한국보건의료기술평가학회 > 학회지 > 시장진입을 위한 치료재료 신의료기술평가 제도: 의료법, 의료기기법, 국민건강보험법 중심으로
전체 글
수치
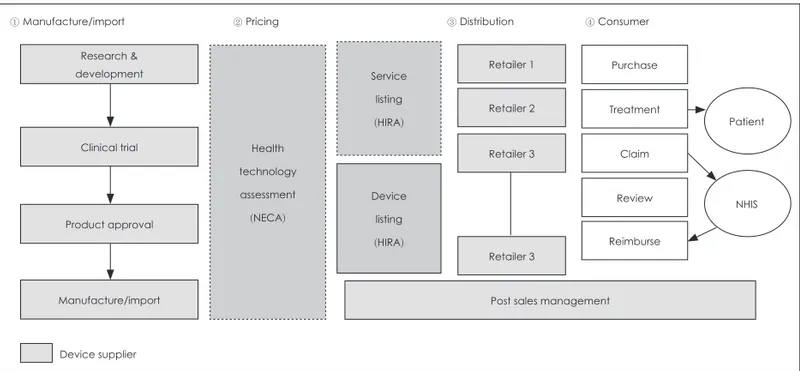
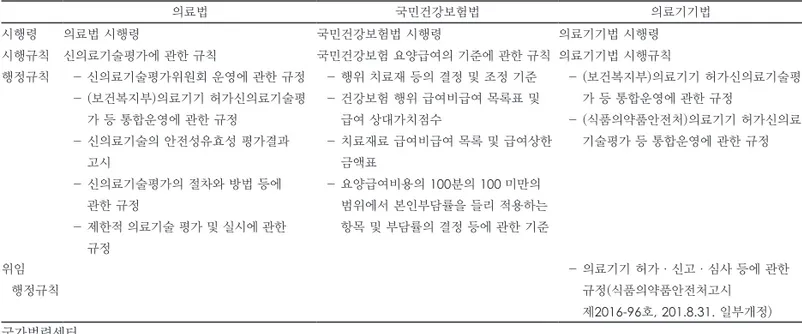
관련 문서
(2020) National Policy on COVID-19 Response & Current Status, Ministry of Health and Welfare, Ministry of Foreign Affairs, Korea Institute of Health and
A fundamental feature of the welfare state is social insurance , a provision common to most advanced industrialized countries (e.g., National Insurance in the United..
Ministry of Economic Affairs, R.O.C. Innovation and Incubation Center, National Kaohsiung University of Science and Technology 、 Institute For Information
Hagen, “The Efficacy Requirements of the Food, Drug and Cosmetic Act”, Food Drug Cosm.. Bodenheimer, “The drug Amendments of 1962 : The anatomy of a Regulatory
Relationship between Metabolic Syndrome and Mental Health in Korean adults; Using the 7th Korea National.. Health and
···108 Table 6.4 An example of safety level adopted by National Environmental Dispute Mediation Commission (Ⅰ) ···109 Table 6.5 An example of safety level adopted
socioeconomic status and thyroid cancer prevalence; Based on the korean National Health and Nutrition Examination Survey2010-2011. Jung YI, Kim
School of Mechanical and Aerospace Engineering Seoul National University..