저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Effect Of Mesenchymal Stem Cells
In Neurodegenerative Disease
by
Hyun Jung Park
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Effect Of Mesenchymal Stem Cells
In Neurodegenerative Disease
by
Hyun Jung Park
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Ph. D. in Biomedical Sciences
Supervised by
Phil Hyu Lee, M.D., Ph.D.
Young Hwan Ahn, M.D., Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Hyun Jung Park is approved.
SUPERVISORY COMMITTEE
Phil Hyu Lee
Young Hwan Ahn
Gwang Lee
In Su Joo
Oh Young Bang
The Graduate School, Ajou University
June, 25nd, 2010
ACKNOWLEDGEMENTS
박사 과정 내내 저에게 많은 도움주신 언제나 든든한 저의 영원한 멋진 스승님 이필휴 교수님과 안영환 교수님, 이광 교수님, 주인수 교수님, 멀리서도 선뜻 달 려와주신 방오영 교수님 머리숙여 깊이 감사드립니다... 급하게 달리기도 하고 저에게 달라진 생활들로 인해 잠시 주춤했던적도 있는 저 의 박사 과정을 이제 마무리 지었습니다. 힘들 때마다 손잡아주고 응원해준 분들 이 있어서 가능했습니다... 아주대에 혼자 남은 외로움을 채워준 우영이, 연주, 정현이, 선아, 주아... 항상 옆에서 도와주고 힘이 되어준 진영이... 멀리있는 다용언니, 근우오빠, 경애언니... 또한 저를 응원해준 모든 분께 감사함을 전합니다.. 그리고, 늦으면 항상 달려와서 기사해주신 울아빠, 언제나 기도하시며 응원해준 울엄마, 동생 잘한다 응원해준 울언니, 학위 과정의 고충을 함께 의논해준 울형 부, 웃게 해준 이쁜 조카 선우... 제가 후회하지 않는 삶을 살게 해주기 위해 본 인의 힘듦은 당연시 해준 내신랑..종민씨... 아름다운 신랑을 낳아주신 울어머님, 엄마가 늦어져도 잘 기다려주고 항상 힘내라고 웃어준 내 딸 소정이 - 울아가 세상의 빛을 본지 일년되는 날을 앞두고 엄마가 이렇게 너한테 박사엄마를 선물 하게 되어 얼마나 기쁜지 몰라...울아가가 있어서 할 수 있었어.. 많이 기뻐하고 많이 축하해줘..딸 -, 아무 걱정없이 실험을 할 수 있게 울딸을 딸처럼 키워주고 계신 울이모님... 내 눈을 응시하며 힘내라고 해준 정진이, 반야, 바라, 밀타... 하 늘에서 지켜보고 응원해주신 외할머니, 꼬모... 정말 고맙고 사랑합니다... 혼자가 아니였기에 이만큼 또 해냈습니다... 여전히 혼자가 아니기에 또 할 수 있 습니다. 다른 길로 또다시 출발하는 시작입니다... 세상의 중심에 서기 위한 다짐 과 설레임으로 또다시 시작하는 이 길의 끝에서 다시 웃겠습니다... 저의 실험을 위해 죽어간 쥐들에게도 감사하고 미안한 마음을...I
shall be telling this with a sigh Somewhere ages and ages hence; Two roads diverged in a wood, and I …… I took the one less travelled by, And that has made all the difference.- ABSTRACT -
Neuroprotective effect of mesenchymal stem cells in
neurodegenerative disease : focusing on Parkinsonian disease
Mesenchymal stem cells (MSCs) are themselves capable of multi-potency, with differentiation under appropriate conditions into chondrocytes, skeletal myocytes, and neurons. MSCs have been also known to pose neuroprotective effects through secreting various cytotrophic factors. Based on our previous studies demonstrating neuroprotective effect of hMSCs in Parkinson’s disease, we extended our investigations into other parkinsonian disease in addition to properties of hMSCs on blood-brain barrier. .
PART A. Human mesenchymal stem cells exerts neuroprotection in an animal model of double lesion-induced multiple system atrophy-parkinsonism (MSA-P).
PART B. Inhibition of blood brain barrier (BBB) permeability by hMSCs.
First, the double lesion-induced MSA-P was established with co-injections of MPTP (10mg/day, total dose 90 mg/kg for 9 days) and 3- NP (total dose 450 mg/kg for 9 days, 12hr interval). At one day after last injection, hMSCs were injected into the tail vain (1X106cells/ml). Three groups of mice were compared (control group, only MPTP+3-NP
group, hMSC treatment in MPTP+3-NP group) through histopathological and behavioral analysis. Compared to only MPTP+3-NP-treated mice, hMSCs treatment in double lesion
mice significantly increased survival of TH- and NeuN-immunoreactive cells in the substantia nigra and the striatum, respectively. Additionally, hMSC treatment significantly decreased Iba-1, GFAP and increased Calbindine immunoreactive cells in the substantia nigra and the striatum. Behavioral analysis showed that the decent times on the top of a vertical wooden pole was significantly decreased in hMSCs-treated double lesion mice, comparable to controls. This study demonstrates that hMSCs treatment had a protective effect on loss of neurons in the substantia nigra and the striatum induced by MPTP+3-NP through a variety of mechanisms, such as anti-inflammatory actions, anti-apoptotic effect.
Second, the neuroinflammation plays collectively suggest that excessive neutrophil infiltration and environmental factors, such as lower astrocyte density and higher BBB permeability, contribute to severe inflammation and neuronal death in the SNpc. At 4hour (4hr) after LPS injection, hMSCs were injected into the tail vain (1X106cells/ml) and three
groups of rat were compared (control group, only LPS group, hMSC treatment in LPS group) through histopathological analysis after 12hr. Compared to only LPS-treated rats, hMSCs treatment in LPS-treated rats significantly showed increase of EBA-immunoreactive cells and reduction of Evans blue- immunoreactive cells in the substantia nigra. Interestingly, Compared to only LPS-treated rats, hMSCs treatment in LPS-treated rats significantly showed increase of the densities of astrocytes, assumed to significantly influence neurovascular structure and integrity and reduction of p-gp-immunoreactive cells, the one BBB transporter. Consequently, hMSC treatment significantly showed reduction of MPO-immunoreactive cells and increase of TH-MPO-immunoreactive cells in the substantia nigra. This
study demonstrates that hMSCs treatment had a protective effect on neuroinflammation plays in the substantia nigra induced by LPS through inhibition of BBB permeability.
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅳ LIST OF FIGURES ··· ⅶ . INTRODUCTION Ⅰ ··· 1A. Human mesenchymal stem cells exerts neuroprotection in an animal model of double lesion-induced multiple system atrophy-parkinsonism (MSA-P) ···2
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs ···3
. MATERIALS AND METHODS Ⅱ ··· 5 A. MATERIALS ··· 5 1. Antibodies ··· 5 B. METHODS ··· 5 1. Animal studies ··· 6 2. Isolation of hMSC··· 6 3. Behavioral test ··· 7
4. Extravasation of Evans Blue Dye ··· 7
5. Tissue preparation··· 8
6. Immunohistochemistry··· 8
7. Western blot analysis ··· 9
8. Stereological cell counts··· 10
. RESULTS
Ⅲ ··· 12
A. Human mesenchymal stem cells exerts neuroprotection in an animal model of double lesion-induced multiple system atrophy-parkinsonism (MSA-P)··· 12
1. Characterization of hMSCs ··· 12
2. Recovery of motor behavior by hMSCs ··· 12
3. Detection of hMSCs in the double toxin-treated SN and striatum··· 13
4. Histological analysis of transplanted hMSCs in double lesion-induced MSA-P model ··· 13
5. Effect of cell therapy with hMSCs on modulation of inflammation and gliosis in animals treated with double toxins··· 14
6. Effect of cell therapy with hMSCs on modulation of cell death signaling pathway ··· 15
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs ··· 15
1. Effect of hMSCs on loss of dopaminergic neuron and modulation of inflammation in LPS-induced animal models··· 15
2. Effect of hMSCs on BBB permeability in LPS-induced animal models··· 16
3. Effect of hMSCs on modulation of the densities of astrocytes at the SN of animals treated with LPS··· 16
4. Effect of hMSCs on modulation of P-glycoprotein at the BBB of LPS-induced animal models ··· 17
. DISCUSSION
Ⅳ ··· 33
A. Human mesenchymal stem cells exerts neuroprotection in an animal model of double lesion-induced multiple system atrophy-parkinsonism (MSA-P)··· 33
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs ··· 36
. CONCLUSION Ⅴ ··· 40
A. Human mesenchymal stem cells exerts neuroprotection in an animal model of double lesion-induced multiple system atrophy-parkinsonism (MSA-P)··· 40
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs ··· 40
REFERENCE ··· 41
LIST OF FIGURES
Fig. 1. Schedule of MSA-P animal model··· 19
Fig. 2. Characterization of MSC··· 20
Fig. 3. Motor behavioral test ··· 21
Fig. 4. Detection of hMSCs in the double-toxin treated mice ··· 22
Fig. 5. Effect of cell therapy with hMSCs on animals treated with 3-NP and MPTP ···23
Fig. 6. Effect of cell therapy with hMSCs on modulation of inflammation and gliosis in animals treated with double toxins ··· 24
Fig. 7. Effect of cell therapy with hMSCs on modulation of cell death signaling pathway·· ··· 25
Fig. 8. Schedule of LPS-induced animal model ··· 26
Fig. 10. Effect of cell therapy with hMSCs on modulation of inflammation
in animals treated with LPS ··· 28
Fig. 11. Effect of cell therapy with hMSCs on BBB permeability in animals treated with LPS ··· 29
Fig. 12. Effect of hMSCs on modulation of the densities of astrocytes
at the SN of animals treated with LPS··· 30
Fig. 13. Effect of hMSCs on modulation of P-glycoparotein at the BBB of animals treated with LPS··· 31
I. INTRODUCTION
Mesenchymal stem cells (MSCs) are present in adult bone marrow and represent <0.01% of all nucleated bone marrow cells. MSCs are themselves capable of multi-potency, with differentiation under appropriate conditions into chondrocytes, skeletal myocytes, and neurons. MSCs have been also known to pose neuroprotective effects through secreting various cytotrophic factors. Previous our study in animal model of Parkinson’s disease (PD) demonstrated that hMSCs had a protective effect on progressive dopaminergic neuronal loss through a variety of mechanisms, such as anti-apoptotic effect, deceasing the polyubiquitinated proteins, and anti-inflammatory actions in addition to possible transdifferentiating effect of MSCs into dopaminergic neurons (Park et al, 2008). Additionally, we also reported in LPS-induced animal model of PD that hMSCs had a neuroprotective property on dopaminergic neurons through a potent anti-inflammatory action (Kim & Park et al, 2009). Furthermore, we recently reported an open-label clinical trial of MSCs in patients with MSA, demonstrating that MSCs injection delayed progression of neurological deficits and improved cerebral glucose metabolism in cerebellum compared to the control patients (Lee et al, 2008).
Based on our previous studies demonstrating neuroprotective effect of hMSCs in animal model of Parkinson’s disease, we extended our investigations into other parkinsonian disease of multiple system atrophy (MSA). In addition, we also evaluate whether hMSCs may modulate blood-brain barrier, which is known to be an important key player in progression of neurodegenerative changes.
A. Human mesenchymal stem cells exerts neuroprotection in an
animal model of double lesion-induced multiple system
atrophy-parkinsonism (MSA-P).
Multiple system atrophy (MSA) is a sporadic neurodegenerative disease of the central and autonomic nervous system. Pathologically, MSA includes striatonigral degeneration, olivopontocerebellar degeneration, astrogliosis and microgliosis. TClinically, the cardinal features include autonomic failure, parkinsonism (MSA-P), cerebellar ataxia, and pyramidal signs in any combination, of which autonomic failure is an integral component in the diagnosis of MSA. Along with progressive supranuclear palsy and corticobasal degeneration, MSA is one of Parkinsonian disease and its prevalence is the second most common following PD. With an identification of α-synuclein-positive glial cytoplasmic inclusions (GCI) as a pathological hallmark, MSA has been regarded as a unique entity within the spectrum of oligodendrogliopathy. Since the prognosis of MSA is fetal, many in vivo and clinical trials have been conducted to archive neuroprotective strategies in MSA. Of those, Stefanova et al. (Stefanova et al, 2008) demonstrated that MAO-B inhibitor had a disease modifying activity in transgenic animal models of MSA, although other clinical trials have been failed to delay disease progression.
The animal model of MSA-P was based on the idea of applying selective degeneration in the nigral and striatal neurons by using 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) and 3-nitropropionic acid (3-NP), which had been previously used to mimic Parkinson’s disease and Huntington’s disease in rodents (Stefanova et al, 2003), respectively. In this double lesion model, the MPTP is known to potentiate striatal damage and its related
with behavioral impairments induced by 3-NP intoxication in mice and thus constitute a useful model of MSA-P. In present study, we investigated whether MSCs has a protective effect on neuronal loss in the substantia nigra (SN) and striatum using double toxins-induced MSA-P animal model.
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs.
The blood-brain barrier (BBB) provides a barrier from potentially toxic molecules, in addition to regulating ion balance and nutrient transport. Recently, BBB impairment has been detected in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, although the integrity of the BBB may depend on the disease severity or duration (Desai et al, 2007). The implications of BBB dysfunction have not been fully elucidated, however, this dysfunction may act as a modifier of disease progression.
Along with the dysregulation of tight junction proteins due to the accumulation of misfolded proteins in the BBB, neuroinflammation is known to be the main contributor to BBB dysfunction in neurodegenerative disease (Desai et al., 2007). Recent studies have suggested that a microglial reaction and inflammatory processes participate in the cascade of neuronal degeneration in neurodegenerative disease (Gao and Hong, 2008). There is in vivo evidence of BBB alterations in both AD and PD. Skoog et al, 1998 identified BBB alterations before the onset of clinical dementia in AD patients and Bowman et al, 2007 suggested that BBB alterations were a modifier of AD progression. In patients with PD, BBB
alterations seemed to depend upon clinical stage; the integrity of the BBB was relatively preserved in early-stage of PD, whereas the BBB was impaired in late-stage PD (Bartels et al, 2008; Kortekaas et al, 2005). Furthermore, we recently demonstrated in vivo evidence of BBB impairment and significant correlations between the BBB indices and clinical severity in patients with MSA, suggesting that BBB dysfunction in MSA may be closely coupled to the extension of MSA pathology (Song et al, 2010).
Based on previous studies, we hypothesize that (i) infiltrated neutrophils by BBB dysfunction are increased inflammation in the brain, (ii) MSCs treatment may exert modulation of BBB integrity through its immunosuppressive properties , and (iii) MSCs may have a neuroprotective effects on dopaminergic neurons though BBB stabilization.
In present study,we employed the LPS-treated animal model and investigated whether hMSCs treatment has a protective effect on inflammation and neuronal loss through BBB dysfunction.
. MATERIALS AND METHODS
Ⅱ
A. MATERIALS
1. Antibodies
mouse anti-tyrosine hydroxylase(TH, 1:2000 dilution for brain tissue, Pel-freez), mouse anti-NeuN (1:500 dilution, Chemicon), mouse anti-nuclear matrix (NuMA, 1:100 dilution, Calbiochem), mouse anti-calbindin-D-28K (Cal, 1:3000 dilution, sigma), rabbit anti-Iba-1 ( 1: 1000 dilution, Wako) and rabbit anti-GFAP ( 1:1000 dilution, Chemicon), Akt, p-Akt, cytochrome C, caspase 3 (1:1000 dilution, cell signaling), Bax, BCL-2, BCL-XL (1:1000 dilution, stressgene), myeloperoxidase (MPO; 1:1000; Dako, Glostrup, Denmark),
endothelial barrier antigen (EBA; 1:1000; Sternberger Monoclonals, Lutherville, MD),
P-glycoprotein (P-gp; 1 : 5000; Becton Dickinson), FITC-labeled tomato lectin (1:1000; Vector Laboratories, Burlingame, CA).
B. METHODS
1. Animal Studies
To introduce double lesions-induced animal model of MSA-P, C57BL/6 adult male mice aged 16 weeks were injected MPTP (10mg/day, total dose 90 mg/kg, i.p.) and 3- NP (10 mg/kg ´ 4, 20 mg/kg ´ 4, 30 mg/kg ´ 4, 40 mg/kg ´ 4 and 50 mg/kg ´ 1, total dose 450 mg/kg, 12 hrs interval, i.p.) for 9-day period (each group, n=5, Fernagut et al, 2004) Control mice (n=5) were injected with saline alone, using the same administration method. At one day after last injection, hMSCs were injected into the tail vain (1 ´ 106 cells/ml).
Histopathological and behavioral analyses were compared among three groups of mice (control group, only MPTP + 3-NP group, hMSCs treatment in MPTP + 3-NP group) (Fig. 1).
For Part B animal model, male SD rats (250–270g) were anesthetized with 10% chloral hydrate for LPS injection. LPS (5mg ⁄ 3mL) was delivered unilaterally into the left SN (5.3 mm posterior, 2.3 mm lateral, 7.7 mm ventral from the bregma) and injected at a rate of 1mL ⁄ 5 min using a 26-gauge Hamilton syringe attached to an automated microinjector using a stereotaxic apparatus (Paxinos & Watson, 1998). The needle was then left in place for an additional 10 min before slow retraction. The control group was injected with phosphate-buffered saline (PBS; 3mL) into the same position and method with LPS injection group. At 4hr after LPS injection, hMSCs were injected into the tail vain (1 ´ 106 cells/ml).
Histopathological analyses were compared among three groups of rats (control group, only LPS group, hMSCs treatment in LPS group) (Fig. 8).
The animal work was approved by the respective Institutional Animal Care and Use Committees of Ajou University.
2. Isolation of hMSC
Bone marrow aspirates (10mL) were obtained from the iliac crests of human donors. The mononuclear cell layer was isolated by Ficoll-Hypaque, washed in PBS, plated in polystyrene plastic 100mm culture dishes, and cultivated in low-glucose Dulbecco modified
Eagles’ medium (Gibco-BRL, Grand Island, NY), containing 10% fetal bovine serum (Hyclone, Irvine, CA) and 1% penicillin/streptomycin (P/S, Sigma, St. Louis, MO) in a humidified incubator at 37°C under 5% CO2. Non-adherent cells were removed after 24 h.
When these primary cultures reached 80% confluence, the cells were harvested using 0.25% trypsin and subcultured. At passage 6, hMSCs were injected into tail vain.
3. Behavioral test
The pole test was performed according to Matsuura et al, 1997 with minor modifications (Fernagut et al, 2002). The mouse was placed head upward on the top of a vertical wooden rough-surfaced pole (diameter: 1 cm, height: 50cm). Each mouse was habituated to the apparatus on the day prior to testing, then allowed to descend five times. The total time until the mouse reached the floor with its four paws was recorded. For each session of five descents, the best performance was kept for the total time. If the mouse was unable to turn completely downwards, fell or slipped down, the default value of 120 s was taken into account. The pole test was performed at baseline, then at day 1, week 1 and 2 after hMSCs transplantation.
4. Extravasation of Evans Blue Dye
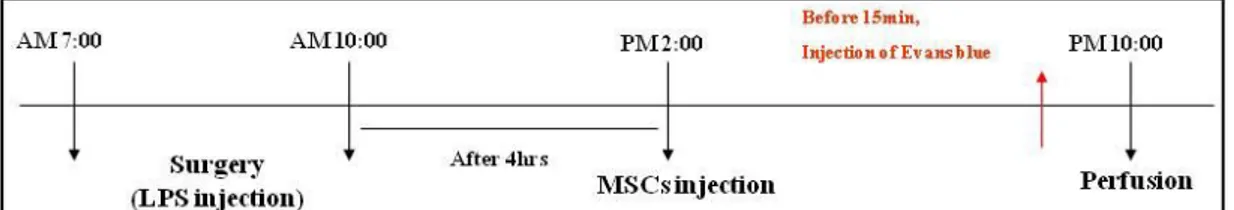
To measure the vascular permeability in the brain, Evans blue (EB) dye (4% in saline, 3 mL/Kg) was intravenously injected. Fifteen minutes later the rats were anesthetized and
perfused. The frozen sections were prepared and the infiltrated EB dye was analyzed using a confocal microscope (Carl Zeiss).
5. Tissue preparation
For immunohisotochemistry, the mice and rats were perfused with saline solution containing 0.5% sodium nitrate and heparin (10mU/mL) and were fixed with 4% paraformaldehyde dissolved in 0.1M PB (~ 50 mL/mouse) at 30 days (mice) or 12hr (rats) after first drug injection. The brains were removed from the skulls, post-fixed overnight in buffered 4% paraformaldehyde at 4 and stored in a 30% sucrose solution for 1 to 2 day℃ s at 4 , until they sank. They were then sectioned to obtain a 30μm coronal sections. The 30μm ℃ coronal sections were stored in tissue stock solution (30% glycerol, 30% ethylene glycol, 30% 3 times distilled water, 10% 0.2M PB) at 4 until required. For Wes℃ tern blotting, the mice were euthanized at 30 days after first injection, and the SN and striatum area were rapidly removed from the brains and frozen at –70 . ℃
6. Immunohistochemistry
The 30μm coronal brain sections were rinsed twice in PBS and incubated in 0.2% Triton X-100 for 30 min at room temperature (RT). They were rinsed three times with 0.5% bovine serum albumin (BSA) in 1× PBS for blocking. After blocking, they were incubated
overnight at 4 with primary antibody; TH, NeuN, NuMA, calbindin℃ -D-28K, Iba-1 and GFAP, MPO, EBA, P-gp.
After overnight, the brain sections were rinsed three times in 0.5% BSA in 1× PBS (10 min/rinse) and incubated with the appropriate biotinylated secondary antibody and avidin– biotin complex (Elite Kit; Vector Laboratories, Burlingame, CA) for 1 h at RT. Bound antibodies were visualized by incubating with 0.05% diaminobenzidine– HCl (DAB) and 0.003% hydrogen peroxide in 0.1M PB. The brain sections were rinsed with 0.1M PB for DAB inhibition. Immunostained cells were analyzed by bright-field microscopy.
For tomato lectin and GFAP doublelabeling, sections were incubated with GFAP antibodies, followed by Texas red-conjugated secondary antibodies and FITC-labeled tomato lectin for 1 h. Subsequently, sections were washed, mounted, and analyzed using confocal microscopy (Carl Zeiss).
7. Western blot analysis
Brain tissues from the ST, SN were dissected and homogenized in ice-cold lysis buffer (20mM Tris-HCl, pH 7.5, 1 mM EDTA, 5 mM MgCl2, 1 mM dithiothretol, 0.1 mM phenylmethylsulfonyl fluoride plus protease inhibitor cocktail (Sigma)). Tissue homogenates was centrifuged (20min, 14,000×g, 4 ) and supernatants were transferred to fresh tubes. ℃ Proteins were analyzed using the Bio-Rad Protein Assay Kit (USA). Equal amounts of protein (50μg) were loaded in each lane with loading buffer containing 0.125M Tris-HCl, pH
6.8, 20% glycerol, 4% SDS, 10% mercaptoethanol, and 0.002% bromophenol blue. Samples were boiled for 5min before gel loading. Proteins were transferred electrophoretically to polyvinylidiene difluoride membranes (Millipore, Bedford, MA). Membranes were washed in Tris-buffered saline solution with 2.5 mM EDTA (TNE) and then blocked in TNE containing 5% skim milk for 1 h. Membranes were incubated overnight at 4 with primary ℃ antibody. After washing, the membranes were incubated with secondary antibodies (1:2000; Amersham) for 1 h at RT, and washed again. The blots were finally developed with the ECL Western blotting detection reagents (Amersham).
For semiquantitative analysis, the densities of the immunoblot bands were measured average of each group (n=3) by computer imaging (Image J; NIH, Bethesda, MD, USA).
8. Stereological cell counts
Unbiased stereological estimations of the total number of the staining cells in the SN and striatum were made using an optical fractionator, as previously described with some modifications (Kirik et al, 1998). This sampling technique is not affected by tissue volume changes and does not require reference volume determinations (West et al, 1991). The sections used for counting covered the entire SN, ST from the rostral tip of the pars compacta back to the caudal end of the pars reticulate. This generally yielded 8-9 sections in a series. Sampling was performed using the Olympus C.A.S.T.-Grid system (Olympus Denmark A/S, Denmark), using an Olympus BX51 microscope, connected to the stage and feeding the computer with the distance information in the z-axis. The SN and striatum were
delineated at 1.25× objective. A counting frame (60%, 35, 650 μm2) was placed randomly on
the first counting area and systematically moved though all counting areas until the entire delineated area was sampled. Actual counting was performed using a 40×oil objective. Guard volumes (4μm from the top and 4-6μm from the bottom of the section) were excluded from both surfaces to avoid the problem of lost cap, and only the profiles that came into focus within the counting volume (with a depth of 10μm) were counted. The total number of stained cells was calculated according to the optical fractionator formula (West et al, 1991).
9. Statistical analysis
The Mann-Whitney test and the Kruskal-Wallis analysis were used to compare the means in pairs of groups and multiple comparisons, respectively. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using commercially available software (version 10.0; SPSS Inc., Chicago, IL).
. RESULTS
Ⅲ
A. Human mesenchymal stem cells exerts neuroprotection in an
animal model of double lesion-induced multiple system
atrophy-parkinsonism (MSA-P).
1. Characterization of hMSCs
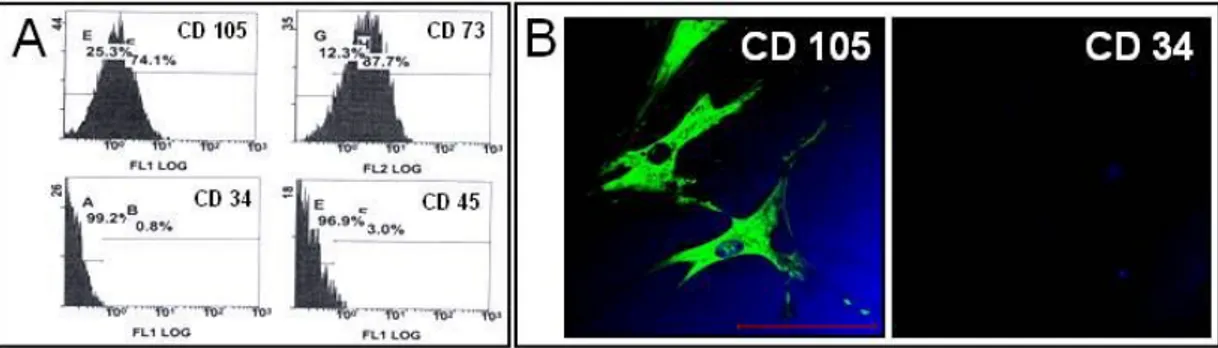
FACS analysis demonstrated that hMSCs expressed CD105 and CD73, positive markers for hMSCs, and did not express CD 45 and CD 34, negative markers for hMSCs (Fig. 2-A). Immunofluorescent labeling showed that hMSCs were positive for CD 105 and negative for CD 34 (Fig. 2-B).
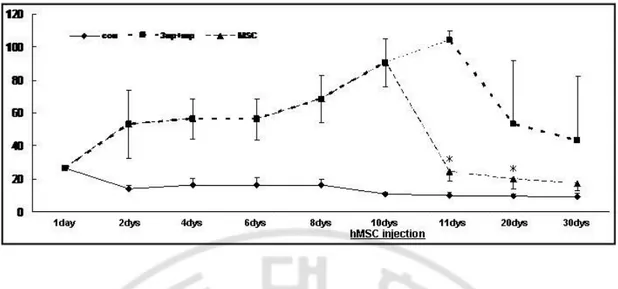
2. Recovery of motor behavior by hMSCs
The total time until the mouse reached the floor with its four paws was significantly increased in mice of double lesion-induced MSA-P model than control group (p<0.05, Fig. 3). Compared to double toxin-treated mice, hMSCs administration in double toxins-treated mice had a significant decrease in the total time and this significant difference was maintained for 10 days after hMSCs administration (p<0.05, Fig. 3).
3. Detection of hMSCs in the double toxin-treated SN and striatum
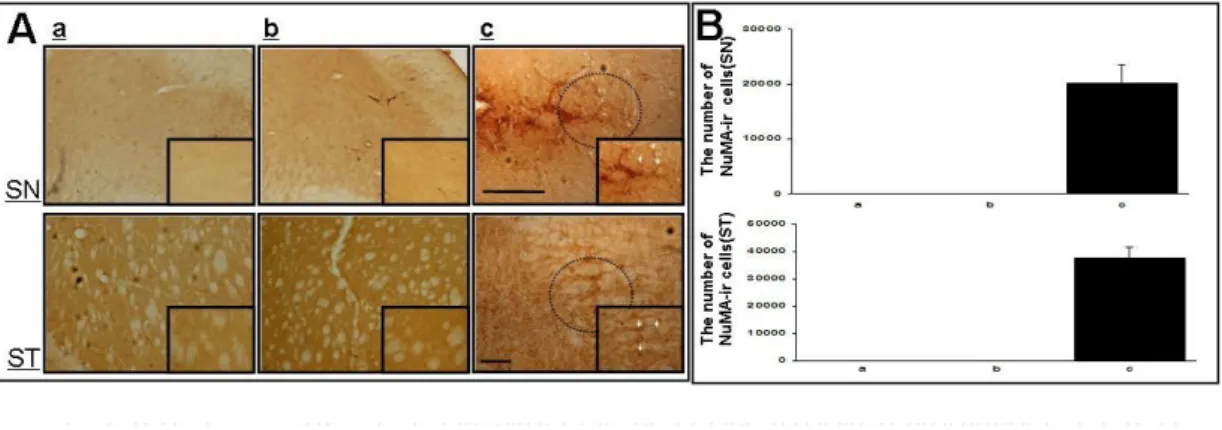
To determine whether transplanted hMSCs would survive, we identified hMSCs in the SN and stratum in double toxin-treated mice using human-specific NuMA immunostaining (Fig. 4). NuMA-ir cells were observed in hMSCs administrated double toxin-treated mice (Fig. 4-A) and the number of NuMA-ir cells in the SN and striatum was 20120 ± 825 and 37859 ± 25, respectively, which corresponded to about 2.0% and 3.8% of the total injected hMSCs (Fig. 4-B).
4. Histological analysis of transplanted hMSCs in double
lesion-induced MSA-P model
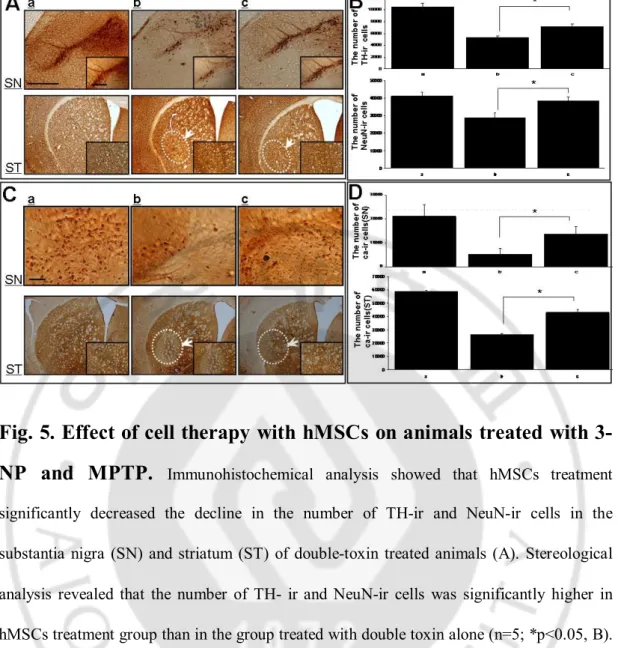
Brain tissue was prepared for immunohistochemical analysis 4 weeks after the first MPTP and 3-NP co-injections. Immunohistochemical analysis showed that administration of both toxins induced a significant decline in the number of TH-immunoreactive (TH-ir) cells in the SN and NeuN-ir cells in the striatum (Fig. 5A). Neuronal loss, as quantified by stereological analysis, revealed that TH-ir and NeuN-ir cells decreased by approximately 48% and 29%, respectively (Fig. 5B; both p < 0.001). However, hMSC administration significantly reduced neuronal loss in the double-toxin-treated SN and striatum (Fig. 5A). Stereological analysis revealed that the number of TH-ir and NeuN-ir cells was significantly greater in the hMSC-treated group than in the MPTP + 3-NP treatment group, showing a 23% and 18% increase in the survival of TH- and NeuN-ir cells in the SN and striatum, respectively (Fig. 5-B; p < 0.05). To evaluate functional neurons, cells in the SN and
striatum were immunostained with Calbindin-D-28kD, a marker for calcium binding protein, which is important to maintain in synaptic transmission and axonal transport. Consistent with the increase of neuronal survival in the SN and striatum of double-toxin-treated mice after hMSC administration, the number of Calbindin-D-ir cells in the SN and striatum was significantly greater in the hMSC-treated group as compared with the double-toxin-treated group (Fig. 5 and D; p < 0.05).
5. Effect of cell therapy with hMSCs on modulation of
inflammation and gliosis in animals treated with double
toxins
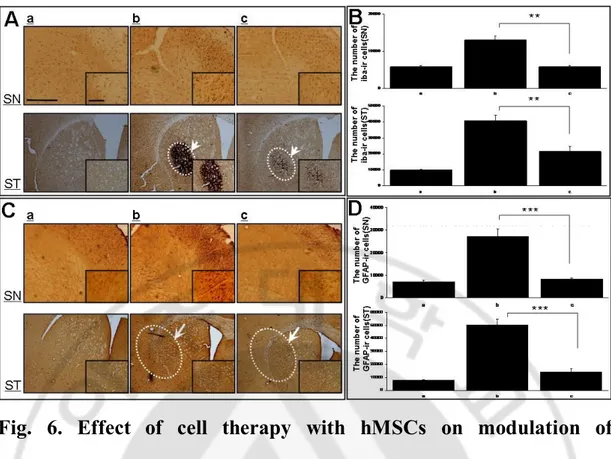
To determine the effects of hMSCs on modulation of inflammation and gliosis, brain tissue in the SN and striatum was immunostained with Iba-1, a marker for activated microglia and GFAP, a marker for activated astrocyte. There was a marked increase in Iba-1 and GFAP-immunoreactivity in double toxin-treated mice (Fig. 6); however, hMSCs treatment in double toxin-treated mice markedly decreased Iba-1 and GFAP immunoreactivity (Fig. 6-A and C). Stereological analysis revealed that the number of activated microglia and astrocytes was significantly decreased in hMSCs treatment group than in the group only treated with double toxins in the SN and striatum (Fig. 6-B and D, p<0.01 in microglia and p<0.001 in astrocytes).
6. Effect of cell therapy with hMSCs on modulation of cell death
signaling pathway
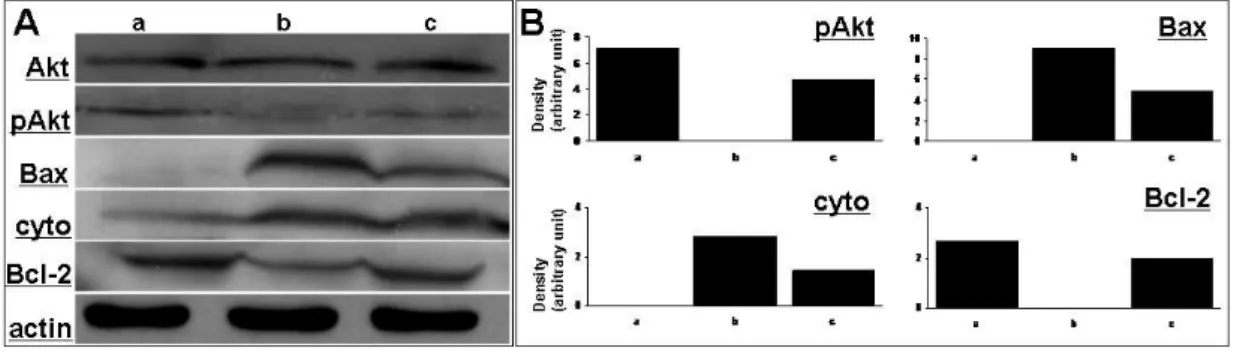
To determine the effects of hMSCs on modulation of cell death signaling pathway, Western blot analysis was performed using brain tissue prepared at 4 weeks after first MPTP and 3-NP injection (Fig. 7A and B). The expression of p-Akt was significantly decreased in double toxin-treated mice compared to controls, however, hMSCs administration in double toxin-treated mice increased the expression of p-Akt. hMSCs treatment decreased significantly Bax expression in double toxin-treated mice, whereas the expression of Bcl-2 after hMSCs administration was increased significantly in double toxin-treated mice. In addition, hMSCs significantly decreased the expression of cytochrome c, which had been elevated after double-toxin treatment.
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs.
1. Effect of hMSCs on loss of dopaminergic neuron and modulation
of inflammation in LPS-induced animal models
To determine the effects of hMSCs on loss of dopaminergic neuron and modulation of inflammation, brain tissue in the SN was immunostained with TH, a marker for dopaminergic neuron and OX42, a marker for activated microglia. There was a marked decrease in TH- -immunoreactivity (ir) and increase in Iba-1-ir in LPS-induced animal models (Fig. 9, 10); however, hMSCs treatment in LPS-induced animal models, markedly
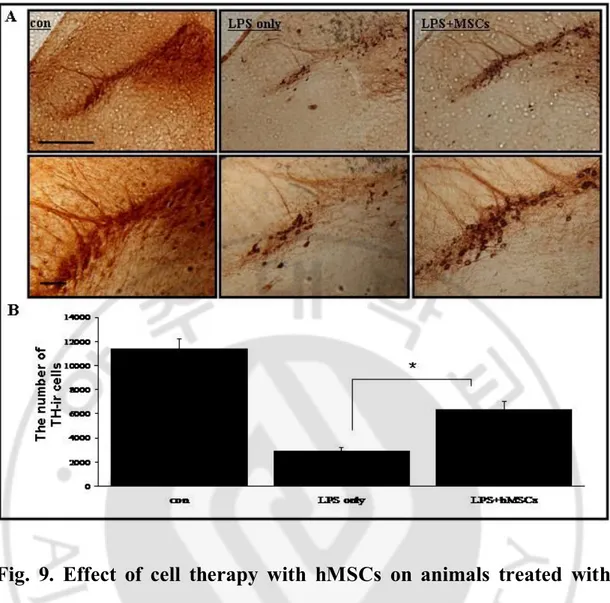
increased TH-ir (Fig. 9-A) and decreased Iba-1-ir (Fig. 10-A). Stereological analysis revealed that the number of dopaminergig neuron was significantly increased in hMSCs treatment group than in the group only treated with LPS in the SN (Fig. 9-B) and the number of activated microglia was significantly decreased in hMSCs treatment group than in the group only treated with LPS in the SN (Fig. 10-B, p<0.01 ).
2. Effect of hMSCs on BBB permeability in LPS-induced animal
models
To determine the effects of hMSCs on BBB permeability, brain tissue in the SN was detected by Evans blue (EB) and immunostained with endothelial-barrier antigen (EBA), a marker for endotherial cell. There was a marked increase in EB and EBA-ir cells in LPS-induced animal models (Fig. 11); however, hMSCs treatment in LPS-LPS-induced animal models markedly decreased EB and EBA-ir cells (Fig. 11-A and B). Stereological analysis revealed that the number of detected EBA-ir cells was significantly decreased in hMSCs treatment group than in the group only treated with LPS in the SN (Fig. 11-C, p<0.01 ).
3. Effect of hMSCs on modulation of the densities of astrocytes at
the SN of animals treated with LPS
astrocytes reduced in the SN of injected animals; however, hMSCs treatment in LPS-induced animal models markedly increased the densities of astrocytes (Fig. 12-A). Astrocyte end feet around vessels displayed using GFAP/tomato lectin double staining. Astrocytes were densely located around vessels in the SN of hMSCs treatment group (Fig. 12-B).
4. Effect of hMSCs on modulation of P-glycoprotein at the BBB of
LPS-induced animal models
To determine the effects of hMSCs on modulation of BBB dysfunction, brain tissue in the SN was immunostained with P-glycoprotein (p-gp), the BBB transporter. There was a markedly increase in p-gp-ir cells in LPS-induced animal models (Fig. 13); however, hMSCs treatment in LPS-induced animal models significantly decreased p-gp-ir cells (Fig. 13-A). Stereological analysis revealed that the number of regulation protein of BBB permeability was significantly decreased in hMSCs treatment group than in the group only treated with LPS in the SN (Fig. 13-B, p<0.01 ).
5. Effect of hMSCs on MPO-1 neutrophil infiltration in the SNpc
To determine the effects of hMSCs on modulation of inflammation by neutrophil infiltration, brain tissue in the SN was immunostained with MPO, a marker for activated neutrophil. There was a markedly increase in MPO-ir cells in LPS-induced animal models (Fig. 14); however, hMSCs treatment in LPS-induced animal models markedly decreased
MPO-ir cells (Fig. 14-A). Stereological analysis revealed that the number of neutrophil infiltration was significantly decreased in hMSCs treatment group than in the group only treated with LPS in the SN (Fig. 14-B, p<0.01 ).
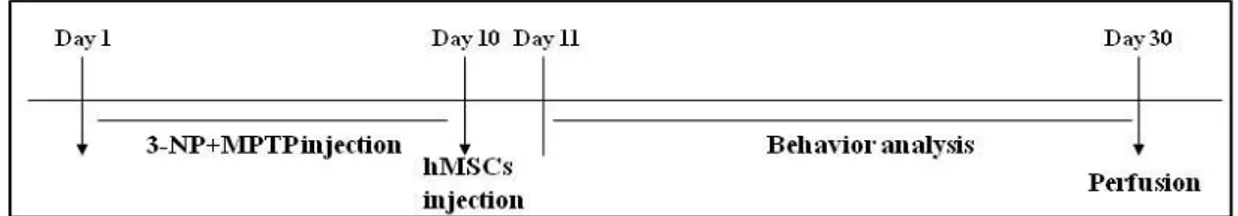
Fig. 1. Schedule of MSA-P animal model.
C57BL/6 adult male mice aged 16 weeks were injected MPTP (10mg/day, total dose 90 mg/kg, i.p.) and 3- NP (10 mg/kg ´ 4, 20 mg/kg ´ 4, 30 mg/kg ´ 4, 40 mg/kg ´ 4 and 50 mg/kg ´ 1, total dose 450 mg/kg, 12 hrs interval, i.p.) for 9-day period (each group, n=5). At one day after last injection, hMSCs were injected into the tail vain (1 ´ 106 cells/ml). The pole test was performed at baseline,then at day 1, week 1 and 2 after hMSCs transplantation.
Fig. 2. Characterization of MSC.
Flow cytometric analysis (A) and immunofluorescent labeling of human mesenchymal stem cells (B) Scale bar: 100μm.Fig. 3. Motor behavioral test.
The pole test was performed at baseline, then at day 1, 10 and 20 after hMSCs transplantation. The total time until the mouse reached the floor with its four paws was significantly increased in mice of double-toxin treated mice than controls (p<005, n=5). Compared to double toxin-treated mice, hMSCs administration in double toxins-treated mice had a significant decrease in the total time and this significant difference was maintained for 10 days after hMSCs administration (n=5, *p<0.05).Fig. 4. Detection of hMSCs in the double-toxin treated mice.
The existence of hMSCs in the substantia nigra (SN) and striatum (ST) of the double toxin-treated mice was identified by human specific NuMA staining (A). The number of NuMA-ir cells in the SN and striatum was 20120 ± 825 and 37859 ± 25, respectively, which corresponded to about 2.0% and 3.8% of the total injected hMSCs (n=5, B). Scale bar:100mmFig. 5. Effect of cell therapy with hMSCs on animals treated with
3-NP and MPTP.
Immunohistochemical analysis showed that hMSCs treatment significantly decreased the decline in the number of TH-ir and NeuN-ir cells in the substantia nigra (SN) and striatum (ST) of double-toxin treated animals (A). Stereological analysis revealed that the number of TH- ir and NeuN-ir cells was significantly higher in hMSCs treatment group than in the group treated with double toxin alone (n=5; *p<0.05, B). Functional neurons immunostained by Calbindin-D was also increased significantly in the SN and ST of double-toxin treated mice after administration of hMSCs (C). Stereological analysis revealed that the number of Calbindin-ir cells was significantly higher in hMSCs treatment group than in the group treated with double toxin alone (n=5; * p<0.05, D). Scale bar:100mmFig. 6. Effect of cell therapy with hMSCs on modulation of
inflammation and gliosis in animals treated with double toxins.
MPTP and 3-NP treatment dramatically led to microglial activation and gliosis in the substantia nigra (SN) and striatum (ST) , however, hMSCs treatment attenuated significantly activation of microgia and gliosis in double-toxin treated SN and ST (A and C). Stereological analysis revealed that the number of activated microglia and astrocytes was significantly lower in hMSCs treatment group than in the group treated with double toxin alone (n=5; ** p<0.01, *** p<0.001 B, D). Scale bar:100mmFig. 7. Effect of cell therapy with hMSCs on modulation of cell death
signaling pathway.
Western blot analysis performed at 4 weeks after first double-toxin injection showed that the expression of p-Akt was significantly decreased in double toxin-treated mice compared to controls, however, hMSCs administration in double toxin-toxin-treated mice increased the expression of p-Akt. hMSCs treatment decreased significantly Bax expression in double toxin-treated mice, whereas hMSCs treatment increased significantly the expression of Bcl-2 in these mice. In addition, hMSCs significantly decreased the expression of cytochrome c, which had been elevated after double-toxin treatment. (n=3,Fig. 8. Schedule of LPS-induced animal model.
male SD rats (250–270g) were anesthetized with 10% chloral hydrate for LPS injection. LPS (5 mg ⁄ 3 mL) was delivered unilaterally into the left SN (5.3 mm posterior, 2.3 mm lateral, 7.7 mm ventral from the bregma) and injected at a rate of 1 mL ⁄ 5 min using a 26-gauge Hamilton syringe attached to an automated microinjector using a stereotaxic apparatus. At 4hr after LPS injection, hMSCs were injected into the tail vain (1 ´ 106 cells/ml). Evans blue (EB) dye(4% in saline, 3 mL/Kg) was intravenously injected. Fifteen minutes later the rats were anesthetized and perfused.
Fig. 9. Effect of cell therapy with hMSCs on animals treated with
LPS.
Immunohistochemical analysis showed that hMSCs treatment significantly increased the decline in the number of TH-ir in the substantia nigra (SN) of LPS- injected animals (A). Stereological analysis revealed that the number of TH-ir cells was significantly higher in hMSCs treatment group than in the group treated with LPS olny (n=5; *p<0.05, B). Scale bar:100mmFig. 10. Effect of cell therapy with hMSCs on modulation of
inflammation in animals treated with LPS.
LPS treatment dramatically led to microglial activation in the substantia nigra (SN), however, hMSCs treatment attenuated significantly activation of microgia in LPS-injected SN (A). Stereological analysis revealed that the number of activated microglia was significantly lower in hMSCs treatment group than in the group treated with LPS only (n=5; ** p<0.01, *** p<0.001 B). Scale bar:100mmFig. 11. Effect of cell therapy with hMSCs on BBB permeability in
animals treated with LPS.
LPS treatment dramatically led to BBB dysfunction in the substantia nigra (SN), however, hMSCs treatment attenuated significantly inhibition of BBB permeability in LPS-injected SN (A,B). EB was examined under a confocal microscope. hMSCs treatment significantly decreased the decline in the detected EB in the SN of LPS- injected animals (A). Immunohistochemical analysis showed that hMSCs treatment significantly decreased the decline in the number of EBA-ir in the SN of LPS- injected animals (B). Stereological analysis revealed that the number of EBA-ir cells was significantly higher in hMSCs treatment group than in the group treated with LPS olny (n=5; *p<0.05, C). Scale bar:100mmFig. 12. Effect of hMSCs on modulation of the densities of astrocytes
at the SN of animals treated with LPS.
Immunohistochemical analysis showed that hMSCs treatment significantly increased the decline in the number of GFAP-ir in the substantia nigra (SN) of LPS- injected animals (A). Astrocyte end feet around vessels displayed using GFAP/tomato lectin double staining. Astrocytes were densely located around vessels in the SN of hMSCs treatment group. Scale bar:100mmFig. 13. Effect of hMSCs on modulation of P-glycoprotein at the
BBB of animals treated with LPS.
Immunohistochemical analysis showed that hMSCs treatment significantly decreased the decline in the number of p-gp-ir in the substantia nigra (SN) of LPS- injected animals (A). Stereological analysis revealed that the number of p-gp-ir cells was significantly lower in hMSCs treatment group than in the group treated with LPS olny (n=5; *p<0.05, B). Scale bar:100mmFig. 14. Effect of hMSCs on MPO-1 neutrophil infiltration in the
SNpc.
LPS treatment dramatically led to neutrophil infiltration in the substantia nigra (SN), however, hMSCs treatment attenuated significantly neutrophil infiltration in LPS-injected SN (A). Stereological analysis revealed that the number of neutrophil was significantly lower in hMSCs treatment group than in the group treated with LPS only (n=5; ** p<0.01, *** p<0.001 B). Scale bar:100mm. DISC
Ⅳ
USSION
A. Human mesenchymal stem cells exerts neuroprotection in an
animal model of double lesion-induced multiple system
atrophy-parkinsonism (MSA-P).
The present study revealed that hMSC treatment significantly protected against neuronal loss induced by MPTP and 3-NP treatment in the SN and striatum with coincident improvement in motor behavior. Neuroprotective mechanisms exerted by hMSCs may be mediated by inflammatory and cell survival and death signaling-pathway modulation as the hMSCs migrated from the peripheral circulation into the SN and striatum. These data suggest that neuroprotective strategies using hMSCs may be applicable in patients with MSA-P.
With advances in the understanding of MSA pathobiologies, it has been suggested that oligodendroglial degeneration resulting from α-synuclein inclusion formation contributes to secondary widespread neuronal degeneration. However, the initial trigger or aggravating mechanism underlying the abnormal accumulation and aggregation of α-synuclein in MSA remains unknown. In case–control epidemiological studies, occupational exposure to pesticides, insecticides, or solvents that interrupt mitochondrial electron transport is associated with increased risk of MSA (Nee et al., 1991; Vanacore et al., 2005). In animal studies, high-dose 3-NP administration also aggravated nigrostriatal and olivopontocerebellar degeneration in MSA transgenic mice using proteolipid protein promoters (Stefanova et al., 2005). Furthermore, we recently reported that 3-NP
administration in transgenic mice led to oxidation-specific modifications of α-synuclein that were concomitant with an exacerbation of behavioral deficits and widespread neuronal and oligodendrocytic pathology in a number of brain regions implicated in MSA (Ubhi et al., 2009). These data support that derangement in mitochondrial function by mitochondrial neurotoxins, such as MPTP or 3-NP used in this study may be a main mediator for progression of MSA pathology.
Our study demonstrated that hMSCs had neuroprotective properties against mitochondria- inhibiting double-toxin-induced neuronal cell loss, showing about a 20% increase in the survival of TH- and NeuN-ir cells in the SN and striatum. A significant improvement of motor behavior after hMSC treatment was in accordance with increased survival of these neuronal cells following hMSC treatment in double-toxin-treated mice, although functional recovery was not maintained in the end of study period possibly due to the effect of spontaneous recovery in double-toxin only treated animals. The neuroprotective effects of MSCs seem to be mediated by complex mechanisms. First, our study has demonstrated that hMSCs can restore the balance between neuronal survival and apoptosis, which is disrupted by mitochondrial neurotoxins. In this study, hMSC treatment significantly increased the expression of the cell survival factor p-Akt in double-toxin treated mice. pAkt activation is modulated by growth factors and prevents apoptotic cell death signaling pathways (Saito et al., 2004). Although we did not investigate the potential factors that induced pAkt activation, MSCs are known to increase the production of various neurotrophic factors, such as NGF, BDBF, or NT-3 (Kim et al., 2010), which may modulate pAkt activation in this study. Along with upregulation of cell survival signaling pathways by
hMSCs, hMSCs also modulated expression of pro-and anti-apoptotic proteins toward suppressing apoptotic cell death signaling, and thus prevented the release of cytochrome c from mitochondria.
Second, hMSC treatment had anti-inflammatory and anti-gliotic effect, showing significantly decreased activation of microglia and astrocytes in the double-toxin-treated SN and striatum. As in PD, microglial reaction and inflammatory processes also participate in the cascade of neuronal degeneration in MSA. In human MSA, neuropathological studies suggest that the mode of microglial activation is system-specific, consistent with the known pattern or system degeneration in MSA, and is significantly correlated with the burden of GCI in the extrapyramidal motor and cerebellar input systems (Ishizawa et al., 2004). A similar pattern of microglial activation was also observed in MSA patients using [11C](R)-PK11195 positron emission tomography study (Gerhard et al., 2003). Additionally, we reported that 3-NP administration in MSA transgenic mice produced marked microglial activation and gliosis (Ubhi et al., 2009). It has been suggested that MSCs can not only inhibit nearly all cells participating in the immune response cell–cell contact-dependant mechanism, but can also release a variety of soluble factors that may be involved in the immunosuppressive activity of MSCs (Karussis et al., 2008; Krampera et al., 2006; Nauta and Fibbe, 2007). Furthermore, we recently demonstrated in vitro and in vivo evidence that hMSCs have a neuroprotective effect on dopaminergic neurons through anti-inflammatory actions, where soluble factors released from MSCs, such as IL-6, IL-10, and TGF-β may regulate the microglial response to inflammatory stimulants (Kim et al., 2009). Accordingly,
our data suggest that the neuroprotective properties of hMSCs via anti-inflammatory effects were also evident in an animal model of MSA.
MSCs characteristically migrate towards injured brain area in various animal models of ischemia and PD, possibly in response to signals that are upregulated under injury condition (Hellmann et al., 2006; Li et al., 2008). Chemokines released from damaged brain cells and their receptors, such as stromal cell-derived factor-1 (SDF-1) and its receptor CXCR4 may play an important role in migration of MSCs (Chamberlain et al., 2007; Stumm et al., 2002). SDF-1 is widely expressed in the brain, including cortex, cerebellum, basal ganglia, and SN pars compacta (Banisadr et al., 2003). Damage in the SN and striatum induced by MPTP and 3-NP may increase the expression of SDF-1 and CXCR4, leading to recruitment of MSCs to these regions. In this study, the number of surviving hMSCs in the SN and striatum 20 days after hMSC administration was approximately 2.0% and 3.8% of the total number of injected hMSCs, respectively. These migrated cells may contribute to modulate the microenvironmental cascade of the neurodegenerative process in the SN and striatum.
B. Inhibition of blood brain barrier (BBB) permeability by hMSCs.
The present study demonstrated that hMSCs treatment significantly stabilized BBB permeability in the SN of LPS-injected animals. The effects of BBB stabilization would be mediated by modulation of inflammatory, protection of astrocytic end feet, and inhibition of BBB transporter by hMSCs migrated from peripheral circulation into the SN.
Ample evidence has suggested that a microglial reaction and inflammatory processes participate in the cascade of neuronal degeneration in neurodegenerative disease through various pathomechanisms (Gao and Hong, 2008). Of those, neuroinflammation-mediated BBB breakdown is one of the main contributors to propagation of neurodegenerative changes (Desai et al., 2007). Additionally, BBB breakdown also disturbs astrocyte end feet and enhance neutrophil infiltration, thus further deteriorating BBB integrity in PD animal model (Ji et al, 2008).
Our study demonstrated that hMSCs had protective effects of BBB function against LPS-induced neutrophil infiltration. The number of EB and EBA-ir after hMSC treatment was in accordance with reduced neutrophil infiltration following hMSC treatment in LPS-induced animal models. The properties of BBB stabilization by MSCs seem to be mediated by complex mechanisms. First, our study has demonstrated that hMSCs can restore the function of astrotic end feet surrounding endotherial cells. In this study, hMSC treatment significantly increased the number of GFAP-ir in LPS-induced animal models. The lower density of astrocytes in the SNpc might be ineffective at downregulating inflammatory responses and at maintaining BBB structure (Ross et al, 1995; Tomas-Camardiel et al, 2004; Vizuete et al, 2000). Astrocytes participate in nutritive and metabolic support of neuron. Thus, astrocytes are in a position to disperse vascular nutrients away from the vessels in support of neural function and astrocytes can down-regulate microglial inflammatory reactions by, for example, reducing iNOS and IL-12 expression (Aloisi et al, 1997; Min et al, 2006; Pyo et al, 2003; Vincent et al, 1996). Furthermore, astrocytes play a critical role in the formation of the BBB (Kacem et al., 1998). Increase in astrocyte density would stabilize
BBB permeability by astrotic end feet surrounding endotherial cells (Ji et al, 2008). Along with increase of GFAP-ir by hMSCs, our data showed that hMSCs can also modulate astrotic end feet surrounding endotherial cells.
Second, hMSC treatment had anti-inflammatory showing significantly decreased activation of microglia in LPS-treated SN. Recently study demonstrated that BBB dysfunction occur by neuroinflammation in 6OHDA-induced animal model (Carvey et al, 2005). Previous our study demonstrated that MSCs treatment reduce microglial activation and neuroinflammation through reduction of TNF-a, pre-inflammatory factor and then MSC released anti-inflammatory factors ; IL-6, TGF-b (Kim & Park et al, 2009). A primary product of microglial activation is TNF-a, which is also known to increase BBB permeability (Tsao et al., 2001; Didier et al., 2003). Accordingly, our data suggest that hMSCs would stabilize BBB in LPS-induced animal models through anti-inflammatory effects.
Third, hMSC treatment can modulate of abnormal transporter activity, showing significantly decreased activation of p-gp in LPS-treated SN. Carvey et al, 2005 demonstrated that BBB dysfunction occur by neuroinflammation and then increase transporter, p-gp in the tight junction in 6OHDA-induced animal model, suggesting the role of p-gp in BBB stabilization.
The consequence of BBB alteration in parkinsonian diseases remains speculative. In the condition of neurodegenerative disease, the neurons or glial cells are confronted with microenvironment of decreased homeostatic reserve and thus become extremely sensitive
and vulnerable to an altered composition of the extracellular milieu (Popescu et al, 2009). In turn, increased BBB permeability may increase the probability of exposing microenvironments in the brain to environmental neurotoxins interrupting mitochondrial electron transport or elements of the peripheral immune system, and subsequently accelerate pathological changes of neuro-glial degeneration and neuroinflammation in the cascade of degenerative processes. Therefore, the strategy for BBB stabilization may act as a modifier of disease progression in neurodegenerative diseases. In this regard, our data suggest that the BBB stabilizing property of hMSCs may provide a useful therapeutic strategy for the prevention and/or treatment of PD.
. CONC
Ⅳ
LUSION
In conclusion, we have shown that hMSCs treatment has a protective effect against neuronal death in the SN and striatum induced by double mitochondrial neurotoxins and protective effects of BBB function against LPS-induced neutrophil infiltration. Modulation of inflammatory actions and cell survival and death signaling pathways by hMSCs may work in the neuroprotective process, which may be applicable clinically in patients with neurodegenerative disease of parkinsonian diseases as one of candidates for neuroprotective strategies.
REFERENCE
1. Aloisi F, Penna G, Cerase J, Menendez IB, Adorini L : IL-12 production by central nervous system microglia is inhibited by astrocytes. J Immunol 159:1604-1612, 1997
2. Banisadr G, Skrzydelski D, Kitabgi P, Rostene W and Parsadaniantz SM : Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons.
Eur J Neurosci 18:1593-1606, 2003
3. Bartelsa AL, van Berckelb BNM, Lubberinkb M, Luurtsemab G, Lammertsmab AA, Leendersa KL : Blood–brain barrier P-glycoprotein function is not impaired in early Parkinson's disease. Parkinsonism & Related Disorders 14:505-508, 2008
4. Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF : Blood-brain barrier impairment in Alzheimer disease: stability and functional significance.
Neurology 68(21):1809-14, 2007
5. Kortekaas R, Leenders KL, van Oostrom CH, Vaalburg W, Bart J, Willemsen TM, Harry Hendrikse N : Blood–Brain Barrier Dysfunction in Parkinsonian Midbrain In Vivo.
6. Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD : 6-Hydroxydopamine-induced alterations in blood–brain barrier permeabilit.
European Journal of Neuroscience 22:1158-1168, 2005
7. Chamberlain G, Fox J, Ashton B and Middleton J : Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739-2749, 2007
8. Desai BS, Monahan AJ, Carvey PM, Hendey B : Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell Transplant 16(3):285-99, 2007
9. Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A : Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86: 246-254, 2003
10. Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM : Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci 98:14669-14674, 2001
11. Fernagut PO, Diguet E, Bioulac B, Tison F : MPTP potentiates 3-nitropropionic acid-induced striatal damage in mice:reference to striatonigral degeneration. Experimental
Neurology 185: 47– 62, 2004
12. Gao HM and Hong JS : Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29: 357-365, 2008
13. Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN et al : [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61: 686-9, 2003
14. Hellmann MA, Panet H, Barhum Y, Melamed E and Offen D : Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett 395:124-128, 2006
15. Hitoshi S : Neuroimaging of PD, PSP, CBD and MSA-PET and SPECT studies. J Neurol 253 (Suppl 3): III30-III34, 2006
16. Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T : Microglial Activation Parallels System Degeneration in Multiple System Atrophy. SourceJournal of
17. Ji KA, Eu MY, Kang SH, Gwag BJ, Jou IL, Joe EH : Differential Neutrophil In.ltration Contributes to Regional Differences in Brain In.ammation in the Substantia Nigra Pars Compacta and Cortex. GLIA 56:1039-1047, 2008
18. Kacem K, Lacombe P, Seylaz J, Bonvento G. : Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 23:1-10, 1998
19. Karussis D, Kassis I, Kurkalli BG and Slavin S : Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 265:131-135, 2008
20. Kim HJ, Lee JH and Kim SH : Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. Journal of Neurotrauma 27:131-138, 2010
21. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO and Lee PH : Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 57:13-23, 2009
22. Kirik D, Rosenblad C, Bjorklund A : Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp. Neurol. 152; 259-277, 1998
23. Kirik D, Rosenblad C and Bjorklund A : Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Experimental Neurology 152:259-277, 1998
24. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S and Annunziato F : Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin
Pharmacol 6:435-441, 2006
25 . Lee PH, Kim JW, Bang OY, Ahn Y H, Joo IS and Huh K : Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther 83:723-730, 2008
26. Li W Y, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G and Bang OY : Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing.
27. Matsuura K, Kabuto H, Makino H and Ogawa N : Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J
Neurosci Methods 73:45-48, 1997
28. Min KJ, Yang MS, Kim SU, Jou I, Joe EH : Astrocytes induce hemeoxygenase 1 expression in microglia: A feasible mechanism for preventing excessive brain in ammation. J Neurosci 26:1880-1887, 2006
29. Nauta AJ and Fibbe WE : Immunomodulatory properties of mesenchymal stromal cells.
Blood 110:3499-3506, 2007
30. Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ : Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin Auton Res 1:9-13, 1991
31. Park HJ, Lee PH, Bang OY, Lee G and Ahn YH : Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. Journal of
Neurochemistry 107:141-151, 2008
32. Pyo H, Yang MS, Jou I, Joe EH : Wortmannin enhances lipopolysaccharide-induced inducible nitric oxide synthase expression in microglia in the presence of astrocytes in rats. Neurosci Lett 346:141-144, 2003
33. Ross HR, Romrell LJ, Kaye GI : Histology: A test and atlas. Baltimore: Williams & Wilkins. p 282, 1995
34. Saito A, Narasimhan P, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH : Neuroprotective role of a proline-rich Akt substrate in apoptotic neuronal cell death after stroke: relationships with nerve growth factor. J Neurosci 24:1584-93, 2004
35. Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K : A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology 50(4):966-71, 1998
36. Song SK, Lee SK, Lee JJ, Lee JE, Choi HS, Sohn YH, Lee PH : Blood–brain barrier impairment is functionally correlated with clinical severity in patients of multiple system atrophy. Neurobiology of Aging 2010, in press
37. Stefanova N, Poewe W and Wenning GK : Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol 210:421-427, 2008
38. Stefanova N, Puschban Z, Fernagut PO, Brouillet E, Tison F, Reindl M, Jellinger KA, Poewe W and Wenning GK : Neuropathological and behavioral changes induced by various treatment paradigms with MPTP and 3-nitropropionic acid in mice: towards a model of striatonigral degeneration (multiple system atrophy). Acta Neuropathol 106:157-166, 2003
39. Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ et al : Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol 166:869-76, 2005
40. Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer, M, Krieglstein J, Hollt V and Schulz S : A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci, 22:5865-5878, 2002
41. Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL : Minocycline reduces the lipopolysaccharide-induced in.ammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis 16:190-201, 2004
42. Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY : Tumour necrosis factor-alpha causes an increase in blood.brain barrier permeability during sepsis. J. Med. Microbiol 50:812-821, 2001
43. Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E et al : Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res 87:2728-39, 2009
44. Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R et al : Case-control study of multiple system atrophy. Mov Disord 20:158-63, 2005
45. Vincent VA, Van Dam AM, Persoons JH, Schotanus K, Steinbusch HW, Schoffelmeer AN, Berkenbosch F : Gradual inhibition of inducible nitric oxide synthase but not of interleukin-1 beta production in rat microglial cells of endotoxin-treated mixed glial cell cultures. Glia 17:94-102, 1996
46. Vizuete ML, Merino M, Venero JL, Santiago M, Cano J, Machado A : Histamine infusion induces a selective dopaminergic neuronal death along with an inflammatory reaction in rat substantia nigra. J Neurochem 75:540–552, 2000.
47. West MJ, Slomianka L and Gundersen HJ : Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anatomical Record, 231:482-497, 1991