A spinal cord injury (SCI) is defined as an insult to the spinal cord that partially or completely interrupts the motor, sensory, and reflex functions of the cord. Our basic under- standing of the pathophysiology of a SCI is based on the stud-
ies of the evolution of the lesion in a SCI experimental model.
These studies suggest a division of the injury into primary and secondary changes. The lack of regenerative properties of the spinal cord is probably the result of a combination of factors, such as the inhibitory character of the glial scars, white matter in the central nervous system, and lack of trophic sup- port. In addition, it is well known that a series of secondary degenerative processes occur, which lead to the further loss of tissue1,2,35). This secondary reaction involves a variety of
522 522
A Comparative Study of Behavioral and immunohistological Changes after Spinal Cord Injury between Young and Adult Rats
Jun-Young Yang, M.D., June-Kyu Lee, M.D., Kwang-Jin Rhee, M.D., Kyung-Cheon Kim, M.D., Ui-Pyo Hong, M.D., and Jung-Bum Lee, M.D.
Department of Orthopedic Surgery, School of Medicine, Chungnam National University, Daejeon, Korea
522 522 Address reprint requests to
Jun-Young Yang, M.D.
Department of Orthopaedic Surgery, School of Medicine, Chungnam National University, 640 Daesa-dong, Jung-gu, Daejeon 301-721, Korea
Tel: +82.42-220-7351, Fax: +82.42-252-7098 E-mail: jyyang@cnuh.co.kr, jyyang@cnu.ac.kr
Purpose: The aim of this study was to compare the behavioral and immunohistochemical changes in a spinal cord injury (SCI) between young and adult rats and to clarify the differences in the mechanism underlying the changes in a SCI between young and adult rats.
Materials and Methods: A total of 25 young and 25 adult male Sprague-Dawley rats (5 weeks and 16 weeks old) were used. The rats were anesthetized with pentobarbital and laminectomies were carried out at the level of the 11th and 12th thoracic vertebra. Using a modified New York University Impactor, a SCI was induced by dropping a 10 gm weight at a height of 20 mm. The bladders were emptied manually twice a day to prevent urinary problems. The animals that received no surgery were used as the normal controls. Behavior tests were performed using the Basso-Beatti-Bresnahan (BBB) scoring system, 1 and 7 days after the weight drop injury. The difference in the BBB score between the young and adult rats were analyzed by a paired t-test with a p value <0.05 considered significant. The injured spinal cords were dissected at 1 and 7 days after surgery. H-E stain and immunohistochemistry for c-Jun and GFAP were performed in the spinal cord sections. The immunoreactions were visualized by incubation for 1 hour at RT in an avidin-biotin-peroxidase complex in PBS and 5-10 min in 0.05% 3,3 -diaminobenzidine and 0.01%
H2O2in 0.1 M PBS.
Results: The hind limbs of young rats were paralyzed 1 day after surgery, but had recovered partially 7 days after surgery. However, the adult rats remained in the paralyzed status 7 days after surgery. The c- Jun expression level increased in the gray matter up to 7 days after the weight-drop injury in adult rats.
The c-Jun expression level increased significantly in the gray matter 1 day after the injury in the young rats. However, the c-Jun expression level decreased significantly in the gray matter 7 days after the injury in the young rats compared with that of the 1 day post-injury. The GFAP expression level in the gray mat- ter increased 1 day after the weigh-drop injury in the adult rats. However, it decreased in the necrotic region 7 days after the injury in the adult rats. GFAP expression in the gray matter increased gradually up to 7 days after the injury in the young rats.
Conclusion: These results suggest that lack of recovery from a SCI in adult rats may be related to the continuous upregulation of c-Jun expression and/or the downregulation of GFAP after the weight-drop injury. In contrast, the upregulation of GFAP expression and/or the downregulation of c-Jun expression in the spinal cord might be related to a partial recovery in young rats after a weight-drop injury.
Key Words: Spinal cord injury, Behavioral test, c-Jun, GFAP
′
neurochemical changes that initiate an excitotoxic cascade leading to changes in the physiological state of the spinal neurons. This excitotoxic cascade includes a series of cyto- plasmic events that cause changes in gene expression.
Following a mechanical injury to the central nervous sys- tem (CNS), the breakdown of the blood-brain barrier results in the accumulation of hematogenous cells at the site of injury. As observed in many other tissues types, the initial infiltration of these cells in an injured CNS has been attribut- ed to chemokines. The production of these factors in the CNS has not only been attributed to the activated lymphoid and mononuclear cells that infiltrated the CNS, but also to the resident glial cells, particularly reactive astrocytes6,13,30).
Gliosis is the most important histopathological indicator of a CNS injury, regardless of the etiology. The glial fibril- lar acidic protein (GFAP), which is a distinctive cytoplasmic intermediate filaments, forms glial filaments in the astrocytes in the CNS and in some Schwann cells in the peripheral nerve. Astrocytes respond to an injury by hypertrophy and hyperplasia. Extraordinary metamorphosis can be demon- strated through a study of the immunohistochemistry for GFAP11). However, the mechanism and factors that initiate astrocytic proliferation and gliosis are poorly understood.
The immediate early genes such as c-fos and c-jun are signal-transducing factors of the activator protein (AP)-1 family4,9). c-fos, which are the first targets of the excitotoxic cascade, is a proto-oncogene that is expressed in cells fol- lowing a prolonged intense or noxious activation2,7,16). One of the earliest and most consistent indications of neuronal damage is the de novo expression of the inducible transcrip-
tion factor c-jun14). The expression of the c-jun protein (c- Jun) is believed to be associated with the death15,17)and regen- eration of neurons23,24,33,34).
The capacity of the CNS for axonal growth decreases with the age of the animal at the time of injury28). However, mech- anism of the changes in the SCI according to age is well estab- lished. The aim of this study was to compare the behavioral and immunohistochemical changes in a SCI between young (5 week-old) and adult (16 week-old) rats and clarify the differences in the underlying mechanism of the changes in the SCI between young and adult rats.
MATERIALS AND METHODS
1. Animal model
A total of 25 young (5 weeks old) and 25 adult (16 weeks old) male Sprague-Dawley rats were used in this study. The adults, weighing 260 gm and the young, weighing 100 gm on average, were kept under standardized conditions ( 5 mice/
cage, 20-24℃, 45-65% humidity, 12 hours daily lighting time) and given food and water (solid food and unrestricted water intake).
2. Operation technique
The rats (20 young and 20 adult rats) were anesthetized with pentobarbital (50 mg/kg, i.p.) and laminectomies were carried put at the level of the 11th and 12th thoracic verte- bra, which is at the L5 spinal cord level (Fig. 1). Using a New York University (NYU) Impactor, a SCI was induced by dropping a 10 gm weight at a height of 20 mm (Fig. 2).
The rod of the Impactor has a 3 mm diameter smooth circu- lar surface. After the injury, the muscles and skin were closed
Fig. 1.Dorsal laminectomies at the level of the 12th thoracic ver- tebra were carried out under phenobarbital anesthesia.
Fig. 2.The exposed dorsal surface of the cord was subjected to weight drop impact using a 10 gm weight at a height of 20 mm.
A B
in layers, and the rats were placed in a temperature (20-24
℃) and humidity (45-65%)-controlled chamber overnight.
The rats were given antibiotics (cefetezol, i.m.) for three days after the injury and the bladders were emptied manually twice a day in order to prevent urinary retention and a uri- nary tract infection. The animals that received no surgery were used as the normal controls (5 young and 5 adult rats).
3. Behavior test
Behavioral tests of the rats subjected to the spinal cord injury were performed using Basso-Beatti-Bresnahan (BBB) scoring. The BBB score for the evaluation of the hind limb function after the SCI ranges from 0 to 217,8). Testing was carried out 1 and 7 days after the injury.
4. Tissue preparation for histology and immunohistoche- mistry for c-Jun and GFAP
The animals were subjected to pentothal sodium anesthe- sia (50 mg/kg, i.p.), 1 and 7 days after the spinal cord injury (10 young and 10 adult rats, respectively). Subsequently, the rats underwent transcardiac perfusion fixation with saline followed by 4% phosphated-buffered paraformaldehyde. For a histological evaluation, a 20 mm cord segment centered at the injury site was removed from the vertebral canal and postfixed in 4% paraformaldehyde overnight at 4℃. Each cord was then dehydrated in a graded series of ethanol, cleared in xylene, embedded in paraffin, and coronal sections (5 m) were cut. The deparaffinized sections were stained with hematoxylin-eosin
5. Immunohistochemistry for c-Jun and GFAP
Immunohistochemistry for c-Jun was carred out on, depa- raffinized sections that had been immersed in 3% H2O2for 30 min in order to inactivate the endogenous peroxidases. The sections were incubated 1 hour at room temperature (RT) in c-Jun (1:250, Santa Cruz) and GFAP Antibodies (1:250, Dako) in 0.1 M PPS, pH 7.4, containing 0.1% TritonX- 100, 1.5% bovine serum albumin (BSA), and 1:200 nor- mal goat serum (NGS), followed by incubation in 1:200 biotinylated goat anti-rabbit IgG (Vector) and 1:200 NGS in PBS. at RT for 1 hour. The immunoreactions were visu- alized by incubation in avidin-biotin-peroxidase complex (1:100, ABC kit, Vector) in PBS for 1 hour at RT and in 0.05% 3,3 -diamino-benzidine (DAB) and 0.01% H2O2in 0.1 M PBS for 5-10 mm.
6. Data analysis
The BBB score in the young and adult rats after the SCI are expressed as the mean SEM. The difference in the BBB score between the young and adult rats were analyzed by a paired t-test with a p value ≤0.05 considered significant.
RESULTS
1. Behavior analysis
Open field motor testing using the BBB Locomotor Rat- ing Scale7,8)showed that the young rats had a substantially im- proved hindlimb function compared with the adult rats (Fig.
3). The adult rats showed low BBB score on the 1st (1.41.2) and 7th day (4.41.5) after the injury. The young rats showed a low BBB score (1.81.5) on day 1, but showed an improved BBB score (12.21.8) on day 7. A significant difference was noted between the two groups on the 7th day (p<0.01).
2. Morphological features 1) Adult rats
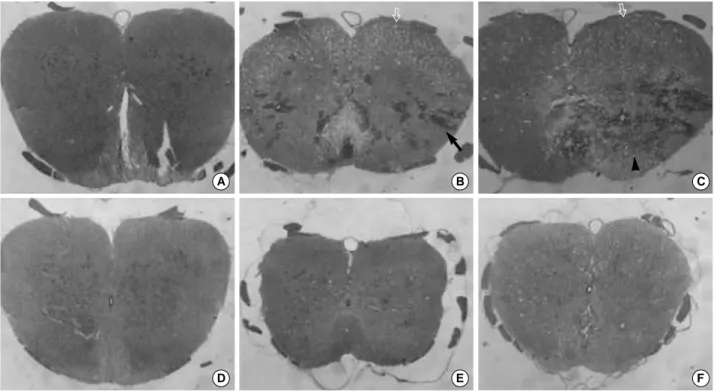
A SCI leads to a focal petechial hemorrhage in many areas of the gray matter and a few areas of the white matter on day 1 (Fig. 4B). Most of the neurons in the gray matter had degenerated and the white matter showed marked vacuo- lar degeneration on day 1 (Fig. 4B). An egg-shaped zone of necrosis appeared in the contused spinal cord on day 7 (Fig.
4C). Most of the neurons disappeared and inflammatory cells infiltrated the necrotic region, and the white matter showed vacuolar degeneration on day 7 (Fig. 4C).
′ Fig. 3.Neurological function of rats after a SCI between young
and adult rats, assessed by the BBB locomotor rating scale. The error bars indicate the SEM; p<0.01.
Open field score
25 20 15 10 5 0
0 day 1 day 7 days
Time post-injury
Adult rats Young rats Behavior Analysis
2) Young rats
A SCI leads to a focal petechial hemorrhage in few area of the gray matter on day 1 (Fig. 4E), and a petechial hem- orrhage was less severe than in the adult rats. A few neurons in the gray matter had degenerated and the white matter showed few vacuolar degeneration on day 1 (Fig. 4E). The egg-shaped zone of necrosis did not appear in the contused spinal cord on day 7 (Fig. 4F). A few neurons had degener- ated in the gray matter, but moderate vacuolar degeneration was observed in the white matter on day 7 (Fig. 4F).
3. Expression of c-Jun 1) Adult rats
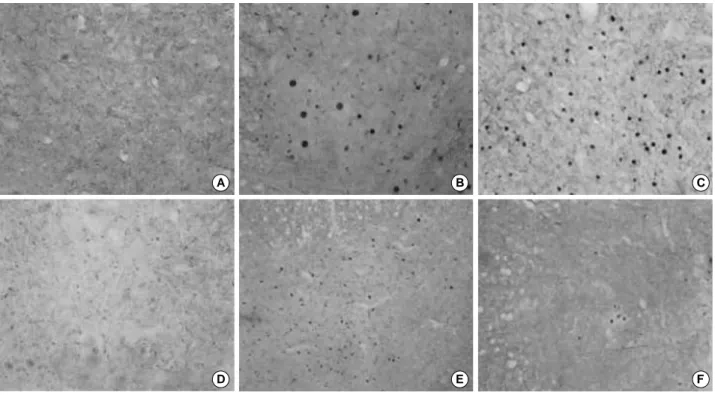
c-Jun immunoreactivity was not detected in the control rats (Fig. 5A). However, c-Jun immunoreactivity increased prominently in the gray matter on day 1 (Fig. 5B), and c- Jun immunoreactivity in the gray matter on day 7 was simi- lar to that observed in the adult rats on day 1 (Fig. 5C).
2) Young rats
c-Jun immunoreactivity was not detected in the control rats (Fig. 5D). c-Jun immunoreactivity increased markedly in the gray matter on day 1, which was similar that of the adult rats on day 1 (Fig. 5E). However, the c-Jun immunore- activity in the gray matter on day 7 decreased significantly compared with that of young rats on day 1 (Fig. 5F).
4. Expression of GFAP 1) Adult rats
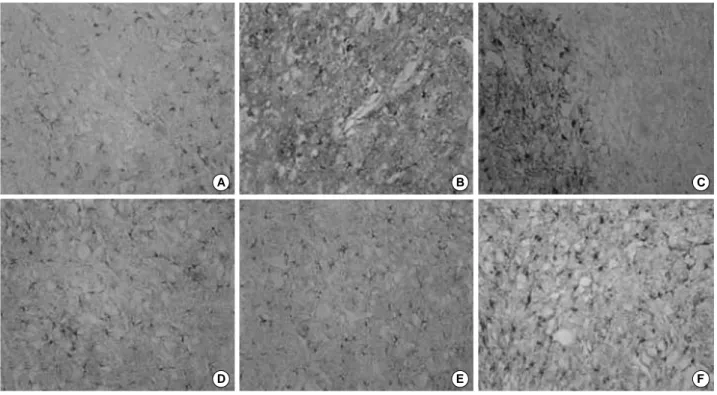
The GFAP immunoreactivity in the gray matter was simi- lar to that of the control rats on day 1 (Fig. 6A, B). Howev- er, on day 7, the GFAP immunoreactivity was totally absent in the necrotic region and increased markedly in the gray matter, which is a relatively intact region close to the necrot- ic region (Fig. 6C).
2) Young rats
GFAP immunoreactivity in the gray matter increased slightly compared with that of the control rats (Fig. 6D, E).
A B C
Fig. 4.Histopathology of the spinal cord after a weight-drop injury. H-E stain, ×40. (arrow, focal petechial hemorrhage; vacant arrow, vacuolar degeneration in the white matter; arrowhead, infiltrated inflammatory cells). (A, D) The spinal cord of adult and young con- trol rat. (B) The spinal cord of an adult rat 1 day after the injury; Focal petechial hemorrhage can be seen particularly in the gray mat- ter, neuronal degeneration and vacuolar formation. (C) The spinal cord of an adult rat 7 days after the injury; Necrosis like Egg-shaped.
(E) The spinal cord of a young rat 1 day after the injury; Less severe focal petechial hemorrhage, neuronal degeneration, and vacuo- lar formation than (B). (F) The spinal cord of a young rat 7 days after the injury; An egg-shaped zone of necrosis did not appear.
D E F
The GFAP immunoreactivity increased significantly in the gray matter on day 7 contrast to those of control rats and young rats on day (Fig. 6F).
DISCUSSION
The task of designing a clinically relevant animal model of SCI is extremely difficult, This is because human injuries are multifactorial such as the mechanism of injury, a number of complex forces, and the difference in the interval between the time of the injury and treatment. Considering these vari- ables, a variety of experimental SCI models have been devel- oped. Among them, the weight drop method closely sim- ulates some of the biomechanics of the human SCI and pro- duces highly reproducible injuries. By recording the height in centimeters and the weight in grams, the magnitude of the injury could be expressed as its product in gram-cen- timeters (g-cm). This study used a modified NYU impactor which was made at our institute, and the SCI was induced by dropping a 10 gm weight at a height of 20 mm, the mag- nitude was 200 g-cm with a constant 3 mm diameter sur- face. It is believed that the impactor is reproducible, easy
to make and economical7,8). The analysis were performed using a behavioral test, as well as histological and immuno- logical stain. Behavioral tests are necessary to clarify the dif- ference in the nerve function between the young and adult rats. The BBB score in the present and previous studies25-27) show that the motor function of the hind limb was impaired with a similar severity a day after th injury. the impairment of the hind limb movement had improved in the young and adult rats on day 7, but the improvement was more pro- nounced in the young rats. A significant difference was noted between the two groups.
A variety of morphologic changes become evident within minutes of the SCI3). These changes consisted of a petechial hemorrhage in the gray matter, small ruptures in the venules, an increase in the size of the extracellular spaces in the gray and white matter, and an enlarged periaxonal space. The results similar to the above-mentioned changes. According to other reports, obvious demyelination was observed in the odrsal columns as early as 21 hours after the SCI. Initially, acute cellular infiltration by polymorphonucleocytes occurs, and it is replaced by an invasion of the macrophages with-
A B C
Fig. 5.c-Jun immunoreactivities in the spinal cord weight-drop injury, ×100. (A, D) c-Jun immunoreactivity of the spinal cord of adult and young control rats; No immunoreactivity was not detected. (B) Increase in the c-Jun immunoreactivity of the gray matter of adult rats 1 day after injury. (C) The immunoreactivity was similar to that observed in the adult rat 7 days after injury. (E) Increase in c-Jun immunoreactivity of the gray matter of a young rat 1 day after the injury. (F) Significant decrease in the c-Jun immunoreactivity of the gray matter of a young rat 7 days after the injury.
D E F
in days of the injury. By day 7, the cystic degeneration of the necrotic area became evident, particularly in the animals that received a more severe injury. In these results, many different histological findings between the young and adult rats in on day 1 and day 7 were identified. The degree of petechial hemorrhage, the degeneration of the neurons, infil- tration of the inflammatory cells, and vacuolar formations were more severe in the adult rats than in the young rats.
The data presented here suggest that adult rats appear to have more severe behavioral dysfunctiona of the hindlimb and neurodegeneration in the spinal cord compared with the young rats on day 7. These results suggest that factors related to neurodegeneration may be expressed dominantly in the spinal cord of adult rats after a weight-drop injury, or that factors related to neuroprotection and/or factors related to functional recovery may be expressed dominantly in the spinal cord of the young rats after a weight-drop injury.
Although differences in the behavioral test were observed between the two groups, an impairment of hindlimb move- ment was improved in the young and adult rats on day 7, but was markedly improved in the young rats. However,
the degeneration in the gray matter increased on day 7 in the adult rats compared with day 1. The degeneration in the gray matter and the white matter was not decreased on day 7 in the young rats compared with day 1. This suggests that morphological features in the spinal cord were not con- sistent with the functional changes in the hindlimb after a weight-drop injury. A functional recovery of the hind limb may be partly independent of the neurodegeneration in the L5 spinal cord after a L5 weight-drop injury. However, fur- ther studies will be needed.
The proto-oncogene c-Jun is believed to play a role in the control of growth and differentiation of many cell types. It was demonstrated previously that damage to the axons of peripheral motor or sensory neurons resulted within 24 hours in line with substantially increases in the levels of the c-Jun gene in the parent cell bodies. These increased levels of the c-Jun protein and messenger RNA are maintained if the damaged nerve is ligated, but return to the basal levels if the peripheral nerve is allowed to regenerate21,22). The induc- tion and phosphorylation of c-Jun may be involved in the cellular events leading to the death of neurons in vivo and
A B C
Fig. 6.GFAP immunoreactivities in the spinal cord weight-drop injury, ×100. (A, D) GFAP immunoreactivity of the spinal cord of adult and young control rat; Some difference was noted between the two groups. (B) No significant findings of GFAP immunoreactivity in the gray matter of an adult rat 1 day after injury. (C) Totally absent in the necrotic area but higher GFAP immunoreactivity of the gray mat- ter can be observed in an adult rat 7 days after injury. (E) Slight increase in the GFAP immunoreactivity of the gray matter of a young rat 1 day after injury. (F) Significant increase in the GFAP immunoreactivity of the gray matter of a young rat 7 days after injury.
D E F
this response can be modulated by the glial-cell-line-derived- neurotrophic factor19,20,29). The c-Jun expression level increased in the gray matter up to 7 days after the weight-drop injury in the adult rats and increased significantly in the gray mat- ter on day 1 in the young rats. However, compared with the 1st day after injury, the c-Jun expression level decreased sig- nificantly in the gray matter on day 7 in the young rats. This indicates that c-Jun expression in the spinal cord may be a maker of the early neuronal damage or death after a weight- drop injury. A decrease in the c-Jun expression level in the spinal cord on day 7 might reflect the decrease in the neu- rodegeneration in the spinal cord after a weight-drop injury in young rats compared with that of adult rats. In contrast, an increase in the c-Jun expression level in the spinal cord on day 7 may be related to severe neurodegeneration after a weight-drop injury in adult rats.
The accumulation of glial fibers is a histological landmark of an astrocyte response to injury, which is appropriately named fibrous gliosis. However, little is known about the disruption of the functional glial-neuronal interactions that can occur when the astrocytes respond to injury. An exam- ple would be the glutamate uptake system in reactive astro- cytes. If the uptake is enhanced, the neurons would acquire extra protection against the toxic effect of the excitatory neu- rotransmitter1,3,5,10,12,18,31,32). Alternatively, considering that the astrocyte machinery is switched to other tasks such as fibrillogenesis, the uptake system may be impaired.
GFAP expression in the gray matter increased 1 day after the weigh-drop injury in the adult rats. However, GFAP expression decreased in the necrotic region on day 7 in the adult rats. On the other hand, GFAP expression in the gray matter in the young rats increased gradually to 7 days after the injury. These results show that an increase in the GFAP expression level in the spinal cord may be closely related to the neuroprotective features in the spinal cord of young rats after a weight-drop injury.
While these results did not show a complete difference between young and adults rats and a means to control spinal cord regeneration, they do highlight areas for further study.
CONCLUSION
These results suggest that the lack of recovery from a spi- nal cord injury in adult rats may be related to the continu- ous upregulation of c-Jun expression and/or the downregu-
lation of GFAP after a weight-drop injury. In contrast, the upregulation of GFAP expression and/or the downregula- tion of c-Jun expression in the spinal cord may be related to a partial recovery in young rats after a weight-drop injury.
REFERENCES
1. Abraham KE, McGinty JF and Brewer KL: The role of kainic acid/AMPA and metabotropic glutamate receptors in the regulation of opioid mRNA expression and the onset of pain-related behavior following excitotoxic spinal cord injury. Neuroscience, 104: 863- 874, 2001.
2. Abraham KE and Brewer K: Expression of c-fos mRNA is in- creased and related to dynorphin mRNA expression following exci- totoxic spinal cord injury in the rat. Neurosci Lett, 307: 187-191, 2001.
3. Amundson RH, Goderie SK and Kimelberg HK: Uptake of [3H]serotonin and [3H]glutamate by primary astrocyte cultures.
II. Differences in cultures prepared from different brain regions.
Glia, 6: 9-18, 1992.
4. Angel P, Hattori K, Smeal T and Karin M: The jun proto-on- cogene is positively autoregulated by its product, Jun/AP-1. Cell, 55: 875-885, 1988.
5. Aoki C, Milner TA, Sheu KF, Blass JP and Pickel VM: Re- gional distribution of astrocytes with intense immunoreactivity for glutamate dehydrogenase in rat brain: implications for neuron- glia interactions in glutamate transmission. J Neurosci, 7: 2214- 2231, 1987.
6. Baldwin SA, Broderick R, Blades DA and Scheff SW: Alter- ations in temporal/spatial distribution of GFAP- and vimentin- positive astrocytes after spinal cord contusion with the New York University spinal cord injury device. J Neurotrauma, 15: 1015- 1026, 1988.
7. Basso DM, Beattie MS and Bresnahan JC: Graded histologi- cal and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol, 139: 244- 256, 1996.
8. Basso DM, Beattie MS and Bresnahan JC: A sensitive and reliable locomotor rating scale for open field testing in rats. J Neu- rotrauma, 12: 1-21, 1995.
9. Bohmann D, Bos TJ, Admon A, Nishimura T, Vogt PK and Tjian R:Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science, 238: 1386-1392, 1987.
10. Bouvier M, Szatkowski M, Amato A and Attwell D: The
glial cell glutamate uptake carrier countertransports pH-changing anions. Nature, 360: 471-474, 1992.
11. Cotran RS, Kumar VK and Robbins SL: Pathologic basis of disease. W.B. Sunders Co., London, pp. 1297, 1994.
12. Derouiche A and Frotscher M: Astroglial processes around iden- tified glutamatergic synapses contain glutamine synthetase evidence for transmitter degradation. Brain Res, 552: 346-350, 1991.
13. Dijkstra S, Geisert EE JR, Gispen WH, Bar PR and Joosten EA:Up-regulation of CD81 (target of the antiproliferative anti- body; TAPA) by reactive microglia and astrocytes after spinal cord injury in the rat. J Comp Neurol, 428: 266-277, 2000.
14. Eilers A, Whitfield J, Babij C, Rubin LL and Ham J: Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci, 18: 1713-1724, 1998.
15. EM Jr: Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apopto- sis. J Cell Biol, 127 (6 Pt 1): 1717-1727, 1994.
16. Griffiths R, Malcolm C, Ritchie L, et al: Association of c-fos mRNA expression and excitotoxicity in primary cultures of mouse neocortical and cerebellar neurons. J Neurosci Res, 48: 533-42, 1997.
17. Guegan C, Levy V, David JP, Ajchenbaum-Cymbalista F and Sola B:c-Jun and cyclin D1 proteins as mediators of neu- ronal death after a focal ischaemic insult. Neuroreport, 8: 1003- 1007, 1997.
18. Hansson E and Ronnback L: Receptor regulation of the gluta- mate, GABA and taurine high-affinity uptake into astrocytes in primary culture. Brain Res, 548: 215-221, 1991.
19. Hayashi M, Ueyama T, Nemoto K, Tamaki T and Senba E:
Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury, J Neurotrauma, 17: 203- 218, 2000.
20. Herdegen T and Leah JD: Inducible and constitutive transcrip- tion factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev, 28: 370-490, 1998.
21. Jenkins R, McMahon SB, Bond AB and Hunt SP: Expres- sion of c-Jun as a response to dorsal root and peripheral nerve sec- tion in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci, 5: 751-759, 1993.
22. Jenkins R, Tetzlaff W and Hunt SP: Differential expression of immediate early genes in rubrospinal neurons following axoto- my in rat. Eur J Neurosci, 5: 203-209, 1993.
23. Jenkins R and Hunt SP: Long-term increase in the levels of c-
jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett, 129: 107- 110, 1991.
24. Eah JD, Herdegen T and Bravo R: Selective expression of Jun proteins following axotomy and axonal transport block in periph- eral nerves in the rat: evidence for a role in the regeneration pro- cess. Brain Res, 566: 198-207, 1991.
25. Li S and Stys PK: Mechanisms of ionotropic glutamate recep- tor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci, 20: 1190-1198, 2000.
26. Liu D, Xu GY, Pan E and McAdoo DJ: Neurotoxicity of glu- tamate at the concentration released upon spinal cord injury. Neu- roscience, 93: 1383-1389, 1999.
27. Liu XZ, Xu XM, Hu R, et al: Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci, 17: 5395-5406, 1997.
28. Nakamura M and Bregman BS: Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol, 169: 407-415, 2001.
29. Oo TF, Henchcliffe C, James D and Burke RE: Expression of c-fos, c-jun, and c-jun N-terminal kinase (JNK) in a develop- mental model of induced apoptotic death in neurons of the sub- stantia nigra. J Neurochem, 72: 557-564, 1999.
30. Pekny M, Johansson CB, Eliasson C, et al: Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol, 145: 503-514, 1999.
31. Schousboe A, Svenneby G and Hertz L: Uptake and meta- bolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem, 29: 999-1005, 1997.
32. Szatkowski M, Barbour B and Attwell D: Non-vesicular release of glutamate from glial cells by reversed electrogenic gluta- mate uptake. Nature, 348: 443-446, 1990.
33. Vaudano E, Rosenblad C and Bjorklund A: Injury induced c-Jun expression and phosphorylation in the dopaminergic nigral neurons of the rat: correlation with neuronal death and modula- tion by glial-cell-line-derived neurotrophic factor. Eur J Neurosci, 13: 1-14, 2001.
34. Vaudano E, Campbell G, Hunt SP and Lieberman AR: Axo- nal injury and peripheral nerve grafting in the thalamus and cere- bellum of the adult rat: upregulation of c-jun and correlation with regenerative potential. Eur J Neurosci, 10: 2644-2656, 1998.
35. Yezierski RP, Santana M, Park SH and Madsen PW: Neu- ronal degeneration and spinal cavitation following intraspinal injec- tions of quisqualic acid in the rat. J Neurotrauma, 10: 445-456, 1993.
목 적: 어린 쥐와 성숙 쥐의 척수 손상에서 나타나는 행동상과 면역 조직 화학상의 변화의 차이를 비교함으로써 척수 손상 에서 나타나는 변화의 기전 차이를 설명하고자 하였다.
대상 및 방법: 생후 5주된 수컷 쥐 25마리와 16주된 수컷 쥐(Spraque-Dawley rat) 25마리가 사용되었다. 쥐들을 페노바비 탈로 마취시킨 후 제 11번, 12번 흉추 위치에서 추궁절제술을 시행하였으며 개량된 뉴욕 대학 충격기(modified New York University impactor)를 이용하여 20 mm 높이에서 10 gm의 충격을 줘서 척수 손상을 유발하였다. 이에 따른 배뇨장애를 방지하기 위해 매일 2회씩 손으로 눌러 방광을 비워주었으며, 수술을 받지 않은 쥐를 정상 대조군으로 사용하였다. 척수 손상을 입은 쥐들에 대한 행동 시험을 Basso-Beatti-Bresnahan (BBB) 가산표를 이용하여 시행하였고, 행동 시험은 척수 손상 후 제 1, 7일에 시행하였다. 쥐들의 손상된 척수를 수술 후 제 1, 7일에 절개하였으며, 척수 절편에 H-E 염색과c-Jun 과GFAP유전자에 대한 면역조직화학 검사를 시행하였다. 면역 반응은 상온에서 1시간 동안 말초 혈액 도말 내의 avidin- biotin-peroxidase 복합체 내에서 그리고 0.05% 3,3 -diaminobenzidine과 0.01% 과산화 수소에서 5-10분간 반응 시켰다.
결 과: 생후 5주된 쥐들의 뒷다리는 수술 1일 이후 마비되었고 수술 7일후에 부분적으로 회복되었으나, 생후 16주된 쥐들 의 경우 수술 후 7일째에도 마비된 상태로 남아있었다. 생후 16주된 쥐들에서는 손상 받은 척수의 회백질에서c-Jun유전 자의 발현이 7일째까지 증가했으며, 생후 5주의 쥐들에서는c-Jun유전자의 발현이 수술 1일째 상당히 증가하였으나, 회
백질의c-Jun유전자의 발현은 수상 후 7일째부터 감소하였다. 회백질의GFAP유전자의 발현은 생후 16주의 성인 쥐들에
서는 수상 후 제 1일째부터 증가되기 시작하였으나 수상 제 7일째부터 괴사부분에서 감소되기 시작하였고, 생후 5주의 어 린 쥐들이 회백질에서는GFAP유전자 발현이 수상 후 7일째까지 점진적으로 증가하였다.
결 론: 나이가 많은 쥐들에서 척수 손상이 회복되지 않은 것은 척수 손상 후c-Jun유전자 발현의 지속적인 상향조절 또는 GFAP유전자의 하향조절과 연관되어 있을 가능성을 제시한다. 반대로, GFAP유전자의 상향조절 또는c-Jun유전자의 하 향 조절은 동일한 손상을 받은 어린 쥐들에서 척수 손상의 부분 회복과 관련되어 있는 것으로 사료된다.
색인 단어: 척수 손상, 행동상 시험, c-Jun, GFAP
어린 쥐와 성숙 쥐에서 척수 손상 후 행동상과 면역조직학상 변화에 대한 비교 연구
양준영ㆍ이준규ㆍ이광진ㆍ김경천ㆍ홍의표ㆍ이정범
충남대학교 의과대학 정형외과학교실
′