Enucleation of Pancreatic Endocrine Tumor Following Pancreatic Duct Stenting: A Case Report
Pancreatic fistula is the most frequent complication after pancreatic resection regardless of the extent of the resection. A 68-year-old woman with B-viral hepatitis was referred with an incidentally detected pancreatic head mass that was diagnosed 4 months previously when performing following up of her liver cirrhosis. She had no specific symptoms, but she had a 1.2 cm sized solitary mass that was suspected to be a pancreatic endocrine tumor and it was located very close to the main pancreatic duct in the pancreas uncinate process on the imaging workup.
Preoperative endoscopic pancreatic stenting was prepared to guide the enucleation of the mass while identifying the pancreatic duct using intraoperative ultrasonography. Precise intraoperative estimation of the mass and the pancreatic duct was possible and the enucleation was successful without injury to the duct. We recommend this operative approach especially when planning local pancreatic resection for tumors in the pancreatic head or uncinate process, as these tumors make the pancreatic duct vulnerable to injury.
Sun Choon Song, M.D., Min Jung Kim, M.D., Woo Seok Kim, M.D., Dong Wook Choi, M.D., Jin Seok Heo, M.D., Seong Ho Choi, M.D.
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine
Corresponding Author Seong Ho Choi
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50, Irwon-dong, Gangnam-gu, Seoul 135-710, Korea
Tel: +82-2-3410-3468 Fax: +82-2-3410-6980
E-mail: sh3468.choi@samsung.com
Key Words : Pancreatic endocrine tumor, Pancreatic duct stent, Pancreatic fistula
Received: 2010. 7. 27.
Accepted: 2010. 10. 1.
Introduction
The rate of pancreatic origin in all gastrointestinal endocrine tumor is reported to be about 43%.1 It is usually non-functional without an associated syndrome and less aggressive than exocrine carcinoma. But more than half of the pancreatic endocrine tumors (PET) are detected to be already metastatic at the time of diagnosis and are known to have more unfavorable outcome than the origin of other organs.2 Overall 5-year survival rate ranges from 30% in
metastatic PET to 100% in benign tumor.2,3
In non-metastatic localized PET, surgery is the treatment of choice with high success rate.2 But even the minor disruption of pancreatic duct during pancreatic resection can lead to pancreatic fistula, stricture or pancreatitis.4 Pancreatic fistula is known to be most frequent compli- cation after pancreatic resection, irrespective of operative approach or the range of resection.5-7 Here, we report the treatment of a case with single, small PET in uncinate process with high risk of postoperative pancreatic fistula by performing enucleation with the aid of intraoperative
ultrasonography after endoscopic pancreatic stenting.
Case Report
A 68- year-old woman admitted with incidental findings of pancreatic head mass diagnosed 4 months ago. In her past history, she has taken medication (CoaprovelⓇ) for hypertension for 5 years ago and was regularly followed up for B viral liver cirrhosis (Child class A) with anti-viral medication (EntecavirvⓇ) from 2 years ago. She had no previous operation history. Her both parents died of liver cirrhosis and her brother died of hepatocellular carcinoma.
At the time of admission, her vital signs showed blood pressure 103/56 mmHg, pulse rate 80/min, respiratory rate 20/min, body temperature 36.6oC and the body mass index (BMI) was 24.3 kg/m2. She did not complain hypoglycemic symptoms, plethora, or any abdominal symptoms such as
abdominal pain, epigastric soreness or nausea. No specific mass-like lesions were palpated on her abdomen. In her peripheral blood sample test at admission showed hemo- globin 12.4 mg/dl, hematocrit 37.3%, white blood cell count 6,850/mm3, platelet count 219,000/mm3, albumin 4.1 g/dl, aspartate transaminase (AST) 22 IU/L, alanine transa- minase (ALT) 20 IU/L, total bilirubin 0.5 mg/dl, inter- national normalized ratio (INR) 1.12, amylase 54.9 IU/L, lipase 35.4 IU/L, alfafeto protein (AFP) 2.4 ng/ml and protein induced by vitamin K absence/antagonist-II (PIVKA-II) to be 15 mAU/ml.
A 1.2 cm sized mass was shown in pancreatic uncinate process with high density in arterial phase of abdominal CT and with low-signal in T1-weighted image with closed to main pancreatic duct in magnetic resonance cholangio- pancreatography (MRCP). In this image, enlarged lymph nodes or distant metastasis were not existed. In endoscopic
Fig. 1. (A) An arterial-enhanced 1.5cm sized well-demarcated mass (solid arrow) was found in pancreatic uncinate process at initial computed tomography (CT). (B) In magnetic resonance cholangiopancreatography (MRCP) a mass (dotted arrow) showed low signal in T1-weighted image, and was located close to main pancreatic duct (arrow head), which was not dilated. (C) A low echoic mass was seen in pancreatic uncinate process in endoscopic ultrasonography (EUS).
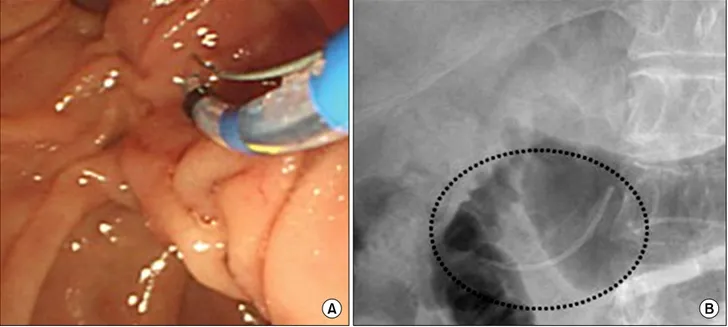
Fig. 2. (A) Preoperative plastic stent was inserted to pancreatic duct for guiding wedge resection or enucleation of pancreatic tumor. (B) X-ray view of pancreatic stent (dotted circle) after placement into the main pancreatic duct.
ultrasonography (EUS), a low echoic mass was found in pancreatic uncinate process (Fig. 1). At the time of diagnosis, she denied to do further management because she had not complained any symptoms related with the tumor.
We planned to perform preoperative endoscopic panc- reatic stenting. So the endoscopic retrograde cholangiopan- creatography (ERCP) was performed at fifth days prior to the surgery. Papilla looked normal finding and after small endoscopic papillary sphincterotomy by papillotome, a 5-Fr 5-cm plastic stent was inserted to pancreatic duct for guiding enucleation or wedge resection of pancreatic tumor (Fig. 2).
In the operative filed, the mass was located in the posterior portion of pancreatic head and it was able to be palpated. Intraoperative ultrasonography showed the distance between the hypoechoic mass and pancreatic duct stent with posterior acoustic shadowing, which had only within 1 cm distance each other (Fig. 3). After careful enucleation of the mass with bipolar electrocauterization, enough remnant pancreatic parenchyma was identified between resection plane and pancreatic duct stent.
Intraoperative frozen biopsy confirmed the enucleated mass to be pancreatic endocrine tumor. Minimal hemorrhage was successfully controlled with fibrin glue. Two closed suction drains (Jackson Pratts) were placed close to enucleation bed.
Any complications did not occur with remaining normal pancreatic enzyme level throughout the postoperative course. The pancreatic stent was removed out at seventh postoperative day and safely discharged on the eighth postoperative day. Pathologic evaluation revealed the specimen was found to a 9×8 mm sized well-differentiated pancreatic endocrine tumor with negative resection margin.
Tumor cells were stained with haematoxylin & eosin and immunohistochemical synaptophysin (Fig. 4). The expre- ssion of Ki-67 was 2% and CK9, CD10, S-100 were negative.
She is currently asymptomatic after 2 months of follow-up.
Discussion
Limited pancreatic resection or enucleation is generally recommended in the treatment of benign or low-grade malignant pancreatic tumor including intraductal papillary
Fig. 3. (A) Intraoperative ultrasonography showed the relationship between the low echoic mass (arrow head) and the pancreatic duct stent (dotted circle) with acoustic shadowing. (B) After enucleation of the mass, the stent was present in the pancreatic duct with enough distance from resection plane.
Fig. 4. Enucleation of pancreatic tumor (A) and cut section of the specimen (B). Tumor cells are arr- anged with trabecular pattern for- ming encapsulated fibrotic mass (H&E stain, ×100) (C). Immuno- histochemical staining with synap- tophysin (D).
mucinous neoplasm, mucinous cystic neoplasm, serous cystadenoma and non-functioning endocrine tumor.6-8 Although it helps to preserve pancreatic parenchyma so to maintain the exocrine and endocrine pancreatic function,
but the pancreatic fistula caused by accessory pancreatic duct injury also can be developed.7 Pancreatic fistula can cause various complications including pancreatitis, abscess, bleeding, pseudocyst or sepsis. The incidence of the fistula
ranges about from 10% to 30% after distal pancreatectomy or pancreatoduodenectomy, but that after pancreatic enucleation with duct stent has not yet been reported.9 The practical uses of pancreatic duct stenting in the treatment of some pancreatic disease associated with pancreatic duct injury are previously reviewed in several reports, such as in blunt traumatic pancreatic injury with ductal disruption, post-ERCP pancreatitis, pancreatic duct stricture, pancreatic divisum, dysfunction of the sphincter of Oddi, malignant pancreatic duct obstruction or prophylaxis of pancreatic fistula after distal pancreatectomy.10 Most of them are aimed to decompress the pancreatic duct so to lower the risk of peripancreatic fluid collection, pancreatic fistula, chronic abdominal pain or pancreatitis.
In this case, preoperative endoscopic pancreatic stenting was designed not only for preventing pancreatic fistula formation by leakage but also for the guidance to intraoperative detection of pancreatic duct. Precise intrao- perative estimation of pancreatic duct was essential because it was found that the mass was very close to pancreatic duct in preoperative image. Intraoperative ultrasonography was very helpful in the localization of the tumor and identification of the relationship between the tumor and pancreatic duct, as previously be reported.7,8 In addition, it is expected that this stenting helps to prevent progressing to more severe complications after the fistula is once developed.
But some disadvantages from the placement of pancreatic stent have to be perceived. Long-term or repeated use the stent can cause ductal stricture in addition to pancreatitis, stent migration or occlusion.6,7,10 In the current case, transient pancreatitis was developed immediately after the placement of the stent, which resulted in delayed surgery, but stent occlusion did not occur until it was removed at seventh day postoperatively. Further investigations about the appropriate interval between the stent placement and surgery or between the surgery and the time of stent removal will be needed.
Widely used laparoscopic pancreatic resection can be
applied with the aid of intraoperative ultrasonography after pancreatic stenting especially in solitary, small tumor located in body or tail portion.5,8 But it has yet some limitations while the tumor located in the posterior aspect of pancreatic head or uncinate process usually have difficulties to expose, localize and to resect the tumor, especially when it is closed with the pancreatic duct or when the tumor involve vascular or surrounding structures.
Some additional surgical techniques might be considerable to prevent thermal injury to adjacent pancreatic duct after enucleation through our experience. Peripancreatic dissection and enucleation of the mass were performed using bipolar electocautery and fibrin glue itself was effective in the hemostasis. Besides, it could influence to seal the pancreatic resection plane.
In summary, preoperative endoscopic stenting was feasible for enucleation of pancreatic head or uncinate process mass with the aid of intraoperative ultrasono- graphy, especially in pancreatic duct which is not dilated and close to the mass making it hard to be identified and vulnerable to be injured.
References
1. Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083-1092.
2. Dimou AT, Syrigos KN, Saif MW. Neuroendocrine tumors of the pancreas: what's new. Highlights from the "2010 ASCO Gastrointestinal Cancers Symposium". Orlando, FL, USA.
January 22-24, 2010. JOP 2010;11:135-138.
3. Pape UF, Böhmig M, Berndt U, Tiling N, Wiedenmann B, Plöckinger U. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci 2004;1014:
222-233.
4. Abe T, Nagai T, Murakami K, et al. Pancreatic injury successfully treated with endoscopic stenting for major pancreatic duct disruption. Intern Med 2009;48:1889-1892.
5. España-Gómez MN, Velázquez-Fernández D, Bezaury P, Sierra M, Pantoja JP, Herrera MF. Pancreatic insulinoma: a surgical experience. World J Surg 2009;33:1966-1970.
6. Hirota M, Kanemitsu K, Takamori H, et al. Local pancreatic resection with preoperative endoscopic transpapillary stenting. Am J Surg 2007;194:308-310.
7. Lin BC, Liu NJ, Fang JF, Kao YC. Long-term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc 2006;20:1551-1555.
8. Kang CM, Lee KG, Pyo JY, et al. Laparoscopic enucleation of a nonfunctioning neuroendocrine tumor of the pancreas.
Yonsei Med J 2008;49:864-868.
9. Abe N, Sugiyama M, Suzuki Y, Yamaguchi T, Mori T, Atomi Y. Preoperative endoscopic pancreatic stenting: a novel prophylactic measure against pancreatic fistula after distal pancreatectomy. J Hepatobiliary Pancreat Surg 2008;15:
373-376.
10. Shimura T, Suehiro T, Suzuki H, et al. Preoperative endoscopic pancreatic stenting for prophylaxis of pancreatic duct disruption during extirpation of a pancreatic head tumor. Am J Surg 2007;194:553-555.