INTRODUCTION
Streptococcus bovisis a member of group D streptococci- common inhabitants of the intestine and causative agents of endocarditis. A well-recognized association has been established between S. bovisendocarditis and colon carci- noma, which led to great interest in the identification of
this organism [1]. The S. bovisgroup is divided into 3 bio- types according to their biochemical characteristics: bio- type I (mannitol fermentation positive), biotype II/1 (man- nitol negative and b-glucuronidase negative), and biotype II/2 (mannitol negative and b-glucuronidase positive) [2].
Each biotype has somewhat different pathogenicity. S.
bovis biotype I has been documented to be the biotype more likely associated with both endocarditis and malig- nant or premalignant colonic lesions [3]. The taxonomy of the S. bovisgroup has been evolving in the last few decades, and a new nomenclature was adopted on the basis of genetic distances and phylogenetic analyses. The new classifica- tion lists S. bovisbiotypes I, II/1, and II/2 as Streptococcus gallolyticussubsp. gallolyticus, Streptococcus infantarius
160 160
A Case of Streptococcus gallolyticus subsp. gallolyticus Infective Endocarditis with Colon Cancer: Identification by 16S Ribosomal DNA Sequencing
Seon Young Kim, M.D., Sei-Ick Joo, M.T., Jongyoun Yi, M.D., and Eui-Chong Kim, M.D.
Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
160 160
Although the association between Streptococcus bovis endocarditis and colon carcinoma is well known, very few cases of S. bovis infection associated with underlying malignancies have been reported in Korea. The S. bovis group has been recently reclassified and renamed as Streptococ- cus gallolyticus and Streptococcus infantarius subspecies under a new nomenclature system. We report a case of infective endocarditis with colon cancer caused by S. gallolyticus subsp. gallolyti- cus (previously named S. bovis biotype I). A 59-yr-old woman presented with a 1-month history of fever. Initial blood cultures were positive for gram-positive cocci, and echocardiography showed vegetation on mitral and aortic valves. Antibiotic treatment for infective endocarditis was started.
The infecting strain was a catalase-negative and bile-esculin-positive alpha-hemolytic Streptococ- cus susceptible to penicillin and vancomycin. The strain was identified as S. gallolyticus subsp.
gallolyticus with the use of the Vitek 2 GPI and API 20 Strep systems (bioMérieux, USA). The 16S rDNA sequences of the blood culture isolates showed 100% homology with those of S. gallolyticus subsp. gallolyticus reported in GenBank. The identification of the infecting organism, and the sub- sequent communication among clinical microbiologists and physicians about the changed nomen- clature, led to the detection of colon cancer. The patient recovered after treatment with antibiotics, valve surgery, and operation for colon cancer. This is the first report of biochemical and genetic identification of S. gallolyticus subsp. gallolyticus causing infective endocarditis associated with underlying colon cancer in a Korean patient. (Korean J Lab Med 2010;30:160-5)
Key Words : Streptococcus gallolyticus, Endocarditis, Colon cancer, rDNA sequencing
Received :November 23, 2009 Manuscript No :KJLM09-135 Revision received :January 14, 2010
Accepted :February 19, 2010
Corresponding author :Eui-Chong Kim, M.D.
Department of Laboratory Medicine, Seoul National University College of Medicine, 28 Yeongeon-dong, Jongno-gu, Seoul 110-744, Korea
Tel : +82-2-2072-3500 Fax : +82-2-764-6542 E-mail : euichong@snu.ac.kr
subsp.coli,and S. gallolyticussubsp. pasteurianus, respec- tively [4]. We report a case of infective endocarditis with colon cancer caused by S. gallolyticussubsp. gallolyticus. Identification of the causative organism was performed by phenotypic characterization and 16S rDNA sequence anal- ysis.
CASE REPORT
A 59-yr-old woman was admitted to Seoul National University Hospital, with a 1-month history of recurrent episodes of fever and chills. She also had arthralgia of both knees and 3 episodes of fecal incontinence for 1 month. She was a carrier of the hepatitis C virus and had a history of stage 4 gastric marginal zone lymphoma, which had been previously treated with chemotherapy over 2 yr ago. She had no evidence of residual lymphoma after treatment. On physical examination, fever was present (38.9℃); lung fields were clear; heart sounds were regular without murmur;
and abdomen was soft, non-distended, and with no pal- pable mass. Digital rectal examination revealed no mele- na, hematochezia, or mass. Specimens for laboratory tests and blood cultures were collected, and empirical antibiotic therapy with intravenous ciprofloxacin (400 mg every 12 hr) was started. Initial laboratory studies showed the fol- lowing results: hemoglobin (11.1 g/dL), white blood cells [12.01×109/L; neutrophils (79%), lymphocytes (14%), and monocytes (4%)], platelets (242×109/L), and C-reactive pro- tein (5.79 mg/dL). Results of the liver function tests and creatinine, glucose, and troponin tests were within the normal range. Stool occult blood test was not performed.
The patient’s chest radiograph was unremarkable. She had persistent fever increasing up to 39.1℃ despite antibiotic therapy, and initial blood cultures were positive for gram- positive cocci in pairs and short chains. Twenty-four hours later, ciprofloxacin was discontinued and vancomycin (1,000 mg every 12 hr) was started after collecting additional blood cultures. An echocardiography was performed, which show- ed vegetations on the mitral and aortic valves. The initial isolate from the first blood cultures was identified as S.
gallolyticussubsp. gallolyticus, which was susceptible to
penicillin. The second blood cultures were also positive for similar alpha-hemolytic streptococci. Vancomycin was dis- continued and antibiotics were replaced by intravenous penicillin G (3,000,000 U every 4 hr) and gentamicin (63 mg every 8 hr). The fever subsided, and the subsequent blood cultures were negative for streptococci. The isolate from the second blood cultures was initially identified as Strep- tococcus mutans; however, the results of retest and fur- ther investigation confirmed that it was the same strain as the isolate from the first blood cultures. The results of blood cultures were discussed among clinical microbiolo- gists and physicians, including the corrected results of the second blood cultures, the information about the causative organism (i.e., S. gallolyticussubsp. gallolyticus, which was named as S. bovisbiotype I in the previous nomenclature system), and the risk of colon cancer. The abdomen com- puterized tomography (CT) image showed newly detected segmental wall thickening of the sigmoid colon. Colono- scopy revealed an ulceroinfiltrative mass, which was con- firmed as adenocarcinoma. The patient underwent opera- tion for valve replacement and anterior resection for colon cancer on the 18th and 50th day of hospitalization, respec- tively. She successfully recovered and was discharged after completing the antibiotic therapy and postoperative care.
Both the first and the second blood cultures showed gram-positive cocci in pairs and short chains after 24 hr of incubation in broths at 37℃ (Bactec, Becton Dickinson, Baltimore, MD, USA). Subsequent subculture on 5% sheep blood and chocolate agars incubated at 35℃ showed small alpha-hemolytic colonies. The isolates were negative for catalase and pyrrolidonylarylaminidase, showed no zone of inhibition around the optochin disk, failed to grow in heart infusion broth containing 6.5% NaCl, and bile-esculin positive after overnight incubation. A tentative identifica- tion of viridans group Streptococcuswas made. The Vitek2 GP identification system (bioMe@rieux, Durham, NC, USA) was used to identify the isolate from the first blood culture as S. gallolyticussubsp. gallolyticus; the isolate from the second culture was identified as S. mutans. The tests were repeated twice for the subculture of the second isolate, and the results were as follows: S. gallolyticussubsp. gallolyti-
cus(excellent identification, probability 98.11%), S. gal- lolyticussubsp. gallolyticus(low discrimination, 50.52%), and S. mutans(49.48%). Both of the repeated tests showed identical biochemical profiles, except that the test with an excellent confidence level showed a positive result in the pullulan acidification test. The API 20 Strep system (bio- Me@rieux, Durham, NC, USA) identified the isolate from the first blood culture as S. bovisbiotype I (S. gallolyticus subsp. gallolyticus) (profile 5000453, 78.1%) and S. bovis biotype II (13.5%), while the isolate from the second culture was identified as S. bovisbiotype I (S. gallolyticussubsp.
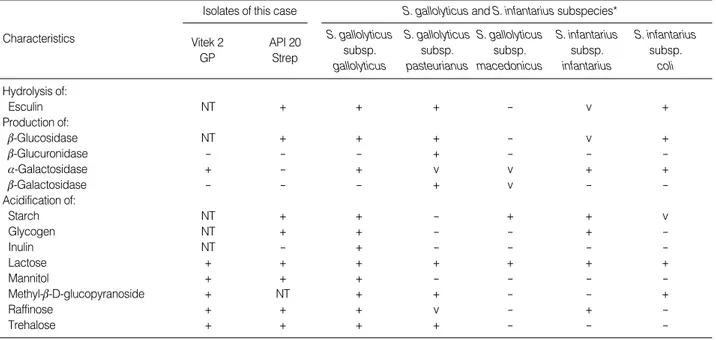
gallolyticus) (profile 5000553, 99.1%). The first isolate show- ed negative reactions with leucine arylamidase and man- nitol, which are atypical for S. gallolyticussubsp. gallolyti- cus. The subculture of the first isolate was tested repeat- edly and identified by the API 20 Strep system as S. gal- lolyticussubsp. gallolyticus, with 99.1% probability. The biochemical profiles of this strain are compared with those of the S. gallolyticusand S. infantariussubspecies in Table 1. Antimicrobial susceptibility testing was performed by E test for penicillin and disk diffusion methods for other anti- biotics. Isolates from the first and second blood cultures
showed identical results: susceptible to penicillin (MIC=
0.032 mg/mL), vancomycin, and chloramphenicol and resis- tant to erythromycin and clindamycin.
We performed 16S rDNA gene sequencing for the isolates from the first and second blood cultures. The forward (27F:
5′-AGAGTTTGATCMTGGCTCAG-3′) and reverse (1492R:
5′-TACGGYTACCTTGTTACGACTT-3′) primers were used to amplify 16S rDNA gene fragments, representing approx- imately 98% of the complete 16S rDNA gene. The following primers were used for sequencing of the fragments obta- ined: 27F, 518F (5′-CCAGCAGCCGCGGTAATACG-3′) and 800R (5′-TACCAGGGTATCTAATCC-3′). Both isolates showed identical 16S rDNA gene sequences and showed 100% (1,480/1,480 bp) homology with those of S. gallolyti- cussubsp. gallolyticus(EU163500, GenBank). Isolates from the first and second blood cultures showed 99.7% (1,476/
1,480 bp) and 98.6% (1,459/1,480 bp) homology with S. gal- lolyticussubsp. pasteurianus(EU163502, GenBank) and S.
infantariussubsp. coli(EU163503, GenBank), respectively [5]. The sequences were analyzed by using MicroSeq ID 16S rDNA 500 library v2.1 and analysis software v2.0 (Applied Biosystems, Foster City, CA, USA), and the isolate was
*The biochemical profiles of the subspecies of S. gallolyticus and S. infantarius were obtained from a report by Schlegel et al. [4].
Abbreviations: NT, not tested; +, ≥80% of strains positive; -, ≤20% of strains positive; v, 21-79% activity compared to the positive control reaction.
Characteristics
Isolates of this case S. gallolyticus and S. infantarius subspecies*
Vitek 2 GP
API 20 Strep
S. gallolyticus subsp.
gallolyticus
S. gallolyticus subsp.
pasteurianus
S. gallolyticus subsp.
macedonicus
S. infantarius subsp.
infantarius
S. infantarius subsp.
coli Hydrolysis of:
Esculin NT + + + - v +
Production of:
b-Glucosidase NT + + + - v +
b-Glucuronidase - - - + - - -
a-Galactosidase + - + v v + +
b-Galactosidase - - - + v - -
Acidification of:
Starch NT + + - + + v
Glycogen NT + + - - + -
Inulin NT - + - - - -
Lactose + + + + + + +
Mannitol + + + - - - -
Methyl-b-D-glucopyranoside + NT + + - - +
Raffinose + + + v - + -
Trehalose + + + + - - -
Table 1. Characteristics of the blood culture isolates of this case in comparison with the subspecies of Streptococcus gallolyticus and Streptococcus infantarius
identified as S. bovis.
DISCUSSION
The relationship between streptococcal endocarditis and colon cancer was first described in 1951 [6], and S. bovis was recognized as the pathogen specifically related to colon cancer in 1977 [1]. Although many other bacterial strains have been associated with gastrointestinal tumors, the association between S. bovisand colon cancer remains the strongest and best documented one. Several prospective studies have suggested that S. bovisinfection could be seen as a first sign of colon cancer [7, 8]. Therefore, clinicians should not readily rule out colon cancer or other malignan- cies when they encounter patients with S. bovisinfection.
In this case, colon cancer can be detected by colonoscopy upon clinical suspicion of S. bovisinfection, even without clinical findings suggesting colon cancer. The pathogene- sis of the association between S. bovisinfection and colon neoplasms has been widely investigated; however, the pa- thogenesis is still not clear. One hypothesis is that the S.
bovisgroup is a normal inhabitant of the gastrointestinal tract and can readily enter the bloodstream upon mucosal disruption or vascular changes [9].
Since the early 1980s, genetic and biochemical diversi- ties among S. bovisbacteria have been recognized. These diversities led to devising schemes to distinguish strains by biotype. S. bovisbacteria are said to be biotype I (or typ- ical) if they ferment mannitol and produce glucan, and biotype II (or variant) if they cannot ferment mannitol or produce glucan. The S. bovisbiotype II strains are further divided into types II/1 and II/2 according to the ability of the latter group to produce b-galactosidase and b-glucu- ronidase and ferment trehalose but not glycogen [10]. In the 1990s, 4 new species were described: Streptococcus gallolyticus, Streptococcus macedonicus, Streptococcus waius, and Streptococcus infantarius[11]. S. gallolyticus, originally isolated from koalas, is phenotypically similar to S. bovisbiotype I [12], and reexamination of strains previ- ously identified as S. bovisby either phenotypic or genetic methods revealed that many of the human strains associ-
ated with endocarditis and animal (goat, pigeon, and cow) strains are identical to S. gallolyticus[13, 14]. Meanwhile, molecular technologies, such as DNA-DNA hybridization or the amplification of selected targets, have been devel- oped to complement and improve the identification of strep- tococci at the species level. Farrow et al. demonstrated that the S. bovis/Streptococcus equinuscomplex comprised 6 DNA groups [15]. Members of DNA group 2 have been re- classified, according to their genetic and biochemical char- acteristics, and renamed as S. gallolyticussubsp. gallolyti- cus, S. gallolyticussubsp. pasteurianus, or S. gallolyticus subsp. macedonicus[4]. Biochemical characteristics, such as gallate hydrolysis activity, are useful for the identifica- tion at the subspecies level. A recent study showed that the published phenotypic characteristics of S. gallolyticus andS. infantariuswere equivocal and did not allow unam- biguous phenotypic differentiation [5]. Beck et al. also show- ed that although the 16S rDNA gene sequences are very similar between subspecies, full-length 16S rDNA gene sequence analysis clearly assigned the subspecies in most cases because of the extremely low base pair variability within one species or subspecies. They showed that only 3 of 29 S. gallolyticussubsp. gallolyticusstrains, 1 of 12 S.
gallolyticussubsp. pasteurianusstrains, and 1 of 17 S. in- fantariusssubsp. colihad single point mutations, whereas other strains showed identical 16s rDNA sequences within each subspecies [5]. Our isolates did not ferment inulin but fermented mannitol and raffinose. They also produced acid from starch, glycogen, methyl-b-D-glucopyranoside, and trehalose, but did not produce b-glucuronidase and b-gal- actosidase (Table 1). On the basis of the biochemical char- acteristics and the 16S rDNA sequencing data, we could identify the infecting organism as S. gallolyticussubsp.
gallolyticuswith high confidence at the subspecies level.
Cases of S. bovis infection associated with underlying malignancies have been rarely reported in Korea. There are 2 previous reported cases of S. bovisendocarditis and associated colon cancer in Korea [16, 17]; however, they did not provide information about the microbiological char- acteristics or S. bovisbiotypes. In a more recent single- center study, the association of S. bovisbiotypes with the
type of clinical infection and data on antimicrobial suscep- tibility was studied. In this study, none of the 13 patients with S. bovisbacteremia had gastrointestinal malignan- cies [18]. More studies on the prevalence, underlying dis- eases, and microbiological characteristics of S. gallolyticus subspecies in Korea are needed.
This case shows that accurate identification of S. gal- lolyticussubsp. gallolyticusendocarditis makes the detec- tion of underlying cancer possible. Much attention should be given when identifying the causative organisms of strep- tococcal endocarditis, and investigation for underlying gas- trointestinal malignancies should be performed when S.
gallolyticusis identified. In addition, some clinicians may not be familiar with the new nomenclature system of S.
bovisbacteria. This lack of awareness can lead to under- diagnosis of serious underlying conditions, including colon cancer; thus, it is important to provide clinicians with suf- ficient information about the nomenclature change when reporting study results. Moreover, although the use of the name S. bovisbiotype I or II/2 instead of S. gallolyticusis common in clinical microbiology despite the clearly estab- lished new nomenclature, it is also important to update and use the proper taxonomic species in reporting, com- munication, or clinical studies to prevent further confu- sion in the future.
REFERENCES
1. Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon.
N Engl J Med 1977;297:800-2.
2. Ruoff KL, Miller SI, Garner CV, Ferraro MJ, Calderwood SB. Bac- teremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J Clin Microbi- ol 1989;27:305-8.
3. Herrero IA, Rouse MS, Piper KE, Alyaseen SA, Steckelberg JM, Patel R. Reevaluation of Streptococcus bovis endocarditis cases from 1975 to 1985 by 16S ribosomal DNA sequence analysis. J Clin Microbiol 2002;40:3848-50.
4. Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. Reappra- isal of the taxonomy of the Streptococcus bovis/Streptococcus equinus
complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp.
nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol 2003;53:631-45.
5. Beck M, Frodl R, Funke G. Comprehensive study of strains previ- ously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J Clin Microbiol 2008;46:2966-72.
6. Mc CW and Mason JM 3rd. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala 1951;21:162-6.
7. Klein RS, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Strep- tococcus bovis septicemia and carcinoma of the colon. Ann Intern Med 1979;91:560-2.
8. Wilson WR, Thompson RL, Wilkowske CJ, Washington JA 2nd, Giuliani ER, Geraci JE. Short-term therapy for streptococcal infec- tive endocarditis. Combined intramuscular administration of peni- cillin and streptomycin. JAMA 1981;245:360-3.
9. Ellmerich S, Scholler M, Duranton B, Gosse F, Galluser M, Klein JP, et al. Promotion of intestinal carcinogenesis by Streptococcus bovis.
Carcinogenesis 2000;21:753-6.
10. Coykendall AL. Classification and identification of the viridans strep- tococci. Clin Microbiol Rev 1989;2:315-28.
11. Poyart C, Quesne G, Trieu-Cuot P. Taxonomic dissection of the Strep- tococcus bovis group by analysis of manganese-dependent superox- ide dismutase gene (sodA) sequences: reclassification of Streptococ- cus infantarius subsp. coli as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype 11.2 as Streptococcus pasteurianus sp. nov.
Int J Syst Evol Microbiol 2002;52:1247-55.
12. Schlegel L, Grimont F, Collins MD, Regnault B, Grimont PA, Bou- vet A. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp.
infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp.
nov., isolated from humans and food. Int J Syst Evol Microbiol 2000;
50 Pt 4:1425-34.
13. Clarridge JE 3rd, Attorri SM, Zhang Q, Bartell J. 16S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Strep- tococcus bovis Biotype II/2 is a separate genospecies and the predom- inant clinical isolate in adult males. J Clin Microbiol 2001;39:1549-52.
14. Devriese LA, Vandamme P, Pot B, Vanrobaeys M, Kersters K, Hae- sebrouck F. Differentiation between Streptococcus gallolyticus strains
of human clinical and veterinary origins and Streptococcus bovis str- ains from the intestinal tracts of ruminants. J Clin Microbiol 1998;36:
3520-3.
15. Farrow JA, Kruze J, Phillips BA, Bramley AJ, Collins ME. Taxo- nomic studies on Streptococcus bovis and Streptococcus equinus: des- cription of Streptococcus alactolyticus sp. nov. and Streptococcus sac- charolyticus sp. nov. Syst Appl Microbiol 1984;5:467-82.
16. Kwack KK, So SC, Park HK, Lee DK, Kim JH, Lyu DY, et al. A case
of subacute infective endocarditis with colon cancer caused by Strep- tococcus bovis. Korean J Med 2000;59:198-202.
17. Koh DW, Choi JH, Kim YS, Koo JS, Cho YJ, Park DK, et al. A case of two synchronous colon cancers accompanied by Streptococcus bovis endocarditis. Korean J Gastrointest Endosc 2001;23:503-6.
18. Uh Y, Kwon O, Yoon KJ, Hwang GY, Kim HY. Underlying diseases associated with Streptococcus bovis bacteremia and antimicrobial sus- ceptibility of the organism. Korean J Clin Microbiol 2006;9:36-41.