Introduction
Advances in cancer therapeutics during recent decades
have resulted in longer disease-free survival periods.
This success in cancer treatment raises concerns about the potential effects of chemotherapeutic regimens on the quality of life of patients after chemotherapy (CTx).
CTx commonly induces a distal, symmetric peripheral polyneuropathy (PPNP), for which, in general, there is no specific treatment.1 Herein, we report a case in which paclitaxel-based CTx aggravated the pre-existing carpal tunnel syndrome (CTS) without the development
파클리탁셀 화학요법 후에 발생한 수근관증후군의 악화에 관한 증례보고
도현정, 김혜림, 김민욱, 장대현
가톨릭대학교 인천성모병원 재활의학과
Aggravated Carpal Tunnel Syndrome after Paclitaxel Chemotherapy without Development of Peripheral Polyneuropathy - A Case Report -
Hyun-Jung Do, Hae-Rim Kim, Min-Wook Kim, Dae-Hyun Jang
Department of Rehabilitation, The Catholic University of Korea, Incheon St. Mary's Hospital, Incheon, Korea
Received December 19, 2014
Revised (1st) February 5, 2015, (2nd) February 26, 2015 Accepted February 26, 2015
Corresponding Author: Dae-Hyun Jang
Department of Rehabilitation Medicine, The Catholic University of Korea, Incheon St. Mary’s Hospital, 56 Dongsu-ro, Bupyeong-gu, Incheon 403- 720, Korea
Tel: 82-32-280-5207, Fax: 82-32-280-5040, E-mail: dhjangmd@naver.com
Functionally disabling peripheral neuropathy is a major side effect of many chemotherapeutic agents. Chemotherapy (CTx) commonly induces distal, symmetric peripheral polyneuropathy (PPNP), for which there is no specific treatment.
We report a case in which paclitaxel-based CTx did not induce PPNP but instead aggravated the pre-existing carpal tunnel syndrome (CTS), which improved after surgical decompression. Because subclinical entrapment neuropathy is not uncommon, we suggest evaluating the pre-existing neuropathy before starting CTx. If a focal entrapment neuropathy, including CTS, is found, patients should be warned of the possibilities of belated aggravation of such neuropathies with CTx, and if possible, regular follow-up with nerve conduction studies and needle electromyography should be performed for early diagnosis. Furthermore, because prompt surgical correction is likely to improve the cases of aggravated CTS and possibly other focal entrapment neuropathies, early diagnosis and immediate intervention will lead to better functional outcomes.
Key Words: carpal tunnel syndrome, paclitaxel, polyneuropathies
Copyright © by Korean Association of EMG Electrodiagnostic Medicine
This is an Open Ac cess article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
J Korean Assoc
Electrodiagn MedEMG CASE REPORT
ISSN 1229-6066 http://dx.doi.org/10.18214/jkaem.2015.17.1.25 J Korean Assoc EMG Electrodiagn Med 17(1):25-30, 2015
of electrodiagnostically confirmed PPNP, which showed improvement after surgical decompression.
Case Report
A 53-year-old female visited our physical medicine and
rehabilitation clinic complaining of numbness in both feet and hands, starting after the initiation of paclitaxel CTx and lasting after the CTx. She had been diagnosed with cancer in her right breast a year ago and had undergone modified radical mastectomy with axillary lymph node dissection. She then received four cycles of CTx with
Table 1. Serial Nerve Conduction Studies
Date Initial visit 4 months 6 months Post-op 2 months
Sensory Amp (mV) Lat Pk (msec) Amp (mV) Lat Pk (msec) Amp (mV) Lat Pk (msec) Amp (mV) Lat Pk (msec) Left median
Wrist Not evoked Not evoked Not evoked Not evoked
(Reference value) (20.0) (3.6)
Palm 4.8 2.30 2.8 2.35 Not evoked Not evoked
Right median Wrist Palm
Not studied 7.9
4.90
10.5 2.35
9.8 5.25
7.9 2.30
8.1 4.75
9.1 2.55 Left ulnar
(Reference value)
19.3 (10.0)
3.1 (3.6)
24 3.2 Not studied 24.5 3.10
Left sural (Reference value)
10.2 (10.0)
3.55 (3.9)
Not studied Not studied 10.2 3.45
Right sural 8.5 3.45 Not studied Not studied 10.6 3.25
Left supf peron (Reference value)
12.0 (5.0)
3.40 (4.2)
Not studied Not studied 8.3 3.50
Right supf peron 9.9 3.50 Not studied Not studied 8.3 3.45
Motor Amp
(mV)
Lat onset (msec)
Amp (mV)
Lat onset (msec)
Amp (mV)
Lat onset (msec)
Amp (mV)
Lat onset (msec) Left median
Wrist
(Reference value) Elbow
3.7 (5.0)
3.6
7.70 (4.2) 49.4*
Not evoked Not evoked 0.5
0.5
12.55
17.95 Right median
Wrist Elbow
Not studied 7.7
4.45
6.9 51.2*
5.7 4.95
5.1 45.0*
6.3 5.3
4.55 53.0*
Left ulnar Wrist
(Reference value) Elbow
9.0 (5.0)
8.7
3.05 (4.2) 59.0*
9.6
9.1
2.85
61.5*
Not studied 9.8
9.2
2.75
60.9*
Right peroneal Ankle
(Reference value) Fibular head
3.9 (2.0)
3.6
3.35 (6.1) 46.4*
Not studied Not studied Not studied
Left peroneal Ankle Fibular head
3.4 3.0
3.95 48.7*
Not studied Not studied Not studied
Right tibial Ankle
(Reference value) Knee
15.5 (5.0) 11.7
3.55 (5.1) 46.3*
Not studied Not studied 19.8
12.8
3.50
48.7*
Left tibial Ankle Knee
17.4 12.7
3.35 46.4*
Not studied Not studied 18.9
13.9
3.85 49.7*
*Velocity (m/s)
Amp: amplitude, Lat: latency, Pk: peak, supf peron: superficial peroneal
cyclophosphamide and doxorubicin at doses of 600 mg/
m2 and 60 mg/m2 per cycle, respectively, without the development of any sensory symptoms. Further, she underwent paclitaxel CTx, and soon after the first cycle of treatment with 175 mg/m2 paclitaxel, she developed distal numbness. She finished four cycles of paclitaxel CTx, and distal numbness persisted even after discontinuing the drug. During or after the CTx, she did not use her hands excessively. She had past history of right carpal tunnel release 14 years ago. After the initial carpal tunnel release surgery, she had no definite CTS-related symptoms in either hand until the CTx was started. The findings of nerve conduction studies (NCSs) and needle electromyography (EMG) showed bilateral, mild CTS with median compound motor action potentials (CMAPs) in the borderline range four years after the first surgery. On physical examination soon after the CTx was finished, motor power was intact and paresthesia was present in all distal extremities with equivocal hypesthesia.
She underwent NCS to identify the possibility of PPNP.
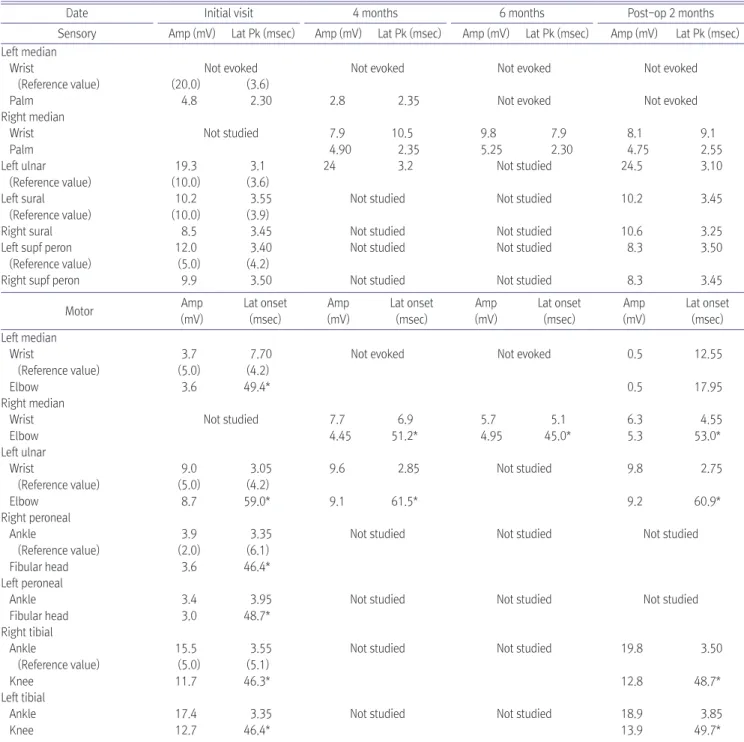
The study showed no definite PPNP but revealed left CTS with mildly decreased CMAP (Table 1, Fig. 1). Four months later, gene ralized paresthesia disappeared, but hypesthesia in the left median dermatome had become evident. Follow-up NCSs showed a full aggravation of left CTS with an absent left median CMAP (Table 1, Fig.
1). On needle EMG, abnormal spontaneous activities and maximally reduced recruitment patterns of motor unit action potentials (MUAPs) were indicated (Table 2). And ultrasonographic findings showed a marked swelling of the left median nerve at the wrist (Fig. 2). Therefore, the patient was advised to undergo carpal tunnel release, but she refused the surgery. Two months later, her thenar muscle atrophy had progressed and a follow-up NCS showed a further aggravated CTS of the left median nerve: absent sensory nerve action potential (SNAP) when stimulated on the palm (Table 1). And left median nerve more swelled on the ultrasonography (Fig. 2). She then
1 2
3
A B
C D
WRIST 1 WRIST 1
30 ms 5 mV 0.00 mA
ELBOW 2 30 ms 5 mV 0.00 mA 30 ms 5 mV 84 mA
1 2
3 ELBOW 2
30 ms 5 mV 100 mA
WRIST 1 30 ms 5 mV 43 mA
ELBOW 2 30 ms 5 mV 43 mA
1 2
3 WRIST 1
50 ms 500 V 33 mAm
50 ms 500 V 33 mAm 1
23
ELBOW 2 L MEDIAN-APB
L MEDIAN-APB L MEDIAN-APB
Fig. 1. Serial compound motor action potentials (CMAPs) on left abductor pollicis brevis muscle. (A) CMAPs at initial visit. (B) CMAPs at 4 months after. (C) CMAPs at 6 months after. (D) CMAPs at post op. 2 months after.
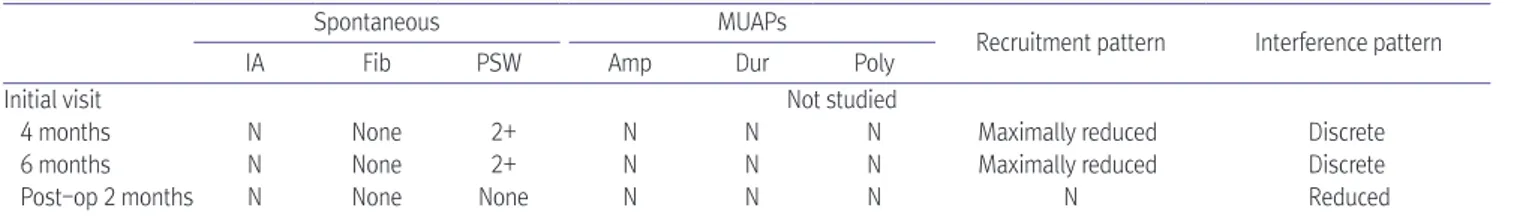
Table 2. Serial Needle Electromyography on Left Abductor Pollicis Brevis Muscle
Spontaneous MUAPs
Recruitment pattern Interference pattern
IA Fib PSW Amp Dur Poly
Initial visit Not studied
4 months N None 2+ N N N Maximally reduced Discrete
6 months N None 2+ N N N Maximally reduced Discrete
Post-op 2 months N None None N N N N Reduced
MUAP: motor unit action potential, IA: insertional activity, Fib: fibrillation potential, PSW: positive sharp wave, Amp: amplitude, Dur: duration, Poly:
polyphasia, N: normal
Other needle electromyography findings showed no abnormality
A B
C D
E F
Fig. 2. Ultrasonographic findings related to the median nerve. (A) Transverse scan of the left median nerve at the wrist (just proximal to pisiform bone) in 4 months after initial visit, showing marked swelling over an area of 0.234 cm2. (B) Transverse scan of the left median nerve at the forearm in 4 months, over an area of 0.043 cm2. (C) Transverse scan of the right median nerve at the wrist in 4 months, over an area of 0.142 cm2. (D) Transverse scan of right median nerve at the forearm in 4 months, over an area of 0.046 cm2. (E) Transverse scan of the left median nerve at the wrist in 6 months, over an area of 0.271 cm2. (F) Transverse scan of the right median nerve at the wrist 6 months, over an area of 0.149 cm2. Outlines indicate the median nerve.
underwent left carpal tunnel release. Two months after the surgery, follow-up NCS and EMG showed a partial recovery of the CMAP amplitude, normalized recruitment patterns of MUAPs, and no evidence of PPNP (Tables 1, 2, Fig. 1).
Discussion
Paclitaxel is used for treating several solid cancers, and it is known to predominantly lead to sensory neuropathy in a dose-dependent manner.2 To the best of our knowledge, there has been no previous report of a case in which paclitaxel CTx aggravated a pre- existing focal entrapment neuropathy, like CTS, without the development of PPNP. The mechanism of enhanced vulnerability to toxic neuropathy in the presence of a pre- existing focal entrapment neuropathy is not known. It can be hypothesized that the focal nerve segment already injured due to entrapment may be more easily affected by the toxic effects of CTx.1 It has been postulated that a decrease in rates of axoplasmic flow resulting from chemotherapy could make the peripheral nerve susceptible to chronic nerve compression.3 Moreover, the aggravation of Charcot-Marie-Tooth-disease upon treatment with vincristine is a well-known phenomenon.2 Pre-existing diabetic or alcoholic neuropathy is known to increase the severity of cases of mixed axonal- demyelinating neuropathy.4 Focal entrapment neuropathy has not been designated as a risk factor for the development of severe neuro pathy after CTx; however, based on the present case, we can deduce that CTx- related vulnerability is more increased in such cases.
In terms of pathophysiology, paclitaxel is believed to cause neuropathy by its effects on microtubules.
Conflicting evidence suggests that paclitaxel-induced sensory neuropathy may be due to an axonopathy, a neuronopathy (dorsal root ganglionopathy), a Schwann cell abnormality, or a combination of these mechanisms. In addition, it was proposed that microtubules in the anterior horn cells play a role in the pathogenesis of weakness resulting from paclitaxel CTx.2 This effect of microtubule dysfunction may have contributed to the aggravation of
the entrapment neuropathy in the present case.
It is noteworthy that the aggravation of CTS was delayed by several months after the last cycle of paclitaxel.
Paclitaxel-induced neuropathy generally is known to start as soon as the first cycle is applied. However, sensory abnormalities and pain due to paclitaxel-induced neuropathy is known to sometimes become a chronic problem.5 For example, van den Bent et al.6 reported the progression of paclitaxel-induced neuropathy following the discontinuation of the drug. The delayed aggravation of the neuropathy in the present case indicates that the toxic effect of CTx in the diseased nerve segment should be considered even long after the cessation of CTx.
Although the causes of CTS are various, the aggravation of CTS was very rapid in this case and the patient did not use her hands excessively after the CTx. So the aggravation of CTS seems to be due to delayed effect of paclitaxel CTx in this case. Further, if the patient had been warned about the possibility of the aggravation of CTS, she could have focused more on the related symptoms and reported them sooner, which could have resulted in an earlier diagnosis and intervention.
The CTx-induced aggravation of focal entrapment neuropathy is noteworthy because it is surgically treatable if found during the critical early period. Although a variety of neuroprotective approaches have been investigated in both experimental studies and clinical trials, there is no available preventive strategy or effective treatment for chemotherapy-induced PPNP.7 On the contrary, in the present case of entrapment neuropathy, even the delayed surgical intervention resulted in both clinical and electrodiagnostic improvements. If the surgery had been performed earlier, the axonal loss shown as an absent median CMAP could have been minimized. An early diagnosis facilitates a prompt intervention; thus, an awareness of the possibility of the aggravation of a pre- existing neuropathy is critical among treating physicians and patients.
In conclusion, an evaluation of the pre-existing neuropathies before the start of CTx can be considered, and if a focal entrapment neuropathy is found, the
patient should be warned of the possibility of a delayed aggravation of their neuropathies. Further more, if possible, regular follow-up NCSs and EMG examinations should be performed. Unlike CTx-induced PPNP, the aggravation of focal entrapment neuropathy can likely be improved by a prompt surgical correction.
References
1. Chaudhry V, Chaudhry M, Crawford TO, Simmons-O'Brien E, Griffin JW. Toxic neuropathy in patients with pre-existing neuropathy. Neurology 2003;60:337-40
2. Freilich RJ, Balmaceda C, Seidman AD, Rubin M, DeAngelis LM. Motor neuropathy due to docetaxel and paclitaxel.
Neurology 1996;47:115-8
3. Upton ARM, McComas AJ. The double crush in nerve entrapment syndromes. Lancet 1973;2:359-361
4. Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity.
Breast Cancer 2004;11:82-5
5. Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 2001;94:293-304 6. van den Bent MJ, van Raaij-van den Aarssen VJ, Verweij
J, Doorn PA, Sillevis Smitt PA. Progression of paclitaxel- induced neuropathy following discontinuation of treatment.
Muscle Nerve 1997;20:750-2
7. Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine 2012;59:3-9