저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Clinical application of potential
biomarkers for renal cell carcinoma
Kwang Hyun Kim
Department of Medicine
The Graduate School, Yonsei University
[UCI]I804:11046-000000516486
[UCI]I804:11046-000000516486
Clinical application of potential
biomarkers for renal cell carcinoma
Kwang Hyun Kim
Department of Medicine
Clinical application of potential
biomarkers for renal cell carcinoma
Directed by Professor Woong Kyu Han
The Doctoral Dissertation
submitted to the Department of Medicine
the Graduate School of Yonsei University
in partial fulfillment of the requirements for the degree
of Doctor of Philosophy
Kwang Hyun Kim
This certifies that the Doctoral
Dissertation of Kwang Hyun Kim is
approved.
---
Thesis Supervisor : Woong Kyu Han
---
Thesis Committee Member#1 : Kyung Sup Kim
---
Thesis Committee Member#2 : Chul Hoon Kim
---
Thesis Committee Member#3: Sang Joon Shin
---
Thesis Committee Member#4: Dong Hyeon Lee
The Graduate School
Yonsei University
ACKNOWLEDGEMENTS
I would like to express my sincerest gratitude to my
supervisor, professor Woong Kyu Han, who supported and
inspired me during my doctoral study. Moreover, he always
instilled enthusiasm for research.
Also, I would like to thank my committee members,
Professor Kyung Sup Kim, Professor Chul Hoon Kim,
Professor Sang Joon Shin and Professor Dong Hyeon Lee for
their generous guidance and constructive criticisms.
The members of HWK Lab has provided the maximal
support to complete my thesis. I really appreciate Kyung Hwa
Choi, Young Eun Yoon, Hyung Ho Lee, Jun Chae Na, Sook
Young Kim and Min Gee Yoon as my colleagues and friends.
My deepest gratitude goes to my family for their love, belief
and full support. I would like to thank my parents, my
parents-in-law, my wife Kyung-ah Jeon and my lovely kids
MJ & YJ.
<TABLE OF CONTENTS>
ABSTRACT ··· 1
I. INTRODUCTION ··· 3
II. MATERIALS AND METHODS ··· 5
1.
Study participants··· 5
2.
Preparation of blood samples··· 5
3.
Assessment of PHD3 and ESM-1 levels··· 6
A.
ELISA for PHD3··· 6
B.
ELISA for ESM-1··· 6
4.
Statistical analysis··· 7
III. RESULTS ··· 8
1.
Patient characteristic··· 8
2.
Diagnostic performance of PHD3 and ESM-1··· 11
A.
PHD3 as diagnostic biomarker··· 11
B.
ESM-1 as diagnostic biomarker··· 12
3.
Serial changes after surgery in RCC patients··· 13
A.
Serial change of PHD3 after surgery··· 13
B
Serial change of ESM-1 after surgery··· 14
IV. DISCUSSION ··· 15
V. CONCLUSION ··· 19
REFERENCES ··· 20
ABSTRACT(IN KOREAN) ··· 27
LIST OF FIGURES
Figure 1 (A) Difference in serum PHD3 level between RCC
patients and controls. The control group included both healthy
kidney donors and patients with benign renal masses. ··· 11
Figure 1 (B) ROC curve for PHD3 comparing RCC patients
(n=56) and controls (n=69). The AUC for PHD3 was 0.668
(95% CI, 0.565-0.769) ··· 11
Figure 2 (A) Difference in serum ESM-1 level between RCC
patients and control subjects. ··· 12
Figure 2 (B) ROC curve for serum ESM-1 comparing RCC
patients (n = 56) and control subjects (n = 56). The AUC for
serum ESM-1 was 0684 (95% CI, 0.628–0.817). ··· 12
Figure 3 Serial changes PHD3 and ESM-1 levels after surgery in
patients with RCC. Both PHD3 and ESM-1 levels declined
after surgery and preoperative and postoperative levels were
compared using paired t-test. ··· 14
LIST OF TABLES
Table 1. Clinical and pathologic characteristics of study cohort
··· 9
Table 2. Serum PHD3 and ESM-1 levels according to
characteristics of RCC ··· 10
Table 2. Multivariate logistic regression analysis to evaluate
1
ABSTRACT
Clinical application of potential biomarkers for renal cell carcinoma
Kwang Hyun Kim
Department of Medicine
The Graduate School, Yonsei University
(Directed by Professor Woong Kyu Han)
Objectives: Most cases of renal cell carcinoma (RCC) are detected incidentally and patients with advanced RCC have unfavorable oncologic and renal function outcomes. Development of a clinically useful RCC biomarker is therefore needed. We aimed to determine the suitability of serum prolyl hydroxylase-3 (PHD3) and endocan (ESM-1) as a diagnostic or monitoring biomarker for RCC.
Methods: Between October 2013 and March 2015, we prospectively recruited participants. The RCC group consisted of 56 patients who underwent radical or partial nephrectomy. The control group included 56 healthy kidney donors and 13 patients with benign renal masses. Blood was sampled prior to surgery, and at 1 and 3 months postoperatively in RCC patients. Serum PHD3 and ESM-1 levels were measured via enzyme-linked immunosorbent assay and compared between RCC patients and controls. Preoperative and postoperative serum PHD3 and ESM-1 levels were also compared. Area under the curve (AUC) was
2
determined using receiver operating characteristic analysis.
Results: RCC patients had higher serum PHD3 and ESM-1 levels than controls (PHD3; 0.79±0.17 ng/ml vs. 0.73±0.09 ng/ml, p=0.023) (ESM-1; 0.59±0.07 ng/ml vs. 0.53±0.09 ng/ml, p<0.001). AUCs for PHD3 and ESM-1 were 0.668 and 0.684, respectively. In subgroup analyses of RCC patients with tumor size >2 cm (n=40), the AUCs for PHD3 and ESM-1 were 0.709 and 0.730, respectively. In patients with RCC, both serum PHD3 and ESM-1 significantly decreased after surgery at postoperative 1 month (PHD3; p=0.050, ESM-1;
p=0.047).
Conclusions: Serum PHD3 and ESM-1 could be a novel RCC biomarker that provides acceptable diagnostic performance. Both serum markers might also be useful for monitoring RCC after surgery.
3
Clinical application of potential biomarkers for renal cell carcinoma
Kwang Hyun Kim
Department of Medicine
The Graduate School, Yonsei University
(Directed by Professor Woong Kyu Han)
I. INTRODUCTION
Renal cell carcinoma (RCC) is the most common solid tumor within the kidney. RCC accounts for approximately 90% of the malignancies in kidney and comprises 2-3% of all malignancies, with a median age of 65 years.1 While
patients with RCC are usually asymptomatic during early in its evolution, patients present with signs or symptoms in advanced stage. Prognosis for patients with metastatic RCCs is quite poor, with a 5-year survival rate of less than 10%. Still, one third of patients with RCC are initially diagnosed with locally invasive or metastatic disease.2 Thus, early diagnosis by means of more
sensitive screening techniques, such as the use of biomarkers, is of critical importance. Noninvasive biomarkers would clearly have a profound clinical impact. Although several markers were identified, those were insufficient for clinical application.3
Currently, no clinically relevant biomarker is
available in RCC.
4
Hypoxia-inducible factor (HIF) is a key regulator of pathogenesis in RCC. HIF regulates at least 500–1000 genes involved in angiogenesis, glucose metabolism, stimulation of growth factors, and cell cycle control.4,5 Although the stability of
HIF in most RCC cells is enhanced due to inactivation of the von Hippel-Lindau (VHL) gene, HIF is rapidly degraded in normal cells via proteasomal degradation.6 Prolyl hydroxylase (PHD) plays a role in the HIF
degradation process via oxygen-dependent hydroxylation.7 PHD family
enzymes (PHD1-3) share a conserved C-terminal hydroxylase domain, but each PHD isoform differs in function and tissue-specific expression pattern.8,9 PHD3
reportedly plays a role in several types of cancer.10,11 Specifically, previous
studies revealed that PHD3 is overexpressed in RCC, suggesting that PHD3 could serve as a biomarker for this disease.12-14
Endocan, or endothelial cell-specific molecule-1 (ESM-1), is a soluble 50-kDa dermatan sulfate proteoglycan expressed by the vascular endothelium 15. ESM-1
is overexpressed in obesity, during sepsis, and under inflammatory conditions, as well as in malignant tumors 16, and is associated with cardiovascular disease 17,18. In RCC tumors, which are typically hypervascular, ESM-1 is upregulated
by angiogenic factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor 19. Transcriptional profile of RCC found that
ESM-1 was overexpressed in tissue samples of RCC 14. The results from a study
of a small number of patients by Leroy et al. 20 suggested that serum ESM-1
5
In this study, we prospectively enrolled patients with and without RCC
and measured serum levels of PHD3 and ESM-1 before and after surgery.
We aimed to evaluate their potential for diagnosing and monitoring RCC.
II. MATERIALS AND METHODS 1. Study participants
Between October 2013 and March 2015, we prospectively recruited participants, after obtaining approval from the Institutional Review Board of Severance Hospital (IRB No. 4-2013-0166). Informed consent was obtained from all participants before study enrollment. All study protocol was carried out in accordance with the Declaration of Helsinki Guidelines. The RCC group consisted of 56 patients who underwent radical or partial nephrectomy for RCC during the study period. The control group included 56 healthy kidney donors and 13 patients with benign renal masses. All RCC and benign renal neoplasm diagnoses were pathologically confirmed by surgical resection. Benign renal neoplasms included five angiomyolipomas, four oncocytomas, three adenomas, and one case of pyelonephritis.
2. Preparation of blood samples
Blood was sampled from RCC patients and controls preoperatively on the day of surgery. To investigate serial changes in PHD3 levels, blood was also sampled 1 and 3 months postoperatively in the RCC group. Blood samples were
6
centrifuged for 15 min at 1000 g within 30 min of collection, and the serum was divided into aliquots and stored at –80°C.
3. Assessment of PHD3 and ESM-1 levels A. ELISA for PHD3
For all serum samples, the level of PHD3 was determined using an ELISA. Anti-PHD3 antibody was purchased from AbFrontier (mouse monoclonal 28A1, Seoul, Korea). Serum was diluted 2-fold before analysis. Recombinant PHD3 protein and diluted serum were coated onto the surface of 96-well ELISA plates overnight at 4°C with 0.1 M sodium bicarbonate buffer (pH 9.6). The coated plates were blocked with 5% skim milk in tris-buffered saline for 1 h at 37°C. After washing with TBS, the plates were incubated with primary antibody (diluted 1:500 in blocking solution) for 2 h, then washed and incubated with secondary antibody (diluted 1:3000 in blocking solution) for 1 hour. After a final washing step, 50 μl of tetramethylbenzidine substrate was added, and the reaction was stopped by addition of 1 N H2SO4. Absorbance was determined at
495 nm using a spectrophotometer. All assays were performed in duplicate. B. ELISA for ESM-1
The level of ESM-1 was quantified using commercially available ELISA kit (Lunginnov s.a.s., Lille, France). Serum was diluted 2-fold before analysis. Experiments were performed according to manufacturer’s instruction. Microwell plates were coated with 100 μL of capture antibody (2 μg/mL) and
7
incubated overnight at 4°C. The coated plates were blocked with 5% skim milk in TBS for 1 hour at 37°C. After a washing step with PBS, the plated were incubated with 100 μL of serum specimen for 1 hour, then washed and incubated with 100 μL of secondary antibody (diluted 1:10,000) for 1 hour at room temperature. After washing, 100 μL of streptavidin-horseradish peroxidase (1:10,000) was added and incubated for 30 minutes. After final washing step, 50 μl of tetramethylbenzidine substrate was added and the reaction was stopped with 1 N H2SO4. The absorbance was determined at 450
nm on a spectrophotometer. All assays were performed in duplicate. 4. Statistical analysis
Both PHD3 and ESM-1 levels in the control group were compared according to the sex and age (≤50 vs. >50 years) of the participants. Serum PHD3 and ESM-1 levels were then compared between controls and RCC patients, as well as according to tumor size, pathologic stage, Fuhrman grade, and histology in RCC patients. Receiver operating characteristic (ROC) curves were generated and the areas under the curves (AUCs) were calculated. The optimal cutoff value, which maximizes sensitivity and specificity, was obtained using the Youden index. In addition, we performed subgroup analyses involving RCC patients with tumor sizes of >2 cm or with clear cell histology. Although RCC and control group were matched by age and sex, patients in RCC group were significantly older than those in control group. We performed multivariate logistic regression analyses to evaluate whether level of ESM-1 is associated
8
with presence of RCC, independently of age. Preoperative and postoperative (at 1 and 3 months) levels were compared. The quantitative values were compared using Student’s t tests or one-way ANOVA, whereas qualitative variables were compared using chi-square or Fisher’s exact tests. Statistical analyses were performed using the Statistical Package for Social Science for Windows, version 18.0 (SPSS, Chicago, IL, USA). A P value of <0.05 was considered significant, and all P values were two-sided. The ROC analyses and plotting graphics were performed using R version 3.2.5 (http://www.r-project.org).
III. RESULTS
1. Patient characteristics
The characteristics of the study cohort are summarized in Table 1. The control group consisted of 56 healthy kidney donors and 13 patients with benign renal masses. Patients in the RCC group were significantly older than the controls (P = 0.027). In control group, PHD3 level did not differ according to age (≤50 vs. >50 years, 0.73±0.09 ng/ml vs. 0.73±0.10 ng/ml; p>0.05). However, mean serum ESM-1 level was higher in older (>50 years) participants than in those that were younger (≤50 years) (0.56 ± 0.06 ng/mL vs. 0.49 ± 0.08 ng/mL, P = 0.001). The mean serum levels of PHD3 and ESM-1 did not vary according to gender, body mass index and presence of hypertension. Additionally, no significant differences in PHD3 and ESM-1 levels were observed between healthy kidney donors and patients with benign renal masses.
9
Table 1. Clinical and pathologic characteristics of study cohort.
RCC Control P value Age (year) mean±SD 52.9±12.6 48.5±9.3 0.024 median (IQR) 54 (44-62) 49 (42-57) Gender, n (%) 0.852 male 35 (62.5) 42 (60.9) female 21 (37.5) 27 (39.1) Hypertension, n (%) 22 (39.3) 7 (10.1) <0.001 Diabetes, n (%) 12 (21.4) 1 (1.4) <0.001
Body mass index (kg/m2)
mean±SD 23.8±3.9 23.5±2.5 0.585 median (IQR) 23.7 (20.8-25.6) 23.9 (21.5-25.5) Size (cm) - mean±SD 3.64±3.36 - median (IQR) 2.7 (2-3.8) - Stage, n (%) - T1a 43 (76.8) - T1b 6 (10.7) - T2a 2 (3.6) - T2b 2 (3.6) - T3a 3 (5.4) - Furmann grade, n (%) - I-II 36 (64.3) - III-IV 20 (35.7) - Cell type, n (%) - clear cell 44 (78.6) - non-clear 12 (21.4) -
RCC=renal cell cancer, SD=standard deviation, IQR=interquartile range
10
clear cell histology RCC. A nonsignificant trend was observed in terms of increasing PHD3 level in patients with larger size, higher grade, T2-stage tumors, and clear-cell histology. The mean serum ESM-1 levels were not different between patients with clear cell RCC and non-clear cell RCC. In patients with clear cell RCC, the serum ESM-1 levels were significantly higher in patients with large-size tumors and higher stages. However, there were no statistically significant differences among patients in the entire RCC group (Table 2).
Table 2. Serum PHD3 and ESM-1 levels according to characteristics of RCC
PHD3 P value ESM-1 P value
ESM-1 (clear cell RCC) P value Tumor size (cm) 0.161 0.095 0.006 ≤2 (n=16) 0.75±0.21 0.56±0.08 0.54±0.08 >2, ≤4 (n=27) 0.78±0.17 0.59±0.06 0.59±0.06 >4 (n=13) 0.87±0.06 0.61±0.05 0.63±0.02 Stage 0.15 0.625 0.024 T1 (n=49) 0.78±0.17 0.59±0.07 0.58±0.07 >T2 (n=7) 0.88±0.05 0.59±0.07 0.63±0.02 Furmann grade 0.086 0.836 0.937 I-II (n=36) 0.76±0.16 0.59±0.06 0.59±0.06 III-IV (n=20) 0.84±0.17 0.58±0.08 0.59±0.07 Cell type 0.535 0.904 clear cell (n=44) 0.80±0.18 0.59±0.06 non-clear (n=12) 0.76±0.12 0.59±0.08
11
2. Diagnostic performance of PHD3 and ESM-1 A. PHD3 as diagnostic biomarker
RCC patients had higher serum PHD3 levels than controls (0.79±0.17 ng/ml vs. 0.73±0.09 ng/ml; p=0.023) (Figure 1A.). In multivariate linear regression analysis, presence of RCC significantly affected serum level of PHD3, regardless of age (P = 0.020). Analyses of ROC curves for all cases (n=56) versus all controls (n=69) revealed an AUC for PHD3 of 0.668 (95% CI, 0.565-0.769) (Figure 1B.). Using a cutoff value of 0.761 ng/ml as calculated according to the Youden index, the sensitivity, specificity, positive predictive value, and negative predictive value were 66.1, 68.1, 28.8, and 37.3%, respectively. ROC curve analyses were also performed for subgroups of RCC cases. The PHD3 AUCs for patients with tumor size >2 cm (n=40) and patients with clear cell histology (n=44) were 0.709 and 0.688, respectively.
Figure 1. (A) Difference in serum PHD3 level between RCC patients and controls. The control group included both healthy kidney donors and patients
12
with benign renal masses. (B) ROC curve for PHD3 comparing RCC patients (n=56) and controls (n=69). The AUC for PHD3 was 0.668 (95% CI, 0.565-0.769).
B. ESM-1 as diagnostic biomarker
Patients with RCC had higher serum ESM-1 levels than control group participants (0.59 ± 0.07 mg/mL vs. 0.53 ± 0.09 ng/mL, P < 0.001) (Figure 2A). The AUC for serum ESM-1 level was 0.684 (95% CI: 0.628–0.817) (Figure 2B). With a cutoff value of 0.568 ng/mL (calculated by the Youden index), the sensitivity, specificity, positive-predictive values, and negative-predictive values were 67.9%, 73.2%, 30.5%, and 28.3%, respectively.
Figure 2. (A) Difference in serum ESM-1 level between RCC patients and control subjects. (B) ROC curve for serum ESM-1 comparing RCC patients (n = 56) and control subjects (n = 56). The AUC for serum ESM-1 was 0684 (95% CI, 0.628–0.817).
13
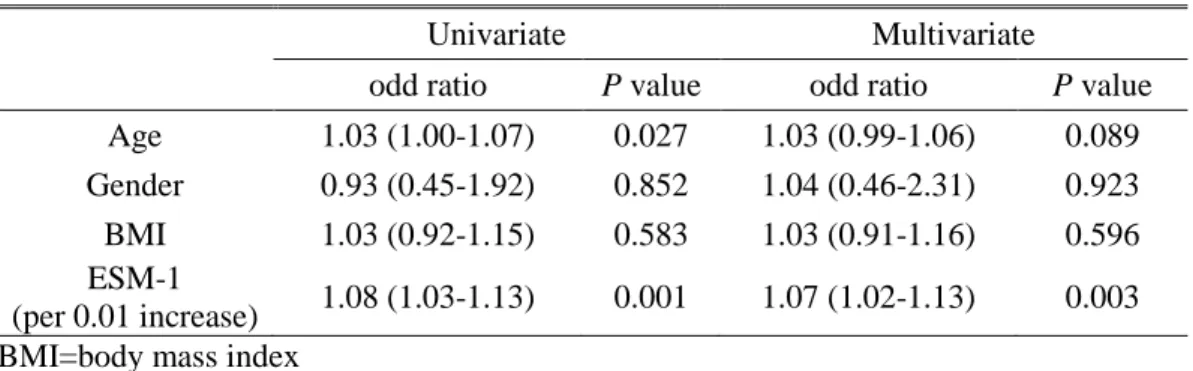
(n = 40) and with clear cell histology (n = 44), AUCs for ESM-1 levels were 0.771 and 0.721, respectively. Because of the older age of patients in the RCC group and the higher levels of serum ESM-1 in older participants, we performed a multivariate logistic regression analysis. After controlling for effects of age, we found that ESM-1 is independently associated with the presence of RCC (P =0.003) (Table 3).
Table 3. Multivariate logistic regression analysis to evaluate whether the ESM-1 is independently associated with RCC.
Univariate Multivariate
odd ratio P value odd ratio P value
Age 1.03 (1.00-1.07) 0.027 1.03 (0.99-1.06) 0.089
Gender 0.93 (0.45-1.92) 0.852 1.04 (0.46-2.31) 0.923
BMI 1.03 (0.92-1.15) 0.583 1.03 (0.91-1.16) 0.596
ESM-1
(per 0.01 increase) 1.08 (1.03-1.13) 0.001 1.07 (1.02-1.13) 0.003 BMI=body mass index
When we divided the entire cohort according to age, the AUCs for ESM-1 were 0.813 in the younger group (age ≤50 years) and 0.637 in the older group (age >50 years).
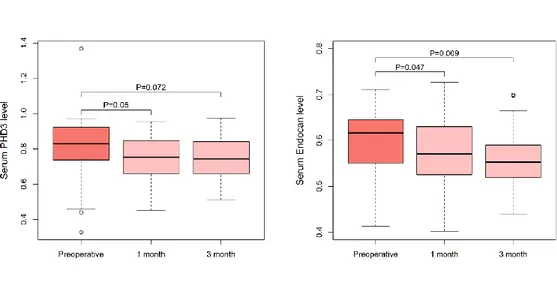
3. Serial changes after surgery in RCC patients A. Serial change of PHD3 after surgery
The mean PHD3 level in RCC patients decreased at postoperative months 1 and 3 (Figure 3A). The mean PHD3 levels at both 1 month and 3 months after surgery were 0.74 ± 0.11 mg/mL. Paired t-test analyses revealed that the PHD3 levels at postoperative months 1 were significantly lower than preoperative
14
PHD3 levels (P = 0.05), but difference between preoperative and postoperative months 3 did not reach statistical significance (P = 0.072).
B. Serial change of ESM-1 after surgery
When we analyzed the serial change in serum ESM-1 levels, the mean ESM-1 levels at 1 month and 3 months after surgery were 0.56 ± 0.07 ng/mL and 0.56 ± 0.06 ng/mL, respectively (Figure 3B). In paired t tests, the serum ESM-1 levels at 1 month and 3 months after surgery were significantly lower than the preoperative serum ESM-1 level (P = 0.047 and P = 0.009, respectively).
Figure 3. Serial changes PHD3 and ESM-1 levels after surgery in patients with RCC. Both PHD3 and ESM-1 levels declined after surgery and preoperative and postoperative levels were compared using paired t-test.
15
IV. DISCUSSION
In this study, we found that serum PHD3 and ESM-1 levels were significantly higher in patients with RCC than in healthy individuals.
The diagnostic
performances of both PHD3 and ESM-1 were improved when examining
only patients with tumor size >2 cm. An active surveillance cohort study
reported no association between tumor size <2 cm and delayed
intervention.
21We suggest that these biomarkers provide better
diagnostic accuracy in relatively significant RCC.
Multiple potential RCC biomarkers have been investigated for diagnostic or monitoring purposes. The urinary markers aquaporin-1 and perilipin-2 have been thoroughly investigated. These tumor-specific proteins showed high diagnostic accuracy for differentiating RCC patients from healthy control or patients with other malignancies.22 However, perilipin-2 measurement involves
Western blotting, which could limit widespread clinical implementation. Various inflammatory or genetic factors have also been investigated as serologic markers for RCC.3 Although the HIF pathway plays an important role in the
development of RCC, the suitability of HIF and/or its target molecule as an RCC biomarker remains unclear.4,23 Carbonic anhydrase IX, an enzyme
downstream of HIF, is a well-known RCC biomarker24 that has demonstrated
excellent prognostic value. With regard to its diagnostic potential, positron emission tomography scanning using an antibody against carbonic anhydrase IX
16
can accurately identified RCC.25 Dong et al. recently examined another
downstream molecule of HIF, angiopoietin-like 4, and found that serum levels are significantly higher in patients with RCC compared with healthy control or patients with other malignancies.26
The PHDs are also closely related to HIF signaling, which is critical in the hypoxic conditions of the tumor microenvironment. Under normoxic conditions, PHDs inactivate HIFs by hydroxylation of a specific domain, resulting in binding of VHL to HIF and subsequent proteasomal degradation.7,27 By contrast,
PHD activity decreases under hypoxic conditions, and subsequent HIF accumulation leads to expression of HIF target genes, enabling tumor cells to survive in the diminished oxygen conditions. The specific role of PHD3 in human cancers has yet to be elucidated, although this has been examined in pancreatic, gastric, breast, and colorectal cancers.10,11,28,29 PHD3 has various
biologic functions, and some studies have shown that PHD3 plays a significant role in tumor suppression by promoting apoptosis of tumor cells.10,11,28-30 A
previous transcriptional profiling study revealed that PHD3 expression is highly up-regulated in RCC compared with normal tissues.14 Sato et al. reported that
PHD3 is a potent immunogenic antigen of RCC.13 In a subsequent study, they
measured levels of serum autoantibodies against PHD3 in 22 RCC patients and 26 healthy controls. The serum anti-PHD3 antibody level in RCC patients was significantly higher than in healthy controls. The anti-PHD3 antibody level decreased postoperatively, suggesting that anti-PHD3 antibody is a potential
17
novel diagnostic biomarker for RCC. The mechanism underlying the up-regulation of PHD3 expression in RCC is unclear. Increased PHD3 expression might be due to response to HIF accumulation. On the other hand, phosphatidylinositol-3 kinase pathway also induced PHD3 expression independently of HIF.31
Increased levels of ESM-1 have been found in sera of patients with colorectal cancer, hepatocellular carcinoma, and acute leukemia.32-34 Moreover,
immunohistochemical studies have shown that elevated ESM-1 expression is associated with unfavorable prognoses in multiple types of cancers, including glioblastoma, colon cancer, hepatocellular carcinoma, and RCC.20,35-37 Tumor
cells in RCC are characterized by an increased stability of hypoxia inducible factor and the subsequent induction of VEGF expression.38 Rennel et al.19 have
demonstrated that ESM-1 is secreted from endothelial cells in response to VEGF, suggesting ESM-1 as potential tumor marker of RCC. Subsequent study has shown that serum ESM-1 levels significantly increased in patients with RCC than healthy control.20 However, the study focused on overexpression of
ESM-1 in clear cell RCC and the small number of patients were included in the ELISA analysis. Moreover, the previous study found that ESM-1 levels were significantly increased in clear cell RCC, but not in papillary RCC, and ESM-1 immunoreactivity was very rare in papillary RCC cells. However, in our study, serum ESM-1 levels did not differ between patients with clear cell and non-clear cell RCC. While activation of HIF and VEGF have been mostly
18
described in clear cell RCC, the role of HIF in other subtypes of RCC has also been suggested.39 Moreover, in our study, diagnostic performance of ESM-1
was not improved in subgroup of clear cell RCC. Thus, we speculate that serum ESM-1 may serve as a more generalized biomarker and is not restricted to clear cell RCC.
In the results of subgroup analysis, the diagnostic performance of ESM-1 was greatly improved in young cohort which included individuals with age 50 or less. We found that the level of serum ESM-1 increased with age. Aging process is closely related to chronic inflammation which also influence the expression of ESM-1. Therefore, although ESM-1 levels were significantly increased in sera from patients with RCC independent of age, it may be more difficult to diagnose RCC in elderly patients by ESM-1 levels alone. However, increasing evidence has shown that small renal masses, which are typically slow-growing nonaggressive tumors, might not affect the survival of elderly patients.40 Thus,
ESM-1 levels in serum may be more useful for diagnosing RCC in younger patients.
Our study is not devoid of limitations. First, the control group had relatively few benign renal masses and did not include any patients with other malignancies, and a larger cohort is needed to investigate the efficacy of serum PHD3 or ESM-1 levels in differentiating RCC from benign renal masses. Second, it is important to note that both biomarkers are also elevated in various other types of cancers, and thus are not specific for RCC. However, unlike
19
hepatocellular carcinoma and colorectal cancer, RCC has no serologic biomarker for diagnosis. Third, a prognostic role in RCC was not assessed. During 27 months of median follow-up, only 2 patients experienced recurrence, which was insufficient for statistical analysis. A longer follow-up study in patients with more advanced stages of RCC is needed to evaluate the prognostic value of PHD3. Lastly, our study protocol did not include postoperative blood sampling in control group. Thus, we could not exclude the effect of renal volume reduction on postoperative decrease in serum PHD3 and ESM-1 levels in RCC group.
V. CONCLUSION
Our findings suggest that serum PHD3 and ESM-1 could serve as novel biomarkers for RCC diagnosis and postsurgical monitoring, providing acceptable diagnostic performance. These serologic biomarkers could help differentiate RCC from benign renal masses. Although our findings should be validated in a larger cohort, serum PHD3 and ESM-1 appear to be suitable for clinical application in diagnosing and monitoring patients with RCC.
20
REFERENCES
1. Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917.
2. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78-83.
3. Ngo TC, Wood CG, Karam JA. Biomarkers of renal cell carcinoma. Urol Oncol 2014;32:243-51.
4. Schödel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, et al. Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur Urol 2016;69:646-57.
5. Mole DR, Blancher C, Copley R, Pollard PJ, Gleadle JM, Ragoussis J, et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 2009;284:16767-75.
6. Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int 2006;69:224-32.
7. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001;292:464-8.
21
DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001;107:43-54.
9. Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med 2010;14:758-70.
10. Su Y, Loos M, Giese N, Hines OJ, Diebold I, Görlach A, et al. PHD3 regulates differentiation, tumour growth and angiogenesis in pancreatic cancer. Br J Cancer 2010;103:1571-9.
11. Cui L, Qu J, Dang S, Mao Z, Wang X, Fan X, et al. Prolyl hydroxylase 3 inhibited the tumorigenecity of gastric cancer cells. Mol Carcinog 2014;53:736-43.
12. Tanaka T, Kitamura H, Torigoe T, Hirohashi Y, Sato E, Masumori N, et al. Autoantibody against hypoxia-inducible factor prolyl hydroxylase-3 is a potential serological marker for renal cell carcinoma. Journal of Cancer Research & Clinical Oncology 2011;137:789-94.
13. Sato E, Torigoe T, Hirohashi Y, Kitamura H, Tanaka T, Honma I, et al. Identification of an immunogenic CTL epitope of HIFPH3 for immunotherapy of renal cell carcinoma. Clin Cancer Res 2008;14:6916-23.
14. Amatschek S, Koenig U, Auer H, Steinlein P, Pacher M, Gruenfelder A, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res
22
2004;64:844-56.
15. Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta 2006;1765:25-37.
16. Delehedde M, Devenyns L, Maurage CA, Vivès RR. Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. Int J Cell Biol 2013;2013:705027.
17. Bechard D, Meignin V, Scherpereel A, Oudin S, Kervoaze G, Bertheau P, et al. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res 2000;37:417-25.
18. Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem 1996;271:20458-64. 19. Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, et
al. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res 2007;313:1285-94. 20. Leroy X, Aubert S, Zini L, Franquet H, Kervoaze G, Villers A, et al.
Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology 2010;56:180-7.
23
Hawken S, et al. Predictors of Delayed Intervention for Patients on Active Surveillance for Small Renal Masses: Does Renal Mass Biopsy Influence Our Decision? Urology: The Gold Journal 2016;98:88-96. 22. Morrissey J, Mellnick VM, Luo J, Siegel MJ, Figenshau RS, Bhayani S,
et al. Evaluation of Urine Aquaporin-1 and Perilipin-2 Concentrations as Biomarkers to Screen for Renal Cell Carcinoma: A Prospective Cohort Study. JAMA Oncol 2015;1:204-12.
23. Eichelberg C, Junker K, Ljungberg B, Moch H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur Urol 2009;55:851-63.
24. Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol 2010;58:75-83.
25. Divgi CR, Uzzo RG, Gatsonis C, Bartz R, Treutner S, Yu JQ, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol 2013;31:187-94.
26. Dong D, Jia L, Zhou Y, Ren L, Li J, Zhang J. Serum level of ANGPTL4 as a potential biomarker in renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations 2017.
24
Rev Mol Cell Biol 2004;5:343-54.
28. Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology 2010;138:606-15.
29. Fox SB, Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. The prolyl hydroxylase enzymes are positively associated with hypoxia-inducible factor-1α and vascular endothelial growth factor in human breast cancer and alter in response to primary systemic treatment with epirubicin and tamoxifen. Breast Cancer Res 2011;13:R16-R. 30. Gossage L, Zaitoun A, Fareed KR, Turley H, Aloysius M, Lobo DN, et
al. Expression of key hypoxia sensing prolyl-hydroxylases PHD1, -2 and -3 in pancreaticobiliary cancer. Histopathology 2010;56:908-20. 31. Tanaka T, Torigoe T, Hirohashi Y, Sato E, Honma I, Kitamura H, et al.
Hypoxia-inducible factor (HIF)-independent expression mechanism and novel function of HIF prolyl hydroxylase-3 in renal cell carcinoma. Journal of Cancer Research & Clinical Oncology 2014;140:503-13. 32. Ozaki K, Toshikuni N, George J, Minato T, Matsue Y, Arisawa T, et al.
Serum endocan as a novel prognostic biomarker in patients with hepatocellular carcinoma. J Cancer 2014;5:221-30.
33. Xu Z, Zhang S, Zhou Q, Wang Y, Xia R. Endocan, a potential prognostic and diagnostic biomarker of acute leukemia. Mol Cell
25
Biochem 2014;395:117-23.
34. Ji NY, Kim Y, Jang YJ, Kang YH, Lee CI, Yeom YI, et al. Identification of endothelial cell-specific molecule-1 as a potential serum marker for colorectal cancer. Cancer Sci 2010;101:2248-53.
35. Maurage C, Adam E, Minéo J, Sarrazin S, Debunne M, Siminski R, et al. Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol 2009;68:633-41.
36. Kim JH, Park MY, Kim CN, Kang HB, Kim KD. Expression of endothelial cell-specific molecule-1 regulated by hypoxia inducible factor-1α in human colon carcinoma: impact of ESM-1 on prognosis and its correlation with clinicopathological features. Oncol Rep 2012;28:1701-8.
37. Chen LY, Liu X, Wang SL, Qin CY. Over-expression of the Endocan gene in endothelial cells from hepatocellular carcinoma is associated with angiogenesis and tumour invasion. J Int Med Res 2010;38:498-510.
38. Kaelin WG. The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res 2007;13:680s-4s.
39. Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005;7:77-85.
26
40. Vetterlein MW, Jindal T, Becker A, Regier M, Kluth LA, Tilki D, et al. Small renal masses in the elderly: Contemporary treatment approaches and comparative oncological outcomes of nonsurgical and surgical strategies. Investigative and Clinical Urology 2016;57:231-9.
27
ABSTRACT (IN KOREAN)
신세포암에서 잠재적 바이오마커의 임상적 적용
<지도교수 한웅규>
연세대학교 대학원 의학과
김광현
목적: 대부분 초기 신세포암은 우연히 발견되며 그 예후가
좋으나, 진행성 신세포암의 경우 신기능의 보존이 어려울 뿐
아니라 종양학적 예후 또한 좋지 않다. 현재 신세포암의 진단은
영상학적 검사에 의존하는 경우가 많으나, 조기 진단을 위해
임상적으로
유용한
바이오마커의
개발이
시급하다.
본
연구에서는 신세포암에서 혈청의 prolyl hydroxylase-3 (PHD3)와
endocan (ESM-1)의 진단 혹은 감시 바이오마커로서 효용성을
확인해 보고자 하였다.
방법: 2013년 10월부터 2015년 3월까지 전향적으로 코호트를
구축하였으며, 코호트는 56명의 신세포암 환자와 69명의
대조군으로 구성되었다. 대조군은 56명의 건강인 신공여자와
13명의
양성신장종양
환자를
포함하였다.
신세포암과
양성신장종양 환자는 모두 근치적 혹은 부분신절제술 후
병리학적으로 확인이 되었다. 혈액 추출은 모두 수술 전에
시행되었고, 신세포암 환자의 경우 수술 후 1개월, 3개월에
추가로
시행되었다.
혈청의
PHD3와
ESM-1
수준은
효소면역측정법을 이용하여 측정되었다. 술 전 신세포암군과
28
대조군의 PHD3, ESM-1 수치가 비교되었고, 신세포암에서는 술
후 수치의 변화를 비교하였다. PHD3와 ESM-1의 진단 성능을
평가하기 위해 Area Under Curve (AUC)를 계산하였다.
결과: 혈청 PHD3와 ESM-1은 신세포암 환자에서 대조군에
비하여 통계적으로 유의하게 높게 관찰되었다 (PHD3; 0.79±0.17
ng/ml vs. 0.73±0.09 ng/ml, p=0.023) (ESM-1; 0.59±0.07 ng/ml vs.
0.53±0.09 ng/ml, p<0.001). PHD3와 ESM-1의 AUC는 각각 0.668과
0.684였다. 종양의 크기가 2 cm이상인 환자들만을 분석하였을 때
PHD3와 ESM-1의 AUC는 각각 0.709와 0.730이었다. 술 후
변화를 확인하였을 때 신세포암 환자에서 술 후 1개월 째 혈청
PHD3와 ESM-1은 유의하게 감소하였다 (PHD3; p=0.050, ESM-1;
p=0.047).
결론: 혈청 PHD3와 ESM-1은 새로운 신세포암의 진단
바이오마커이며 적절한 진단 성능을 보여주었다. 또한 PHD3와
ESM-1은 신세포암에서 수술 후 감시 바이오마커로서 효용성이
있을 것으로 보인다.
핵심되는 말 : 신세포암; 바이오마커; 조기진단
29