were analyzed with 1.5% agarose gel electrophoresis and ethidium bromide staining in order to identify three patterns: I/I (a 479 bp fragment), D/D (a 191 bp fragment) and I/D (both 479 and 191 bp fragments) (NCBI: AF118569; repeat region 14094 14381).
3. Statistical analysis
The distribution of the genotypes among schizophrenia, lung cancer, and the controls was estimated using the χ2 test (3×2 contingency table). To compare the allelic frequencies of the two groups, a 2×2 contingency (χ2) test was used. Odds ratio (OR) and 95% confidence interval (CI) were used to quantify any association between ACE polymorphism and the groups.
The SPSS statistical package (Version 11.0) was used for statistical analysis. p values less than 0.05 were considered as statistically significant.
RESULTS
1. The genotype and allele frequencies of ACE gene between lung cancer and controls
The genotypic distributions and allelic frequencies of I/D polymorphism of ACE gene in controls and lung cancer are shown in Table 1. The frequencies of homozygosity for I/I and D/D were 26.99% and 25.57% in the control group, 43.62%
and 15.96% in the lung cancer group. The frequency of heterozygosity for I/D was 47.43% in the control group, 40.43% in the lung cancer group, respectively. The allelic frequencies of I and D were 50.71% and 49.29% in controls, 63.83% and 36.17% in lung cancer, respectively. There was statistically significant difference in genotypic distribution and
Table 2. Angiotensin converting enzyme (ACE): genotype and allele frequencies in Korean control individuals and patients with schizophrenia
Genotype distribution [n (%)] Allele frequency [n (%)]
I/I I/D D/D I D
Schizophrenia (n=89) 22 (24.72) 50 (56.18) 17 (19.10) 94 (52.81) 84 (47.19)
Controls (n=1267) 342 (26.99) 601 (47.43) 324 (25.57) 1,285 (50.71) 1,249 (49.29)
χ2 value 2.8695 0.2931
df 2 1
p value 0.2382 0.5882
OR 1.0877
(95% CI) (0.8022−1.4747)
n: number, df: degree of freedom, OR: odds ratio, CI: confidence interval, I: insertion, D: deletion.
Table 1. Angiotensin converting enzyme (ACE): genotype and allele frequencies in Korean control individuals and patients with lung cancer
Genotype distribution [n (%)] Allele frequency [n (%)]
I/I I/D D/D I D
Lung cancer (n=94) 41 (43.62) 38 (40.43) 15 (15.96) 120 (63.83) 68 (36.17)
Controls (n=1267) 342 (26.99) 601 (47.43) 324 (25.57) 1,285 (50.71) 1,249 (49.29)
χ2 value 12.7572 12.0621
df 2 1
p value 0.0017 0.0005
OR 1.7153
(95% CI) (1.2612−2.3328)
n: number, df: degree of freedom, OR: odds ratio, CI: confidence interval, I: insertion, D: deletion.
allelic frequencies between the control and the lung cancer groups [genotype, p=0.0017; allele, p=0.0005; OR (95% CI), 1.7153 (1.2612−2.3328)] (Table 1).
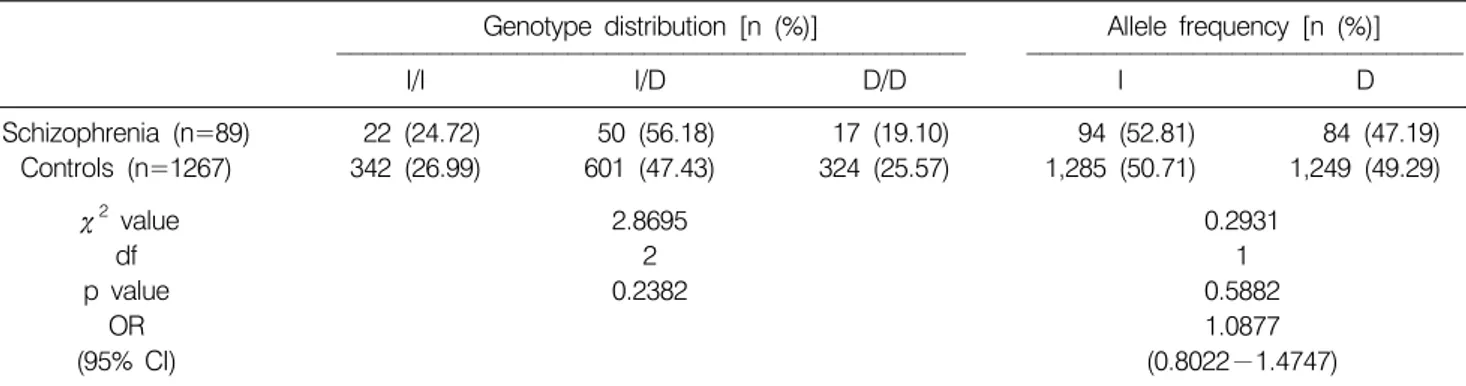
2. The genotype and allele frequencies of ACE gene between schizophrenia and controls
The genotypic distributions and allelic frequencies of I/D polymorphism of ACE gene in controls and schizophrenia are shown in Table 2. The frequencies of homozygosity for I/I and D/D were 26.99% and 25.57% in the control group, 24.72%
and 19.10% in the schizophrenia group. The frequency of heterozygosity for I/D was 47.43% in the control group, 56.18% in the schizophrenia group, respectively. The allelic frequencies of I and D were 50.71% and 49.29% in the control group, 52.81% and 47.19% in the schizophrenia group, respectively. There was no statistically significant difference in genotypic distribution and allelic frequencies between the
control and the schizophrenia groups [genotype, p=0.2382;
allele, p=0.5882; OR (95% CI), 1.0877 (0.8022−1.4747)]
(Table 2).
3. The genotype and allele frequencies of ACE gene between lung cancer and schizophrenia
The genotypic distributions and allelic frequencies of I/D polymorphism of ACE gene in schizophrenia and lung cancer are shown in Table 3. The frequencies of homozygosity for I/I and D/D were 24.72% and 19.10% in the schizophrenia group, 43.62% and 15.96% in the lung cancer group. The frequency of heterozygosity for I/D was 56.18% in the schizophrenia group, 40.43% in the lung cancer group, respectively. The allelic frequencies of I and D were 52.81%
and 47.19% in schizophrenia, 63.83% and 36.17% in lung cancer, respectively. There was statistically significant difference in genotypic distribution and allelic frequencies between the
Table 3. Angiotensin converting enzyme (ACE): genotype and allele frequencies in Korean schizophrenia and lung cancer patients Genotype distribution [n (%)] Allele frequency [n (%)]
I/I I/D D/D I D
Schizophrenia (n=89) 22 (24.72) 50 (56.18) 17 (19.10) 94 (52.81) 84 (47.19)
Lung cancer (n=94) 41 (43.62) 38 (40.43) 15 (15.96) 120 (63.83) 68 (36.17)
χ2 value 7.3604 4.5733
df 2 1
p value 0.0252 0.0325
OR 0.6341
(95% CI) (0.4173−0.9636)
n: number, df: degree of freedom, OR: odds ratio, CI: confidence interval, I: insertion, D: deletion.
lung cancer and the schizophrenia groups [genotype, p=0.0252;
allele, p=0.0325; OR (95% CI), 0.6341 (0.4173−0.9636)]
(Table 3).
DISCUSSION
In this study, we compared the frequency of a functional insertion/deletion (I/D) polymorphism in the 16th intron of the ACE gene between lung cancer and schizophrenia patients.
Plasma ACE levels are associated with the I/D polymorphism of ACE.11,12) Individuals who were homozygous for the D/D allele showed the high activity of ACE and those individuals who had either I/D or I/I ACE genotype showed the low activity of ACE. Illi et al.13) suggested that an interaction with low activity of COMT and high activity of ACE genotype was associated with poor response to conventional neuroleptics.
Cambien et al.14) demonstrated familial resemblance for plasma ACE activity in 87 healthy families. Results of genetic analysis showed that the ACE gene may affect the interindividual plasma ACE activity. Okabe et al.15) also found a family in which an abnormal elevation in plasma ACE levels was transmitted as the autosomal dominant trait.
Yoshida et al.16) showed that the deletion polymorphism in the ACE gene, particularly the homozygote DD, is a risk factor for progression to chronic renal failure in IgA nephropathy.
This deletion polymorphism may be appeared to predict the therapeutic efficacy of ACE inhibition on proteinuria and progressive deterioration of renal function. Williams et al.17) demonstrated that some benefits may be associated with the lower ACE activity of the I allele, suggesting the beneficial effects of ACE inhibitors on myocardial cell survival during ischemia and on the survival of patients with cardiac dys-
function. Winnicki et al.18) reported that the development of cardiovascular complications was associated with a deletion polymorphism of the ACE gene. Keramatipour et al.19) reported that the ACE I allele is associated with increased risk for ruptured intracranial aneurysms. Zhang et al.20) showed the association of the ACE ID polymorphism with the depressor response to mild exercise therapy in patients with mild to moderate essential hypertension. Age-related macular degene- ration-1 (ARMD1) is the most common cause of blindness in the elderly. Hamdi et al.21) demonstrated the association between an Alu polymorphism in the ACE gene with the dry/atrophic form of ARMD1. Suehiro et al.22) reported that the D allele polymorphism was higher expression of the ACE mRNA and may affect the rennin-angiotensin system.
In this study, genotypic distribution and allelic frequencies of ACE I/D polymorphism between controls and schizophrenia patients were no statistically significant difference, respectively [genotype, p=0.2382; allele, p=0.5882; OR (95% CI), 1.0877 (0.8022−1.4747)] (Table 2). However, there was statistically significant difference in genotypic distribution and allelic fre- quencies between controls and lung cancer patients [genotype, p=0.0017; allele, p=0.0005; OR (95% CI), 1.7153 (1.2612−
2.3328)] (Table 1). In Table 3, allelic frequencies of I and D were 52.81% and 47.19% in schizophrenia, 63.83% and 36.17% in lung cancer group, respectively [genotype, p=
0.0252; allele, p=0.0325, OR (95% CI), 0.6341 (0.4173−
0.9636)]. There were statistically significant differences in genotypic distributions and allelic frequencies between lung cancer and schizophrenia patients.
With the results of the present study, we suggest that genotype distribution of ACE I/D polymorphism is different between lung cancer and schizophrenia patients, and may, in
part, be related on a medical puzzle about reduced cancer risk in schizophrenia patients.
ACKNOWLEDGMENTS
This study was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R11-2005-014), and partially supported by the Governance Program of Kyung Hee University.
REFERENCES
1) Ananth J, Burnstein M. Cancer: less common in psychiatric patients? Psychosomatics 18, 44-46, 1977.
2) Coakley DV. No lung cancer in schizophrenics? Br J Psychiatry 134, 649, 1979.
3) Jablensky A, Lawrence D. Schizophrenia and cancer: is there a need to invoke a protective gene? Arch Gen Psychiatry 58, 579-580, 2001.
4) Gulbinat W, Dupont A, Jablensky A, Jensen OM, Marsella A, Nakane Y, Sartorius N. Cancer incidence of schizophrenic patients. Results of record linkage studies in three countries.
Br J Psychiatry Suppl 75-83, 1992.
5) Mortensen PB. The occurrence of cancer in first admitted schizophrenic patients. Schizophr Res 12, 185-194, 1994.
6) Lim YJ, Kim JW, Song JY, Hong MS, Jin SY, Yoon SH, Park HJ, Choe BK, Lee JJ, Yim SV, Hong SI, Baik HH, Ha E, Park YH. Epidermal growth factor gene polymorphism is different between schizophrenia and lung cancer patients in Korean population. Neurosci Lett 374, 157-160, 2005.
7) Park JK, Lee HJ, Kim JW, Park YH, Lee SS, Chang HI, Song JY, Yoon DJ, Bahn GH, Shin YH, Kim YJ, Kim SA, Choe BK, Kim CJ, Chung JH. Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res 67, 71-74, 2004.
8) Conrady AO, Kiselev IO, Usachev NI, Krutikov AN, Ya- kovleva OI, Polunicheva EV, Ovchinnikova OA, Panov AV.
Effect of 24-week treatment with telmisartan on myocardial structure and function: relationship to insertion/deletion poly- morphism of the angiotensin-converting enzyme gene. J Int Med Res 33 Suppl 1, 30A-38A, 2005.
9) Saeed M, Saleheen D, Siddiqui S, Khan A, Butt ZA, Frossard PM. Association of angiotensin converting enzyme gene poly- morphisms with left ventricular hypertrophy. Hypertens Res 28, 345-349, 2005.
10) Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 51, 197-205, 1992.
11) Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P,
Soubrier F. An insertion/deletion polymorphism in the angio- tensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86, 1343-1346, 1990.
12) Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res 20, 1433, 1992.
13) Illi A, Kampman O, Anttila S, Roivas M, Mattila KM, Lehtimaki T, Leinonen E. Interaction between angiotensin- converting enzyme and catechol-O-methyltransferase geno- types in schizophrenics with poor response to conventional neuroleptics. Eur Neuropsychopharmacol 13, 147-151, 2003.
14) Cambien F, Alhenc-Gelas F, Herbeth B, Andre JL, Rakotovao R, Gonzales MF, Allegrini J, Bloch C. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet 43, 774-780, 1988.
15) Okabe T, Fujisawa M, Yotsumoto H, Takaku F, Lanzillo JJ, Fanburg BL. Familial elevation of serum angiotensin convert- ing enzyme. Q J Med 55, 55-61, 1985.
16) Yoshida H, Mitarai T, Kawamura T, Kitajima T, Miyazaki Y, Nagasawa R, Kawaguchi Y, Kubo H, Ichikawa I, Sakai O. Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest 96, 2162- 2169, 1995.
17) Williams AG, Rayson MP, Jubb M, World M, Woods DR, Hayward M, Martin J, Humphries SE, Montgomery HE. The ACE gene and muscle performance. Nature 403, 614, 2000.
18) Winnicki M, Accurso V, Hoffmann M, Pawlowski R, Dorigatti F, Santonastaso M, Longo D, Krupa-Wojciechowska B, Jeunemaitre X, Pessina AC, Somers VK, Palatini P. Phy- sical activity and angiotensin-converting enzyme gene poly- morphism in mild hypertensives. Am J Med Genet A 125, 38-44, 2004.
19) Keramatipour M, McConnell RS, Kirkpatrick P, Tebbs S, Furlong RA, Rubinsztein DC. The ACE I allele is associated with increased risk for ruptured intracranial aneurysms. J Med Genet 37, 498-500, 2000.
20) Zhang B, Sakai T, Miura S, Kiyonaga A, Tanaka H, Shindo M, Saku K. Association of angiotensin-converting- enzyme gene polymorphism with the depressor response to mild exercise therapy in patients with mild to moderate essential hypertension. Clin Genet 62, 328-333, 2002.
21) Hamdi HK, Reznik J, Castellon R, Atilano SR, Ong JM, Udar N, Tavis JH, Aoki AM, Nesburn AB, Boyer DS, Small KW, Brown DJ, Kenney MC. Alu DNA polymorphism in ACE gene is protective for age-related macular degeneration.
Biochem Biophys Res Commun 295, 668-672, 2002.
22) Suehiro T, Morita T, Inoue M, Kumon Y, Ikeda Y, Hashimoto K. Increased amount of the angiotensin-converting enzyme (ACE) mRNA originating from the ACE allele with deletion. Hum Genet 115, 91-96, 2004.
![Table 3. Angiotensin converting enzyme (ACE): genotype and allele frequencies in Korean schizophrenia and lung cancer patients Genotype distribution [n (%)] Allele frequency [n (%)]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/5454789.239968/4.892.81.815.176.371/angiotensin-converting-genotype-frequencies-schizophrenia-genotype-distribution-frequency.webp)