저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
의학 석사학위 논문
The role of immunosenescence in
autoimmune/autoinflammatory
pathogenesis of Behçet's disease
아 주 대 학 교 대 학 원
의 학 과
The role of immunosenescence in
autoimmune/autoinflammatory
pathogenesis of Behçet's disease
지도교수 이 은 소
이 논문을 의학 석사학위 논문으로 제출함.
2016 년 8 월
아 주 대 학 교 대 학 원
의 학 과
양 지 영
양지영의 의학 석사학위 논문을 인준함.
심사위원장 이 은 소 (인)
심
사
위
원 손 성 향 (인)
심
사
위
원 박 선 (인)
아 주 대 학 교 대 학 원
2016 년 7 월 5 일
감사의 글
본 논문을 완성할 때까지 물심양면으로 도움을 주시고 지도와 조언을 아끼지 않으셨던 지도교수이신 이은소 교수님께 진심으로 감사 드립니다. 또한 좋은 연구를 할 수 있도록 부족한 제게 많은 조언과 격려를 주신 박선 교수님, 손성향 교수님께 깊은 감사의 마음을 전합니다. 그리고 연구 기간 동안 도움을 주신 피부과학교실의 모든 선생님들과 실험 여러 과정을 도와주신 박미진 선생님께 감사 드립니다. 언제나 아낌 없는 사랑으로 지원해 주시는 부모님, 남편을 비롯한 가족들에게도 깊은 감사의 마음을 전합니다. 2016 년 7 월 저자 씀i -ABSTRACT-
The role of immunosenescence in autoimmune/autoinflammatory
pathogenesis of Behçet's disease
Background and objectives: Behçet’s disease (BD) is a chronic inflammatory disease characterized by recurrent mucocutaneous ulceration and complications such as blindness and large vessel inflammation. Immunosenescence, aging of immune system, is related to increased susceptibility to infectious diseases, vaccine failure, and chronic low grade systemic inflammation. The role of immunosenescence in BD is not well understood. Therefore, the aim of this study was to investigate the differences in the frequencies of immunosenescent cells in the peripheral blood mononulclear cells in BD patients and controls.
Material and methods: Peripheral blood mononulclear cells (PBMCs) were extracted from age-matched active BD patients (n=19), inactive BD patients (n=20), disease controls (DCs, n=15) and healthy controls (HCs, n=15). Using flow cytometry, the frequencies of senescent CD4+ T cells (CD3+ CD4+ CD27- CD28- cells), CD8+ T cells (CD3+ CD8+ CD27- CD28- cells), and B cells (CD19+ CD27- IgD- cells) were analyzed. The differences among the groups, the correlation with chronological age in HC group, and whether the clinical indicators of disease severity, ongoing steroid treatment, or specific organ involvement affected the frequencies of senescent immune cells were investigated. In addition, senescence-associated β galactosidase (SA-β-Gal) activity was investigated in CD8+ T cells, using flow cytometry with 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (C12FDG).
Results: In active BD patients, the frequency of CD3+ CD8+ CD27- CD28- cells was significantly higher than in DCs and in HCs, respectively. Also, the frequency of CD3+ CD8+ CD27- CD28- cells increased significantly with the age in HCs, in accordance with the previous literature. Other senescent immune cells did not show significant
ii
differences. The levels of both C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) which indicate disease activity did not correlate with increased frequencies of senescent immune cells. Neither the steroid treatment nor specific organ involvement had significant influence on frequencies of senescent immune cells. Frequencies of SA-β-Gal+ cells among CD8+ T cells were significantly higher in active BD and in inactive BD compared to those in DCs and HCs, respectively.
Conclusion: CD3+ CD8+ CD27- CD28- cells, or senescent CD8+ T cells, were increased in the peripheral blood of patients with BD. Functional study or senescence-inducing study are needed to further elucidate the role of immunosenescence in the pathogenesis of BD.
Keyword: Behçet's disease, immunosenescence, CD8+ T cell, flow cytometry, senescence-associated β galactosidase
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ...iii
LIST OF FIGURES ... v
LIST OF TABLES ... vi
INTRODUCTION ... 1
MATERIAL AND METHODS ... 3
A. Subjects ... 3
B. Isolation of peripheral blood mononuclear cells (PBMCs) ... 3
C. Cell surface marker flow cytometry ... 4
D. Measurement of senescence-associated β galactosidase (SA-β-Gal) activity by flow cytometry... 4
E. Statistical analysis ... 5
RESULTS ... 6
A. Subject characteristics ... 6
B. Correlation between the chronological age and the frequency of senescent CD8+ T cells in HC group ... 8
C. Frequencies of immunosenescent cells in PBMCs in active BD, inactive BD, DCs, and HCs ... 9
D. Relation between disease activity, steroid treatment, or specific organ involvement and frequencies of senescent immune cells in BD patients ... 12 E. SA-β-Gal activity of CD8+ T cells in active BD, inactive BD, DCs, and HCs . 13
iv
DISCUSSION ... 15
CONCLUSION ... 19
REFERENCES ... 20
v
LIST OF FIGURES
Fig. 1. Correlation between the chronological age and the frequency of senescent CD8+ T cells among CD8+ T cells (%) of HC group ... 8
Fig. 2. Dot plots of senescent CD4+ T cells, CD8+ T cells, and B cells in representative subjects in PBMCs of active BD, inactive BD, DCs, and HCs ... 10
Fig. 3. Frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells in PBMCs of active BD, inactive BD, DCs, and HCs ... 11
Fig. 4. Dot plots of CD8+ SA-β-Gal+ cells in representative subjects in PBMCs of active BD, inactive BD, DCs, and HCs ... 13
Fig. 5. Freuqency of CD8+ SA-β-Gal+ cells in PBMCs of active BD inactive BD, DCs, and HCs ... 14
vi
LIST OF TABLES
Table. 1. Baseline demographics of active BD, inactive BD, DCs, and HCs ... 3
- 1 -
INTRODUCTION
Behçet’s disease (BD) is a chronic multisystemic inflammatory disease characterized by recurrent mucocutaneous ulceration, ocular involvement, and skin lesions, presenting with episodes of exacerbation. It can also involve multiple joints, gastrointestinal system, cardiovascular system, and central nervous system (Sakane et al., 1999; Hatemi et al., 2008). The pathogenesis of BD is not fully understood, but is known to be related with immune dysfunction in genetically predisposed hosts, which has characteristics of both autoimmune disease and autoinflammatory disease (Takeuchi et al., 2015). Excessive T helper type 1 (Th1) response, increased proinflammatory cytokines such as interleukin (IL) 2 and interferon gamma (IFNγ) (Ben Ahmed et al., 2004), association with IL-17 (Na et al., 2013; Ekinci et al., 2010), and suggested role of human heat-shock protein (HSP) 60 as a candidate autoantigen (Direskeneli et al., 2003) are features of autoimmunity. On the other hand, the features of autoinflammation are; lack of significant high-titer of auto-antibodies or antigen-specific T-cells, increased susceptibility of BD in patients with M694V MEFV mutation (Imirzalioglu et al., 2005), increase of C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) in active state, and dysregulation of IL-1β (Liang et al., 2013; Yüksel et al., 2014). The link between autoimmune feautures and autoinflammatory features is to be elucidated.
Immunosenescence, age-related changes in the structure and function of the immune system, is known to contribute to the increased susceptibility of elderly persons to infectious diseases, vaccine failure, and possibly autoimmunity and cancer (Pawelec, 1999; Targonski et al., 2007). The age-related T cell immunity is characterized by decreased number of naïve T cells due to thymic activity shrinkage, uneven and less diverse repertoire of T cells with expansions of clonal populations, and loss of expression of co-stimulatory molecules, such as CD28, CD27, and CD40L. (Goronzy et al., 2007; Weiskopf et al., 2009). Meanwhile, the age-related B cell immunity is characterized by decreased generation of B cell precursors, decreased number of naive B cells and diversity of the B cell repertoire, loss of expression of costimulatory molecules,
- 2 -
such as CD27 and CD40, reduced ability to isotype switch, and increased autoreactive serum antibodies (Weiskopf et al., 2009; Ademokun et al., 2010). Immunosenescent cells are thought to contribute to chronic inflammation and aging of other cells by secreting inflammatory cytokines and cytotoxic molecules. In particular, the role of immunosenescence in autoimmune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus, and type 1 diabetes mellitus is being actively researched. It is demonstrated that newly-diagnosed RA patients have accumulated deoxyribonucleic acid (DNA) damage in both naïve and memory T-cells, which is related to decreased levels of the DNA repair kinase ataxia telangiectasia mutated (ATM). ATM deficiency was shown to recapitulate the pre-aged phenotype as seen in RA T-cells whereas overexpression of ATM reconstituted DNA repair capabilities (Shao et al., 2009; Shao et al., 2010) In addition, increased frequency of the CD14bright CD16+ monocytes, a senescent subpopulation, in RA is demonstrated as well, and is known to be related with the expansion of T helper 17 (Th17) cells (Rossol et al., 2012). In patients with SLE, mesenchymal stem cells showed senescent phenotype and expression of p16(INK4A) was increased (Gu et al., 2012).
Chronic inflammation of BD may give rise to, or be the consequence of immunosenescence. Currently, the role of immunosenescence in the pathogenesis of BD has not been studied. In this study, we investigated the type of immunosenescent cells related to BD and possible factors related to immunosenescence, such as clinical symptoms, steroid treatment, and disease activity.
- 3 -
MATERIALS AND METHODS
A. Subjects
On approval by the Institutional Review Board (IRB number: AJIRB-BMR-GEN-14-462), the patients with BD were enrolled either who presented themselves for the first time or who were on follow-up visits at the outpatient clinic of Department of Dermatology, Ajou University Hospital from March 2015 to February 2016. The diagnosis was made upon both the BD criteria of International Study Group and the Japanese Criteria (International Study Group for Behçet’s Disease, 1990; Lee, 1997). Thirty-nine patients in total were divided into two groups; 19 in active group and 20 in inactive group. The patients in the active group had at least one of the BD symptoms despite treatment and those in the inactive group were in well-controlled states by taking anti-inflammatory medication. Patients with acute and chronic infection, liver or renal failure, diabetes mellitus, malignancies, other autoimmune or autoinflammatory disease, and BD patients with insufficient medical records were excluded. The disease control (DC) group consisted of age-matched 15 patients with recurrent aphthous ulcer only, and the healthy control (HC) group consisted of age-matched 15 healthy volunteers (Table. 1.). Informed consent was obtained from all of the participants prior to enrollment.
Table. 1. Baseline demographics of active BD, inactive BD, DCs, and HCs total number M:F Mean age, yr (SD, range) active BD 19 6:13 43.5 (7.7, 34-58) inactive BD 20 8:12 45.1 (7.8, 35-58) DC 15 7:8 44.1 (8.2, 30-56) HC 15 7:8 44.3 (8.2, 34-59)
B. Isolation of peripheral blood mononuclear cells (PBMCs)
- 4 -
(Vacutainer® Tubes; BectonDickinson, Stockholm, Sweden). PBMCs were isolated by Ficoll-Paque density gradient centrifugation (Ficoll Paque™ plus; StemCell Technologies, Vancouver, BC, Canada) from each sample and suspended in RPMI 1640 (HyClone; Thermo Scientific, UT, U.S.A.) containing 25 mg/ml gentamicin and 5% human serum.
C. Cell surface marker flow cytometry
Expression of different cell surface markers was analyzed by flow cytometry including markers of immunosenescent cells. PBMCs were labelled with conjugated monoclonal antibodies as follows: fluorescein isothiocyanate (FITC)-anti-CD3, allophycocyanin (APC)-cyanine (Cy)7-anti-CD4, peridinin chlorophyll (PerCP)-Cy5.5-anti-CD8, PerCP-Cy5.5-anti-CD19, phycoerythrin (PE)-Cy7-anti-CD27, APC-anti-CD28, and APC-anti-IgD (BD Biosciences Pharmingen, San Diego, CA, U.S.A.).
All antibodies were titrated to optimal concentrations according to the manufacturer’s protocol. PBMCs were incubated with the fluorescent-labeled monoclonal antibodies in dark at 4°C for 30 minutes. Cell surface expression of each molecule was then analyzed by a multi-parameter flow cytometer (BD FACS CANTO II; BD Biosciences, Moutain View, CA, U.S.A.). The frequencies of senescent CD4+ T cells (CD3+ CD4+ CD27- CD28- cells), senescent CD8+ T cells (CD3+ CD8+ CD27- CD28- cells), and senescent B cells (CD19+ CD27- IgD- cells) were analyzed.
D. Measurement of senescence-associated β galactosidase (SA-β-Gal) activity by flow cytometry
SA-b-Gal activity was measured according to the method described previously (Debacq-Chainiaux et al., 2009). Briefly, to induce lysosomal alkalinization, PBMCs were pretreated with 100 nM bafilomycin A1 in fresh cell culture medium at 37°C for 1 hour. Then fluorogenic substrate 5-Dodecanoylaminofluorescein
Di-β-D-- 5 Di-β-D--
Galactopyranoside (C12FDG) (C12FDG lacZ Gene Expression Kit, Thermo Fisher Scientific, MA, U.S.A.) was added to the cell culture medium to yield a final concentration of 33 μM. After 1-hour incubation cells were harvested with trypsin/EDTA, resuspended in phosphate buffered saline and analyzed immediately with multi-parameter flow cytometer (BD FACS CANTO II; BD Biosciences, Moutain View, CA, U.S.A.).
E. Statistical analysis
Subject characteristics of each group were compared using Pearson chi-square test and Fisher's exact test for dichotomous and nominal variables and independent t test for continuous variables with appropriate transformations.
The associations between the age and the frequencies of immunosenescent cells for each type of immune cells in HC group were assessed using Pearson correlation analysis. The differences of the frequencies of each type of immunosenescent cells among the groups were analyzed using one-way analysis of variance (ANOVA) when transformation did achieve a normal distribution. If not, Kruskal-Wallis test was used. Pearson correlation analysis was done to analyze whether the levels of CRP or ESR in BD group affected the frequencies of senescent immune cells. Independent t test was used when analyzing whether the current status of steroid treatment or specific organ involvement in BD group affected the frequencies of senescent immune cells. All statistical analyses were performed using statistical software (SPSS, Version 22.0, IBM, NY, U.S.A.). For all analyses, p value less than .05 was accepted as significant.
- 6 -
RESULTS
A. Subject characteristics
A total of 69 subjects entered the study (39 patients with BD, 15 DC, and 15 HC). Baseline clinical characteristics of patients with BD are summarized in Table 2. Nineteen patients were in clinically active state with a variety of symptoms at the time of enrollment, and 20 were in inactive state without any symptom. All of the patients with BD complained of mucocutaneous symptoms, including oral ulcer, genital ulcer, or skin manifestation at diagnosis. Ocular, joint, and gastrointestinal symptoms were less common. ESR and CRP levels were measured in blood samples of patients with BD. Active BD group had significantly higher level of both. Serum 25-hydroxyvitamin D [25(OH)D] level was also measured, but there was no difference between active and inactive BD groups.
- 7 - Table 2. Clinical characteristics of BD patients
active BD inactive BD p value
Symptoms at diagnosis Oral ulcer, No (%) 19 (100%) 20 (100%) - Genital ulcer, No (%) 12 (63%) 15 (75%) 0.423 Skin lesion, No (%) 15 (79%) 12 (60%) 0.200 Ocular lesion, No (%) 7 (37%) 8 (40%) 0.839 Arthritis, No (%) 9 (47%) 7 (35%) 0.433 Gastrointestinal, No (%) 2 (11%) 1 (5%) 0.605 Cardiovascular, No (%) 0 (0%) 3 (15%) 0.231 Central nervous system, No (%) 0 (0%) 0 (0%) -
Symptoms at enrollment Oral ulcer, No (%) 10 (53%) - - Genital ulcer, No (%) 4 (21%) - - Skin lesion, No (%) 11 (58%) - - Ocular lesion, No (%) 5 (26%) - - Arthritis, No (%) 3 (16%) - - Gastrointestinal, No (%) 2 (11%) - - Cardiovascular, No (%) 0 (0%) - - Central nervous system, No (%) 0 (0%) - -
Systemic steroid treatment at enrollment, No (%) 12 (63%) 3 (14%) 0.001*
ESR, mm/hr, mean (SD) 1.19 (1.62) 0.23 (0.35) 0.02†
CRP, mg/dL, mean (SD) 30.2 (20.8) 16.0 (14.2) 0.017†
Serum 25(OH)D, ng/ml, mean (SD)‡ 24.3 (7.16) 25.0 (9.00) 0.84
HLA-B51, No (%)§ 6 (50%) 5 (35.7%) 0.540
ESR, erythrocyte sedimentation rate. CRP, C-reactive protein.
*Calculated by Pearson chi-square test. †Calculated by independent t test.
‡Measured in 11 active BD patients and 15 inactive BD patients. §Measured in 12 active BD patients and 14 inactive BD patients.
- 8 -
B. Correlation between the chronological age and the frequency of senescent CD8+ T cells in HC group
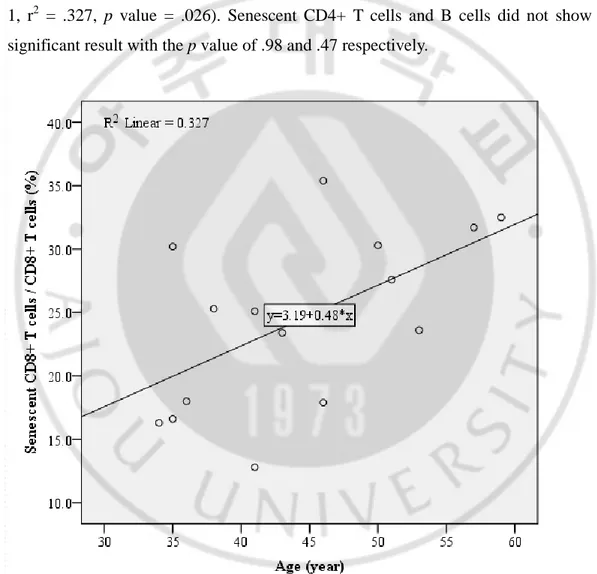
To examine whether the surface markers used in this study are related to senescence, the association between the chronological age and the frequencies of immunosenescent cells in HC group were analyzed. Among 3 types of immunosenescent cells, only senescent CD8+ T cells showed a significant correlation with the chronological age (Fig. 1, r2 = .327, p value = .026). Senescent CD4+ T cells and B cells did not show any significant result with the p value of .98 and .47 respectively.
Fig. 1. Correlation between the chronological age and the frequency of senescent CD8+ T cells among CD8+ T cells (%) of HC group. Senescent CD8+ T cells correlated with the chronological age with significance. (r2 = .327, p value = .026).
- 9 -
C. Frequencies of immunosenescent cells in PBMCs in active BD, inactive BD, DCs, and HCs
To examine which type of immunosenescent cells is associated with BD, cell surface expressions of CD27, CD28, and IgD as senescent markers was investigated in PBMCs using flow cytometry. The percentage of CD27- CD28- cells among CD3+ CD4+ cells, the percentage of CD27- CD28- cells among CD3+ CD8+ cells, and the percentage of CD27- IgD- cells among CD19+ cells was compared between groups (Fig. 2).
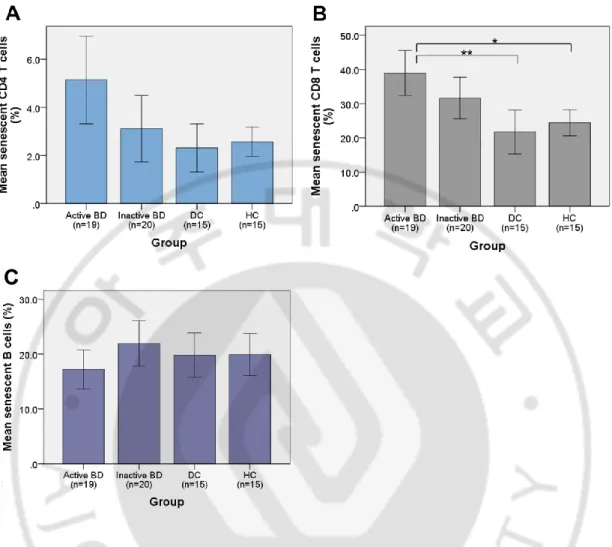
The mean percentage and SD of senescent CD4+ T cells were 5.1 ± 3.8 %, 3.1 ± 2.9 %, 2.3 ± 1.8 %, and 2.6 ± 1.1 % in active BD (n=19), inactive BD (n=20), DCs (n=15), and HCs (n=15), respectively. The frequency of senescent CD4+ T cells were increased in active BD and inactive BD compared to control groups but did not show statistical significance (Fig. 3A).
Regarding senescent CD8+ T cells, the mean percentage and SD were 39.0 ± 13.6 %, 31.6 ± 13.0 %, 21.7 ± 11.6 %, and 24.4 ± 6.9 % in active BD (n=19), inactive BD (n=20), DCs (n=15), and HCs (n=15), respectively. The frequency of senescent CD8+ T cells were increased in active BD and inactive BD compared to control groups. However, only active BD showed statistical significance with the p value of .001 for DC group and the p value of .005 for HC group (Fig. 3B).
The mean percentage and SD of senescent B cells were 17.2 ± 7.4 %, 21.9 ± 8.9 %, 19.8 ± 7.3 %, and 19.9 ± 6.6 % in active BD (n=19), inactive BD (n=20), DCs (n=15), and HCs (n=15), respectively. They neither showed any trend nor statistical significance (Fig. 3C).
- 10 -
Fig. 2. Dot plots of senescent CD4+ T cells, CD8+ T cells, and B cells in
representative subjects in PBMCs of active BD, inactive BD, DCs, and HCs. In each group, the percentage of CD27- CD28- cells among CD3+ CD4+ cells (A), the
percentage of CD27- CD28- cells among CD3+ CD8+ cells (B), and the percentage of CD27- IgD- cells among CD19+ cells (C) were analyzed. Numbers shown are mean values of each group.
- 11 -
Fig. 3. Frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells in PBMCs of active BD, inactive BD, DCs, and HCs. The frequency of senescent CD4+ T cells was increased in active and inactive BD compared to control groups but did not show any statistical significance (A). The frequency of senescent CD8+ T cells were
significantly increased in active BD compared to DC and HC (B, *p value = .001, **p value = .005). The frequency of senescent B cells did not show any statistical
- 12 -
D. Relation between disease activity, steroid treatment, or specific organ involvement and frequencies of senescent immune cells in BD patients
To determine whether the disease activity affected the senescence of the immune system, the correlation between the levels of CRP or ESR, and the frequencies of senescent immune cells were analyzed in BD group. The level of CRP did not correlate with the frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells significantly, with the p value of .200, .236, and .359, respectively. Neither did the level of ESR with the frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells (p value = .458, .944, .476, respectively).
In BD group, 15 patients were receiving systemic steroid treatment and 14 were not. The mean frequencies of senescent immune cells were compared. There was no difference in the mean frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells (p value = .292, .414, .162, respectively).
HLA-B51 was positive in 11 among 26 BD patients. The mean frequencies of senescent immune cells between HLA-B51 postiive group and HLA-B51 negative group were compared. There was no difference in the mean frequencies of senescent CD4+ T cells, CD8+ T cells, and B cells (p value = .095, .447, .056, respectively).
In BD group, all the patients had oral ulcers. There were 69.2% of BD patients with genital ulcer, 69.2% with cutaneous involvement, 38.5% with ocular involvement, 41.0% with joint involvement, 7.7% with gastrointestinal involvement, and 7.7% with cardiovascular involvement. The mean frequencies of senescent immune cells were compared between BD patients who had specific organ involvement and those who didn’t. None of the systems involved had effect on the frequencies of senescent immune cells with the p value > .05.
- 13 -
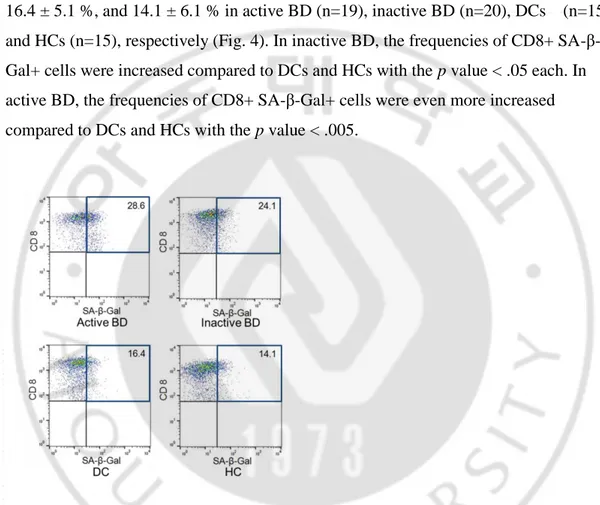
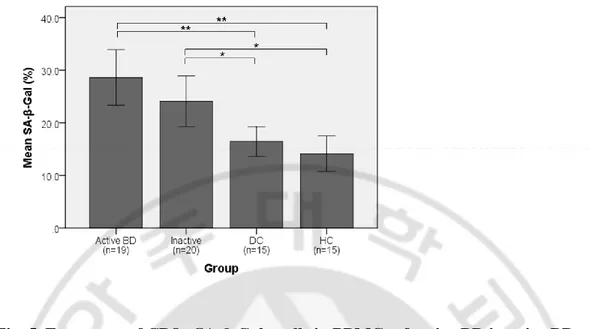
E. SA-β-Gal activity of CD8+ T cells in active BD, inactive BD, DCs, and HCs To confirm the senescence of CD8+ T cells, the activity of SA-β-Gal, a senescent marker, was measured. The percentage of SA-β-Gal+ CD8+ cells in CD8+ cells in each group was analyzed. The mean percentage and SD were 28.6 ± 11.0 %, 24.1 ± 10.3 %, 16.4 ± 5.1 %, and 14.1 ± 6.1 % in active BD (n=19), inactive BD (n=20), DCs (n=15), and HCs (n=15), respectively (Fig. 4). In inactive BD, the frequencies of CD8+ SA-β-Gal+ cells were increased compared to DCs and HCs with the p value < .05 each. In active BD, the frequencies of CD8+ SA-β-Gal+ cells were even more increased compared to DCs and HCs with the p value < .005.
Fig. 4. Dot plots of CD8+ SA-β-Gal+ cells in representative subjects in PBMCs of active BD, inactive BD, DCs, and HCs. Percentage of SA-β-Gal+ cells among CD8+ cells was analyzed in each group. Numbers shown are mean values of each group.
- 14 -
Fig. 5. Frequency of CD8+ SA-β-Gal+ cells in PBMCs of active BD inactive BD, DCs, and HCs. The frequencies of CD8+ SA-β-Gal+ cells were increased in active BD and inactive BD compared to controls with statistical significance (*p < .05, **p < .005).
- 15 -
DISCUSSION
The aim of the study was to investigate the frequencies of immunosenescent cells in peripheral blood mononulclear cells in BD patients and controls. The loss of expression of co-stimulatory molecules such as CD28 in CD4+ and CD8+ T cells is known to increase in an age-dependent manner in healthy subjects (Fagnoni et al., 1996; Vallejo et al., 1998). CD19+ CD27- IgD- cells, known as double-negative B cells, have also shown age-related increase in healthy subjects (Colonna-Romano et al., 2009). However, in the present study, only the frequency of senescent CD8+ cells revealed age-related increase with statistical significance in HC group. It could be partly explained that such phenotypic changes are much more frequently and dramatically found in the CD8+ T cells than in the CD4+ T cells (Fagnoni et al., 1996; Vallejo et al., 1998). Moreover, age-related loss of B cell function is a result of weakened interactions among immune cells, especially between B cells and senescent CD4+ T cells (Lazuardi et al., 2005). Due to the sample size of HC group, frequencies of both the senescent CD4+ cells and senescent B cells might have failed to show age-related increase. Larger sample size might be necessary to meet with age-related increase in these senescent cells with significance.
SA-β-Gal activity is a β-galactosidase activity detectable at pH 6.0 expressed by senescent cells, distinct from the β-galactosidase activity detectable at pH 4.0 which is present in proliferating and quiescent cells (Dimri et al.,1995). It is now widely used as a biomarker of cellular senescence in culture and in vivo (Dimri et al., 1995). Cytochemical assay using the chromogenic substrate 5-bromo-4-chloro-3-indoyl β-D-galactopyranoside (X-gal) is the first used method of detection and is popularly used until now, but has limitations such as low sensitivity. In this study, using fluorescence-based assay coupled to flow cytometry, the SA-β-Gal activity could be detected more sensitively and quantitatively (Debacq-Chainiaux et al., 2009). The result confirmed the
- 16 -
increased percentage of senescent CD8+ T cells in active and inactive BD compared to controls.
In the pathogenesis of BD, T cell response is accepted as more important than B cell response. For example, pathogenesis of BD is related to with increased Th1 cytokines such as IL-2 and IFNγ (Ben Ahmed et al., 2004). Recently, association with IL-17 has been also reported (Na et al., 2013; Ekinci et al., 2010). Moreover, the association of human leukocyte antigen (HLA)-B51 with BD implicate a role for class I molecules with the involvement of CD8+ T cells in disease development (Yu et al., 2004; Yasuoka et al., 2004). The results of this study were coinciding in that the frequency of senescent B cells did not show any trend or statistical significance. The frequency of senescent CD4+ T cells had a tendency to increase along the presence and activity of BD, but without significance. It suggests that the senescence of CD4+ T cell may be less related to BD in the pathogenesis of BD.
The study result shows that senescence of CD8+ T cells is associated with the immune dysfunction of BD. Considering the proportions of CD3+ CD8+ cells in the PBMCs were not significantly different between BD and controls (Bank et al., 2003), only the percentage of the senescent subpopulation has changed. The result does not explain whether the senescence is the cause or the consequence of the chronic inflammation in BD. Assuming the senescence of CD8+ T cells as the cause of the inflammation, tissue inflammation may result from proinflammatory cytokines and cytotoxic molecules released by senescent T cells. The loss of CD28 in T cells is associated with an increased production of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα) and IFNγ, and increased cytotoxicity, via expression of perforin and granzyme B and a propensity to migrate into tissues (Almanzar et al., 2004). However, there is a tendency for a higher incidence of BD in the third decade of life, which cannot be explained if the senescence is associated with the cause of inflammation. Therefore, it would be more natural to explain the senescence of CD8+ T cells as the result. There are several possible explanations. First, it could be the result of viral infection. Viruses such as
- 17 -
herpes simplex I virus (HSV-I) and cytomegalovirus (CMV) are of the most explored viral etiology (Galeone et al., 2012). HSV-I DNA was detected more often in the saliva and the peripheral blood leukocytes of the patients with BD compared to those of HCs (Lee et al., 1996; Studd et al., 1991), suggesting HSV-I infection as one of the etiologic or triggering factors in BD. In addition, CMV infection is reported in relation with clinical flares of BD (Martin et al., 2010), and its DNA was detected more frequently in the blood of BD group compared to that of controls, but without statistical significance (Irschick et al., 2011). CMV infection is well known to induce the senescence of CD8+ T cells (Pawelec, 2014). Chronic antigenic load by HSV infected cells might as well result in the senescence of CD8+ T cells, as shown in HSV-infected mice (Lang et al., 2009). Second, the immune activation by HSP may lead to the senescence of T cells. HSP is a immunoreactive protein produced in response to stressful conditions. Microbial 65-kDa HSP shows significant homology with the human 60-kDa mitochondrial HSP and shares antigenicity with an oral mucosal antigen (Lehner et al., 1991). T cells specific to HSP are known to mediate tissue damage by both the production of Th1 cytokines, such as IFN-r and TNF-a, and activation of cell-mediated cytotoxicity (Benagiano et al., 2005). The increased cell-mediated cytotoxicity may result in the senescence of CD8+ T cells. Third, chronic inflammation caused by T cells in the involved tissues might bring about the immunosenescence. Infiltrates consisting of self-reactive lymphocytes and neutrophils are associated with skin lesion of BD (Mochizuki et al., 1997). Both the CD4+ and CD8+ T cells are known to regulate neutrophilc inflammation in autoinflammatory diseases (Keller et al., 2005). Chronic activation of T cells in the site of inflammation might be the cause of the senescence of T cells.
Meanwhile, accelerated immunosenescence of T cells is well known to occur in RA patients. Accumulation of DNA and impaired DNA repair mechanisms in naïve T cells facilitate their differentiation towards the senescent state and compensatory expansion of the senescent T cells in the peripheral blood, finally resulting in synovial inflammation by synovial senescent T cells (Chalan et al., 2015). This can explain why the incidence
- 18 -
of RA increases with age. Other types of senescence cells related to BD needs to be further investigated.
The limitation of this study is that it only revealed phenotypic features of senescent immune cells. To further confirm the role of immunosenescence in pathogenesis of BD, functional studies are necessary. The effect of cytotoxic molecules and proinflammatory cytokines released by senescent T cells on inflammation of BD needs to be investigated. Perforin, granzyme, TNFα, and IFNγ may be candidates, as they are known to be associated with inflammation in BD. (Accardo-Palumbo et al., 2010; Ahn et al., 2005). In addition, investigating the proneness to immunosenescence in patients with BD would help elucidate the role of immunosenescence. Oxidizing or DNA damaging agents can be used to induce senescence (Chrétien et al., 2008).
- 19 -
CONCLUSION
In this study, CD3+ CD8+ CD27- CD28- cells, or senescent CD8+ T cells, were increased in the peripheral blood of patients with BD. It had a tendency to be more increased in active BD compared to inactive BD. Functional study or senescence-inducing study are necessary to further elucidate the role of immunosenescence in the pathogenesis of BD.
- 20 -
REFERENCES
1. Accardo-Palumbo A, Giardina AR, Ciccia F, Ferrante A, Principato A, Impastato R, Giardina E, Triolo G: Phenotype and functional changes of Vgamma9/Vdelta2 T lymphocytes in Behçet's disease and the effect of infliximab on Vgamma9/Vdelta2 T cell expansion, activation and cytotoxicity. Arthritis Res Ther 12:R109, 2010
2. Ademokun A, Wu YC, Dunn-Walters D: The ageing B cell population: composition and function. Biogerontology 11:125-137, 2010
3. Ahn JK, Chung H, Lee DS, Yu YS, Yu HG: CD8brightCD56+ T cells are cytotoxic effectors in patients with active Behcet's uveitis. J Immunol 175:6133-6142, 2005 4. Almanzar G, Schwaiger S, Jenewein B, Keller M, Grubeck-Loebenstein B, Würzner
R, Schönitzer D: IFN-gamma production by CMV-specific CD8+ T cells is high in elderly donors. Exp Gerontol 39: 863. author reply 867-868, 2004
5. Bank I, Duvdevani M, Livneh A: Expansion of gammadelta T-cells in Behçet's disease: role of disease activity and microbial flora in oral ulcers. J Lab Clin Med 141:33-40, 2003
6. Benagiano M, D'Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombolà G, Romagnani S, Cassone A, Del Prete G: Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J
Immunol 174:6509-6517, 2005
7. Ben Ahmed M, Houman H, Miled M, Dellagi K, Louzir H: Involvement of chemokines and Th1 cytokines in the pathogenesis of mucocutaneous lesions of Behçet’s disease. Arthritis Rheum 50:2291-2295, 2004
8. Chalan P, van den Berg A, Kroesen BJ, Brouwer L, Boots A: Rheumatoid Arthritis, Immunosenescence and the Hallmarks of Aging. Curr Aging Sci 8:131-146, 2015
- 21 -
9. Chrétien A, Dierick JF, Delaive E, Larsen MR, Dieu M, Raes M, Deroanne CF, Roepstorff P, Toussaint O: Role of TGF-beta1-independent changes in protein neosynthesis, p38alphaMAPK, and cdc42 in hydrogen peroxide-induced senescence-like morphogenesis. Free Radic Biol Med 44:1732-1751, 2008
10. Colonna-Romano G, Bulati M, Aquino A, Pellicanò M, Vitello S, Lio D, Candore G, Caruso C: A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev 130:681-690, 2009
11. Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O: Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798-1806, 2009
12. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J: A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363-9367, 1995
13. Direskeneli H, Saruhan-Direskeneli G: The role of heat shock proteins in Behçet's disease. Clin Exp Rheumatol 21:S44-S48, 2003
14. Ekinci NS, Alpsoy E, Karakas AA, Yilmaz SB, Yegin O: IL-17A has an important role in the acute attacks of Behcet’s disease. J Invest Dermatol 130:2136-2138, 2010 15. Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P: Expansion of cytotoxic CD8+ CD28-T cells in healthy ageing people, including centenarians. Immunology 88:501-507, 1996
16. Galeone M, Colucci R, D'Erme AM, Moretti S, Lotti T: Potential infectious etiology of Behçet's disease. Patholog Res Int 2012:595380, 2012
- 22 -
17. Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol 42:400-406, 2007
18. Gu Z, Cao X, Jiang J, Li L, Da Z, Liu H, Cheng C: Upregulation of p16INK4A promotes cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Cell Signal 24:2307-2314, 2012
19. Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, Houman MH, Kötter I, Olivieri I, Salvarani C, Sfikakis PP, Siva A, Stanford MR, Stübiger N, Yurdakul S, Yazici H; EULAR Expert Committee: EULAR ecommendations for the management of Behçet disease. Ann Rheum Dis 67:1656-1662, 2008
20. Imirzalioglu N, Dursun A, Tastan B, Soysal Y, Yakicier MC: MEFV gene is a probable susceptibility gene for Behçet's disease. Scand J Rheumatol 34:56-58, 2005 21. International Study Group for Behçet’s Disease: Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet 335:1078-1080, 1990
22. Irschick EU, Philipp S, Shahram F, Schirmer M, Sedigh M, Ziaee N, Gassner C, Schennach H, Meyer M, Larcher C, Herold M, Schoenitzer D, Fuchs D, Schoenbauer M, Maass M, Huemer HP, Davatchi F: Investigation of bacterial and viral agents and immune status in Behcet's disease patients from Iran. Int J Rheum
Dis 14:298-310, 2011
23. Keller M, Spanou Z, Schaerli P, Britschgi M, Yawalkar N, Seitz M, Villiger PM, Pichler WJ: T cell-regulated neutrophilic inflammation in autoinflammatory diseases.
- 23 -
24. Lang A, Brien JD, Nikolich-Zugich J: Inflation and long-term maintenance of CD8 T cells responding to a latent herpesvirus depend upon establishment of latency and presence of viral antigens. J Immunol 183:8077–8087, 2009
25. Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B: Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology 114: 37, 2005
26. Lee S, Bang D, Cho YH, Lee ES, Sohn S: Polymerase chain reaction reveals herpes simplex virus DNA in saliva of patients with Behçet's disease. Arch Dermatol Res 288:179-183, 1996
27. Lee S: Diagnostic criteria of Behçet's disease: problems and suggestions. Yonsei
Med J 38:365-369, 1997
28. Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun 59:1434-1441, 1991 29. Liang L, Tan X, Zhou Q, Zhu Y, Tian Y, Yu H, Kijlstra A, Yang P: IL-1β triggered by
peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome-dependent pathways is involved in ocular Behçet's disease. Invest
Ophthalmol Vis Sci 54:402-414, 2013
30. Mochizuki M, Morita E, Yamamoto S, Yamana S: Characteristics of T cell lines established from skin lesions of Behçet's disease. J Dermatol Sci 15:9-13, 1997 31. Na SY, Park MJ, Park S, Lee ES: Up-regulation of Th17 and related cytokines in
Behçet's disease corresponding to disease activity. Clin Exp Rheumatol 31:32-40, 2013
- 24 -
32. Pawelec G: Immunosenescence: impact in the young as well as the old? Mech
Ageing Dev 108:1-7, 1999
33. Pawelec G: Immunosenenescence: role of cytomegalovirus. Exp Gerontol 54:1-5, 2014
34. Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U: The CD14 bright CD16 + monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 64:671–677, 2012
35. Sakane T, Takeno M, Suzuki N, Inaba G: Behçet's disease. N Engl J Med 341:1284-1291, 1999
36. Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM: Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med 206:1435-1449, 2009 37. Shao L, Goronzy JJ, Weyand CM: DNA-dependent protein kinase catalytic subunit
mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med 2:415-427, 2010 38. Studd M, McCance DJ, Lehner T: Detection of HSV-1 DNA in patients with
Behçet's syndrome and in patients with recurrent oral ulcers by the polymerase chain reaction. J Med Microbiol 34:39-43, 1991
39. Takeuchi M, Kastner DL, Remmers EF: The immunogenetics of Behçet's disease: A comprehensive review. J Autoimmun 64:137-148, 2015
40. Targonski PV, Jacobson RM, Poland GA: Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine 25:3066-3069, 2007
- 25 -
41. Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ: Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem 273:8119-8129, 1998
42. Weiskopf D, Weinberger B, Grubeck-Loebenstein B: The aging of the immune system. Transpl Int 22:1041-1050, 2009
43. Yasuoka H, Okazaki Y, Kawakami Y, Hirakata M, Inoko H, Ikeda Y, Kuwana M: Autoreactive CD8+ cytotoxic T lymphocytes to major histocompatibility complex class I chain-related gene A in patients with Behçet’s disease. Arthritis Rheum 50: 3658-3662, 2004
44. Yu HG, Lee DS, Seo JM, Ahn JK, Yu YS, Lee WJ, Chung H: The number of CD8+ T cells and NKT cells increases in the aqueous humor of patients with Behçet’s uveitis. Clin Exp Immunol 137: 437-443, 2004
45. Yüksel Ş, Eren E, Hatemi G, Sahillioğlu AC, Gültekin Y, Demiröz D, Akdiş C, Fresko İ, Ö zören N: Novel NLRP3/cryopyrin mutations and pro-inflammatory cytokine profiles in Behçet's syndrome patients. Int Immunol 26:71-81, 2014
- 26 - - 국문요약 -
베체트병의 자가면역/자가염증 기전에서 면역노화의 역할
아주대학교 대학원 의학과 양 지 영 (지도교수: 이 은 소) 연구배경: 베체트병은 점막 및 피부, 눈, 심혈관계, 신장, 위장관계, 신경계를 침 범하는 임상적 특징을 가지는 만성 재발성 전신 질환이다. 면역노화는 연령 증가 에 따른 면역체계의 변화로, 감염성 질환, 백신 실패와 악성 종양, 만성 염증 등 과 관련이 있다. 현재까지 베체트병의 병인 기전에서 면역노화의 역할은 밝혀진 바 없다. 연구목적: 따라서 본 연구에서 베체트병 환자군과 대조군의 말초혈액 단핵세포에 서 면역노화 세포의 빈도의 차이가 있는지 알아보고자 하였다. 연구방법: 39명의 베체트병 환자군과 연령 일치된 질환대조군 15명, 정상대조군 15명을 대상으로 말초혈단핵세포를 분리하였다. 유동세포계수법(flow cytometry)를 이용하여 말초혈단핵세포에서 노화 CD4+ T 세포 (CD3+ CD4+ CD27- CD28- 세포), 노화 CD8+ T 세포 (CD3+ CD8+ CD27- CD28- 세 포), 노화 B 세포 (CD19+ CD27- IgD- 세포)의 빈도를 측정하였다. 언급한 세 가지 면역노화 세포 빈도의 각 군 간 차이, 질병활성도에 따른 차이, 스테로 이드 유무 및 특정 장기 침범 유무에 따른 차이를 분석하였다. 또, 형광원으로 5-dodecanoylaminofluorescein di-β-D-galactopyranoside(C12FDG)를 이 용하여 CD8+ 세포에서 노화 관련 베타갈락토시다아제(senescence-associated β galactosidase) 활성도를 측정하였다. 연구결과: 활동성 베체트병 환자군의 말초혈액에서 CD3+ CD8+ CD27- CD28- 세포의 빈도가 질병대조군 및 정상대조군에 비해 통계적으로 유의하게- 27 -
높았다. 정상대조군에서 이미 알려진 바와 같이 CD3+ CD8+ CD27- CD28- 세포의 빈도가 연령에 따라 증가하는 경향을 보였다. 각 군에서 CD3+ CD4+ CD27- CD28- 세포 나 CD19+ CD27- IgD- 세포의 빈도는 통계적으로 유의 한 차이를 보이지 않았다. 베체트병 활성도와 관련한 C 반응성 단백(C reactive protein) 및 적혈구 침강 속도(erythrocyte sedimentation rate)와 면역 노화 세포의 빈 도 간 유의한 관련성은 보이지 않았다. 또, 스테로이드 치료 유무 및 다른 장기 침범 유무 역시 면역 노화 세포의 빈도에 영향을 미치지 않았다. 활동성 및 비활 동성 베체트변 환자군의 CD8+ 세포에서 노화 관련 베타갈락토시다아제의 활성 이 대조군과 비교했을 때 통계적으로 유의하게 증가되어 있는 것을 확인하였다. 결론: 본 연구 결과 대조군과 비교했을 때 베체트병 환자의 말초혈액에서 노화 CD8+ T 세포의 빈도가 증가되어 있음을 확인하였다. 더 나아가 베체트병 환자의 노화 CD8+ T 세포의 기능 및 노화 유도에 대한 반응에 관한 연구가 필요할 것이 다. 핵심어: 베체트병, 면역노화, CD8+ T 세포, 유동세포계수법, 노화 관련 베타갈 락토시다아제