The Protective Effects of Green Tea
Polyphenol against
Ischemia/Reperfusion Injury in the
Rabbit Kidney
Joong Hyuk Yim
Department of Medicine
The Protective Effects of Green Tea
Polyphenol against
Ischemia/Reperfusion Injury in the
Rabbit Kidney
Directed by Professor Beyoung Yun Park
A Dissertation Submitted to the Department of
Medicine, the Graduate School of Yonsei University
in partial fulfillment of the requirements for the
degree of Doctor of Philosophy
Joong Hyuk Yim
De
De
De
This certifies that the dissertation of Joong Hyuk Yim
is approved
Thesis Supervisor : Beyoung Yun Park
The Graduate School
The Graduate School
The Graduate School
The Graduate School
Yonsei University
Yonsei University
Yonsei University
Yonsei University
De
De
De
감사의
감사의
감사의
감사의 글
글
글
글
긴 시간 동안의 작은 결실은 저를 아껴주신 많은 선생님들의 도움이 있었기에 가능했습니다. 본 논문을 완성하기 까지 여러가지로 부족한 제게 세심한 지도와 격려를 아끼지 않으신 박병윤 지도 교수님께 진심으로 감사 드립니다. 바쁘신 와중에도 논문 지도를 맡아주셔서 많은 조언과 도움을 주신 나동균 교수님, 외과학교실의 김유선교수님, 병리학교실의 양우익 교수님, 신장내과의 최규헌 교수님께도 감사의 말씀을 올립니다. 여러 교수님들의 깊은 뜻을 항상 명심하며 교수님들의 뜻에 어긋나지 않게 노력하겠습니다. 또한 연구의 방향과 진행에 많은 도움을 주신 의과대학 의용공학교실의 박종철 교수님, 한동욱 박사님, 백현숙 연구원 및 성형외과학교실의 탁관철 주임교수님 이하 모든 교실원들에게도 감사의 뜻을 전하고 싶습니다. 성형외과란 학문과 진료의 길을 가면서 최선의 지도를 받을 수 있도록 이끌어주시고 격려를 해주신 교실의 교수님 여러분들께도 깊은 감사를 드리면 여러 동료와 후배들과도 기쁨을 나누고 싶습니다. 한결 같은 마음으로 곁에서 힘을 북돋워준 가족들과 이 기쁨을 함께 나누며, 끝으로 태어나면서부터 지금까지 많은 것들을 내어주시면서도 조용히 옆에서 지켜봐 주신 아버님과 어머님께 깊은 존경과 사랑의 마음으로 이 논문을 바칩니다.
2006
2006
2006
2006
년
년
년
년 12
12
12
12
월
월
월
월
임
임
임
임 중혁
중혁
중혁 올림
중혁
올림
올림
올림
Table of contents
ABSTRACT……….. 1
I. INTRODUCTION……… 4
II. MATERIALS AND METHODS……… 8
1. In vitro study ……… 8
A. Endothelial cell culture ……… 8
B. Oxidative stress induction ……….. 8
C. Polyphenol treatment ……….. 9
D. Cell viability measurements by flow cytometry ……….. 10
2. In vivo study ………. 11
A. GTP preparation ……… 11
B. Animals and experimental groups ……… 11
C. Surgical procedures and treatments ……… 12
D. Blood sampling and renal function ……… 14
E. Histological analysis ……… 15
3. Statistical analysis ……… 15
III. RESULTS ……… 17 1. Protective effects of GTP against H2O2-induced oxidative
Stress ……… 17
2. Protective effects of GTP against Xn/XO-induced oxidative stress ……… 22
3. The effects of GTP pretreatment on body weight and mortality ……… 23
4. The effects of GTP pretreatment on renal function …………... 25
5. The effects of GTP pretreatment on renal morphology ……… 27
IV. DISCUSSION ………... 35 V. CONCLUSION ……….. 41 REFERENCES ……… 42 국문요약 ……….. 48
List of Figures
List of Figures
List of Figures
List of Figures
Figure. 1 Ischemia-reperfusion injury procedures of rabbits ……… 14 Figure. 2. ROS-induced cytotoxicity in HUMVECs. ………. 19 Figure. 3. Effect of GTP on ROS-induced cytotoxicity of
HUMVECs. ……… 20 Figure. 4 Optical microscopic photographs (200×) of the
ROS-damaged HUMVECs pretreated with or without 10 μg/ml GTP. ……… 21 Figure. 5 Changes of body weights according to ischemic time. ….. 24 Figure 6. The effects of green tea polyphenol (GTP) pretreatment
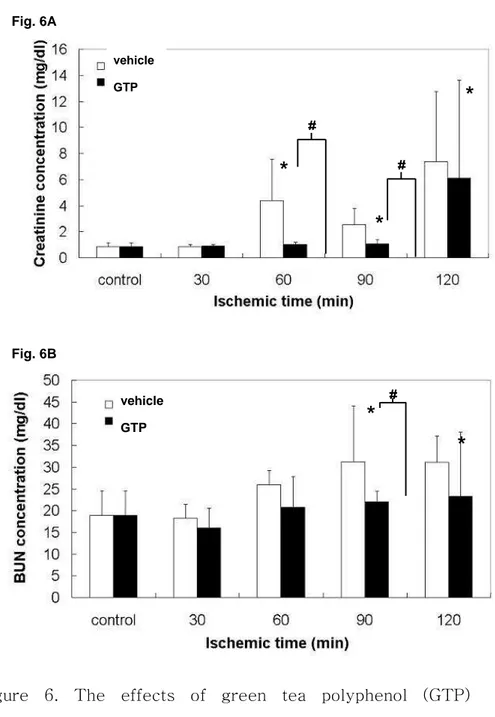
on renal function. Serum creatinine (SCr) level (A) and blood urea nitrogen (BUN) level (B) are expressed as milligrams per deciliter. ……… 26 Figure 7. The renal histology of sham-operated kidney (A),
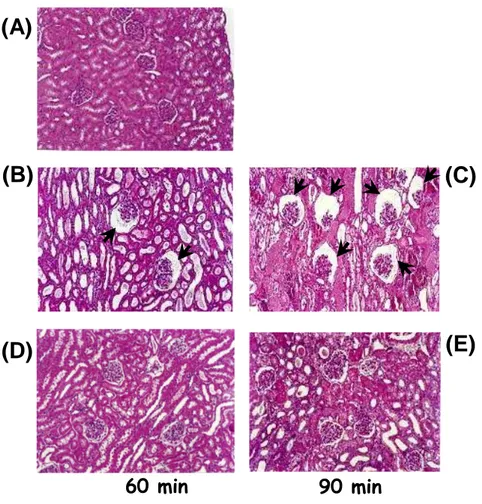
vehicle-treated kidney (B, C) and GTP-treated kidney (D, E) after 60 minutes(B, D) and 90 minutes(C, E) of ischemia, followed by 24 hours of reperfusion. The photographs shown in this figure are representative of 6 independent rabbits showing similar results. The
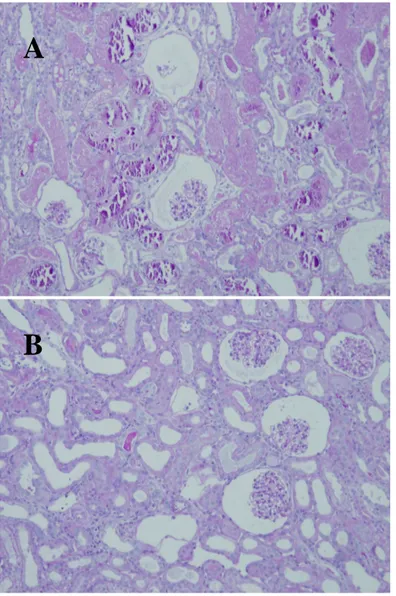
arrows represent the glomerular collapse (H&E stain, Original magnification × 100). ……… 30 Figure 8. The renal histology of vehicle-treated kidney (A) and
GTP-treated kidney (B) after 90 minutes of ischemia, followed by 24 hours of reperfusion. Collapsed glomeruli, luminal calcification, and sloughing of epithelial cells into the tubular lumen were observed in the vehicle-treated kidney (A). But, GTP-treated kidney (B) demonstrated intact tubular lumen and glomerulus. (H & E stain, Original magnification × 100)……… 31 Figure 9. The PAS stain of sham-operated kidney (A),
vehicle-treated kidney (B, C) and GTP-vehicle-treated kidney (D, E) after 60 minutes(B, D) and 90 minutes(C, E) of ischemia, followed by 24 hours of reperfusion.The arrows represent intraluminal calcification and the asterisks(*) in (B) represent PAS positive materials in the tubular lumens(PAS stain, Original magnification × 100)…….. 32 Figure 10. The renal histology of vehicle-treated kidney (A) and
GTP-treated kidney (B) with PAS stain after 90 minutes of ischemia, followed by 24 hours of reperfusion. Collapsed glomeruli, luminal calcification, and disfigurations of basement membrane on the tubules (A). But, GTP-treated kidney (B) was observed some intact basement membrane and glomeruli………… 33 Figure 11. The tubular injury score of vehicle-treated and GTP-
A A A
Abstractbstractbstractbstract
The Protective Effects of Green Tea Polyphenol against
The Protective Effects of Green Tea Polyphenol against
The Protective Effects of Green Tea Polyphenol against
The Protective Effects of Green Tea Polyphenol against
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
JJJJoo
oo
oong Hyuk
oo
ng Hyuk
ng Hyuk
ng Hyuk Y
Y
Yim
Y
im
im
im
Department of Medicine
Department of Medicine
Department of Medicine
Department of Medicine
The Graduate School, Yonsei University
The Graduate School, Yonsei University
The Graduate School, Yonsei University
The Graduate School, Yonsei University
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
Reactive oxygen species have been implicated in the pathogenesis of renal injury after ischemia/reperfusion (I/R). Recently, green tea polyphenol (GTP) has been found to protect the myocardium and liver against I/R injury. Less attention, however, has been paid to the protective effects of GTP with respect to the kidneys. This study was designed to investigate the potential protective roles played by GTP against the injurious effects of reactive oxygen species in human microvascular endothelial cells (HUMVECs) and to
determine whether GTP could protect renal cells from ischemic reperfusion injury of rabbits.
In the in vitro study, oxidative stress was induced in cultured HUMVECs, either by adding 10 mM H2O2 or by the
action of 10 U/l xanthine oxidase (XO) in the presence of xanthine (250 μM). In the in vivo study, the rabbits were divided into three groups of equal size: control (sham-operated), I/R + vehicle (normal saline) and I/R + GTP groups. Each group consisted of six rabbits. Animals underwent 30, 60, 90 and 120 minutes of ischemia, followed by 24 hours of reperfusion, respectively. GTP (200μg/kg) or the vehicle was administered 45 minutes prior to commencement of I/R.
Oxidative stress by H2O2, xanthine+XO treatments produced
a significant reduction (to 68% and 71%, respectively) in HUMVEC viability. On the microscopic observations, the morphological changes and necrotic detachment were appreciably induced by both treatments. The H2O2-induced
pre-incubating the ECs with 10 μg/ml GTP for 1 h. When the oxidative stress was induced by XO, the cell viability and morphology were also significantly maintained at the same GTP concentration. In the in vivo study, the results demonstrated that GTP administration resulted in a significant (P < 0.05) reduction of renal damage after 90 minutes of ischemia, as indicated by the decreased levels of creatinine and urea nitrogen in serum. These results were confirmed by histological examinations, which showed that GTP pretreatment inhibited necrosis and sloughing of the proximal tubules induced by I/R. Examinations also showed decreased necrotic areas in the medulla and decreased glomerular collapse in the I/R-injured rabbits.
The results of this study suggest that GTP can reduce renal injury by preventing the oxidative stress dependent injuries on I/R and may be used in renal transplantation as an antioxidant.
Key Words: green tea polyphenol, ischemia/reperfusion injury, anti-oxidants, rabbit kidney
The Protective Effects of Green Tea Polyphenol
The Protective Effects of Green Tea Polyphenol
The Protective Effects of Green Tea Polyphenol
The Protective Effects of Green Tea Polyphenol against
against
against
against
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
Ischemia/Reperfusion Injury in the Rabbit Kidney
Joong Hyuk Y
Joong Hyuk Y
Joong Hyuk Y
Joong Hyuk Yim
im
im
im
Department of Medicine
Department of Medicine
Department of Medicine
Department of Medicine
The Graduate School, Yonsei University
The Graduate School, Yonsei University
The Graduate School, Yonsei University
The Graduate School, Yonsei University
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
(Directed by Professor Beyoung Yun Park)
I. I. I.
I. INTRODUCTIONINTRODUCTIONINTRODUCTIONINTRODUCTION
Renal ischemia initiates a complex and interrelated sequence of events, resulting in the injury and death of renal cells.1 Reperfusion, although essential for the survival of
ischemic renal tissue, causes additional damage (reperfusion injury).2 Together, ischemia/reperfusion (I/R) of the kidney
contribute to the renal dysfunction and injury associated with ischemic acute renal failure.3,4 Although the exact
mechanisms involved in the pathogenesis of acute renal failure have not been fully elucidated, it is generally believed
that reactive oxygen species (ROS) and reactive nitrogen species are the key mediators of I/R-induced damage to the kidney. Oxidative and nitrosative stress cause lipid peroxidation of cell membranes,5, 6
protein and enzyme oxidation,7 and some irreversible DNA changes,8, 9
collectively leading to the loss of cell viability, either via necrotic or apoptotic pathways.10, 11 One possible way of
preventing ROS-mediated cellular injury is to augment the endogenous oxidative defenses by the dietary intake of antioxidants such as vitamins A, C and E. Recently, a great deal of attention has been focused on a variety of non-vitamin antioxidants such as phenolic compounds, which may also contribute to the cellular antioxidative defense mechanisms, and which can be found in many plant species, such as green tea, fruits and vegetables.
One of the approaches to limit apoptotic or necrotic cell death in response to I/R injury may be antioxidant therapy. Some antioxidants such as vitamins C and E have been shown to have protective properties against ischemia-induced tissue
damage.12, 13 Recently, considerable attention has been
focused on a variety of non-vitamin antioxidants such as phenolic compounds, including quercetin, curcumin, resveratrol etc., which might contribute to renal protection.14,
15
Hyon performed one of the first studies suggesting that non-vitamin antioxidants may offer tissue preservation; he observed that green tea polyphenol (GTP) may contribute to the physiological preservation of tissues or organs, particularly in rat pancreatic islets.16
Since then, little evidence has been accumulated showing these beneficial preservative effects of GTP compared to other effects such as anticarcinogenic and anti-inflammatory activities. The extension of Hyon’s observation regarding the preservation of tissues for transplantation will make it possible to store tissues or organs for longer periods by a concentration-adjusted GTP treatment. Previous studies have demonstrated that GTP can reduce I/R-induced injuries in the livers, hearts, intestines and nerves of rats or mice.17-20 Only a few reports,
however, have focused on its protective activities against I/R injury in rabbit kidneys.21, 22
Consequently, we sought to determine whether GTP pretreatment would protect kidneys from cellular damage after I/R injury. I/R kidneys represent a suitable and well-characterized model for studying free radical-induced oxidative stress and inflammatory response, which can contribute significantly to tissue injury and the functional damage of renal tissues. Our principal hypothesis was that the feasible protective mode of action of GTP in a rabbit model of renal I/R injury would be through prevention of oxidative stress dependent injuries by its anti-oxidant effects.
II. II. II.
II. MATERIALS AND METHODSMATERIALS AND METHODSMATERIALS AND METHODS MATERIALS AND METHODS
1. In vitro study 1. In vitro study 1. In vitro study 1. In vitro study A A A
A. En. En. En. Endothelial cell culturedothelial cell culturedothelial cell culturedothelial cell culture
The HUMVECs were purchased from Young Science (Seoul, Korea) and used between passages 5 and 10. The cells were routinely maintained in Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO, USA) supplemented with 5% fetal bovine serum (Sigma) and a 1% antibiotic antimycotic solution (including 10,000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B per ml, Sigma) at 37 °C in a humidified atmosphere of 5% CO2 in air.
B B B
B. Oxidative stress induction. Oxidative stress induction. Oxidative stress induction. Oxidative stress induction
The oxidative stress was induced by two exogenous methods: (1) 0.1, 1 and 10 mM H2O2 addition and (2) an
enzymatic system, composed of different concentrations (0.1, 1 and 10 U/l) of xanthine oxidase (XO) and its substrate
xanthine (Xn, 250 μM). After 24 h of incubation, the cell viability and morphology were respectively measured.
C C C
C. Polyphenol treatment. Polyphenol treatment. Polyphenol treatment. Polyphenol treatment
The polyphenolic compounds extracted from green tea were kindly supplied by PFI Inc., Kyoto, Japan. The mixture was mainly composed of (−)-epigallo-catechin-3-O-gallate (28%), (−)-gallocatechin-3-O-gallate (11.6%), (−)-epicatechin-3-O-gallate (4.6%), (−)-epigallocatechin (15.0%), gallocatechin (14.8%), (−)-epicatechin (7.0%), and (+)-catechin (9.5%), and its purity exceeded 90%. In order to examine the ability of these compounds to protect the HUMVECs against ROS-mediated oxidative stress, the cells were pre-incubated for 1 h in the presence of GTP at a final concentration of 1 or 10 μg/ml in the medium, which was added to the cell suspension. After pre-incubation, the excess GTP was completely removed and the medium was exchanged before adding the oxidative stress-inducing agents. This avoided a direct reaction between the
polyphenol and the oxidant source in the medium. Finally, the cell viability and morphology were evaluated.
D D D
D. Cell viability measurements by flow cytometry. Cell viability measurements by flow cytometry. Cell viability measurements by flow cytometry . Cell viability measurements by flow cytometry
The toxic effect of ROS on HUMVECs was assessed by using a viability test with fluorescence double staining, followed by flow cytometry (FCM, FACSCalibur, Becton Dickinson, San Jose, CA, USA), as described previously 33, 36.
After inducing oxidative stress, the medium was removed and the cells were washed twice with phosphate-buffered saline (PBS), followed by centrifugation. The precipitates were resuspended in PBS. Afterwards, 5(6)-carboxyfluorescein diacetate (cFDA, 2 μM, Sigma) and propidium iodide (PI, 20 μM, Sigma) were added to the suspension containing 1 × 105
cells/ml. The cFDA/PI-double stained cells were detected by FCM, and the unstained cells were regarded as the negative control. The data obtained was analyzed using the dot plot and histogram of CELLQuest™ software, written by Mac-App (Becton Dickinson).
2. In vivo study 2. In vivo study 2. In vivo study 2. In vivo study A A A
A. . . . GTP preparation GTP preparation GTP preparation GTP preparation
Polyphenolic compounds extracted from green tea were kindly supplied by Pharma Foods International Co. Ltd., Kyoto, Japan. A GTP-containing solution (final concentration: 20 μg/ml) was freshly prepared and stored at 4°C under light protection prior to use.
B B B
B. . . Animals and experimental groups . Animals and experimental groups Animals and experimental groups Animals and experimental groups
Male New Zealand white rabbits (Samtaco Inc., O San, Gyunggi-do, Korea) weighing 2.7 ± 0.3 kg each were housed in metabolic cages for 3 days before surgery and until the day of sacrifice. All animals had free access to water. Animals were fed with a low nitrite/nitrate diet for at least 4 days before the surgery. All experiments were performed between the hours of 09.00 and 17.00. Animal care followed the criteria of the Animal Care Committee of the Yonsei University for the care and use of laboratory animals in research.
sham-operation (control), (II) I/R with vehicle (normal saline) and (III) I/R with GTP (200μg/kg) 45 minutes before I/R. Renal damage measurements were carried out in six animals for each group.
C C C
C. . . Surgical procedures and treatments . Surgical procedures and treatments Surgical procedures and treatments Surgical procedures and treatments
Rabbits were anesthetized with an intramuscular injection of 50 mg/kg ketamine-HCl. The experimental solutions containing GTP (20 μg/ml) or the vehicle were infused at a rate of 1ml/minute for 30 minutes through the ear vein before 45 minutes of ischemia. The abdomen was shaved and the animals were placed on a heating table kept at 39°C to maintain constant body temperature. A midline incision was made and both renal pedicles were cross-clamped. To maintain thermoregulation during surgery, the abdomen contents were replaced and the abdomen was temporarily closed with several sutures. After 30, 60, 90 and 120 minutes of ischemia, the abdomen was reopened and the clamps were removed. The kidneys were inspected for restoration of blood flow, and 1 ml of warm (37°C) normal saline was
instilled into the abdominal cavity. The abdomen was closed in two layers and the animals were placed in a room at the constant temperature of 30°C. The animals were sacrificed after 24 hours of reperfusion. Sham-operated groups were subjected to identical surgical procedures, except for renal I/R and maintained under anesthesia for the duration of the experiment. Both kidneys were resected for a histological analysis. All experiments were performed in accordance with the guidelines of the Animal Research Committee, Yonsei University, Korea.
Figure. 1 Ischemia-reperfusion injury procedures of rabbits.
D D D
D. . . . Blood sampling and renal function Blood sampling and renal function Blood sampling and renal function Blood sampling and renal function
Blood samples were obtained from the ear vein immediately before ischemia and at the end of reperfusion. EDTA was used an anticoagulant. To estimate renal function, serum creatinine (SCr) and blood urea nitrogen (BUN) levels were measured by an automated biochemical analyzer (TBA-2FR
GTP/saline infusion
Cross-clamped Ischemic time for
30, 60, 90 and 120 min Rep
erfu sion time
Neo, Toshiba Co., Ltd., Tokyo, Japan). E
E E
E. . . . HistoloHistoloHistoloHistological analysis gical analysis gical analysis gical analysis
At the end of each experiment, the kidneys were removed. After removal, representative sections from the kidneys were fixed in 10% neutral buffered formalin solution and then embedded in paraffin after several steps of chemical treatments. Serial sections were cut using the same microtome at a thickness of 5 μm and stained with hematoxylin-eosin (H&E) and periodic acid Schiff (PAS). The histological sections were examined with an Olympus BX40 optical microscope (Olympus Optical Co., Osaka, Japan) and photographed. An established tubular injury score was used23
: grade 0 for less than 5% of the region having necrosis, grade 1 for 5% to less than 25% necrosis, grade 2 for 25% to less than 50% necrosis, grade 3 for 50% to less than 75% necrosis, and grade 4 for above 75% necrosis.
3. 3. 3.
3. Statistical analysis Statistical analysis Statistical analysis Statistical analysis
least six animals per group. A one way ANOVA, which was followed by a Tukey HSD test for multiple comparisons was used to detect the differences between groups. A P value of less than 0.05 was considered statistically significant. In addition, the effects of increasing H2O2 and XO concentrations
III. III. III.
III. RESULTS RESULTS RESULTS RESULTS
1. 1. 1.
1. Protective effects of GTP against HProtective effects of GTP against HProtective effects of GTP against HProtective effects of GTP against H2222OOOO2222--induced oxidative --induced oxidative induced oxidative induced oxidative
stress stress stress stress
To investigate the ROS-induced cytotoxic effects on the HUMVECs, increasing H2O2 concentrations were added to the
medium and after incubation the cellular and morphological alterations were examined. To characterize in greater detail the overall cellular injury by the agent, a simple assay in microplate format was performed. In FCM assay in combination with a cFDA/PI double staining, the enzymatic activity of viable cells was used as a parameter of quantitative cytotoxicity. Because the flow cytometric techniques depend on fluorescence, both cFDA and PI are ideal for a quick evaluation of cell viability and FCM allows the simultaneous determination of the number of viable and non-viable cells. Therefore, the differences in the amount of both the fluorescent dyes incorporated by the cells indicate either a variation in the number of the cells or simply a
change in their physiological state. Incubating the cells in the presence of millimolar H2O2 concentrations resulted in a
significant (P < 0.05) dose-dependent decrease in EC viability. After 24 h of treatment with 10 mM H2O2, an
approximate 32% loss of cell viability was observed (Figure. 2, 4). These markers were then used to verify the protective effect of GTPs against H2O2-induced oxidative stress to the
ECs. When the cells were pretreated with 10 μg/ml GTP prior to being challenged with 10 mM H2O2, in conditions similar to
those used in the experiments mentioned above, a complete protection of cell viability was observed, suggesting that the GTP suppresses the H2O2-induced cytotoxicity attributed to
Figure. 2. ROS-induced cytotoxicity in HUMVECs. The cell viability was measured by FCM analysis. The results are reported as a mean ± S.D. (n = 6). The data is analyzed by Student's t-test, and the values marked with asterisks are significantly different from the non-treated control (P < 0.05).
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Figure. 3. Effect of GTP on ROS-induced cytotoxicity of HUMVECs. The cell viability was measured by FCM analysis. The results are reported as a mean ± S.D. (n = 6). The data is analyzed by Tukey HSD tests, and the values marked with asterisks are significantly different from the H2O2-treated or
XO-treated groups without GTP pretreatment (P < 0.05).
*
*
*
* *
*
*
*
Figure. 4 Optical microscopic photographs (200×) of the ROS-damaged HUMVECs pretreated with or without 10 μg/ml GTP. The ROS-treated cells were sequestered with detachment and necrosis, whereas the GTP-treated cells
(e) H
2O
2+ GTP
(b) GTP-pretreated
(f) Xn/XO + GTP
(c) H
2O
2(d) Xn/XO
50 µµµµm(b) GTP-pretreated
(a) control
prior to the oxidative stress induction showed the same local attached sites as incubated only in the presence of GTP: (a) non-treated control; (b) incubated after GTP treatment; (c) 10 mM H2O2-treated; (d) 10 U/l XO-treated; (e) 10 mM
H2O2-treated after GTP treatment; (f) 10 U/l XO-treated
after GTP treatment. The photographs shown in this figure are representative of six independent experiments, showing similar results. The arrows represent the detached cells with necrosis.
2. 2. 2.
2. Protective effects of GTP against Xn/XOProtective effects of GTP against Xn/XOProtective effects of GTP against Xn/XOProtective effects of GTP against Xn/XO----induced oxidative induced oxidative induced oxidative induced oxidative stress
stress stress stress
The cytotoxic effects induced in the ECs were then investigated by another ROS-generating system, consisting of XO and its substrate Xn. This system enzymatically generates superoxide radicals and H2O2 during the conversion of Xn to
uric acid. Figure. 2, 4 show the effects of XO activity on EC viability, as determined by FCM analysis. When the cells
were incubated with increasing XO concentrations in the presence of 250 μM Xn, a marked decrease in viability was observed. About 29% decrease in viability was observed after incubating the cells treated with 10 U/l of the enzyme. The addition of XO or Xn alone did not affect the cell viability (data not shown). Subsequently, the protective effect of GTPs against Xn/XO-induced oxidative stress in the ECs was also investigated under these experimental conditions. Pretreating the cells with 10 μg/ml GTP significantly prevented the Xn/XO-induced loss of the EC viability, indicating that the GTP acted as a biological antioxidant (Figure. 3, 4).
3. 3. 3.
3. The effects of GTP pretreatment on body weight and The effects of GTP pretreatment on body weight and The effects of GTP pretreatment on body weight and The effects of GTP pretreatment on body weight and mortality
mortality mortality mortality
There was no difference in body weight between the vehicle-treated and GTP-treated groups at each ischemic time (Figure. 5). Mean arterial blood pressure measured during the experiments was not different between sham-operated and I/R-injured rabbits. The mortality rate induced by I/R was approximately 45%, whereas GTP administration
decreased the mortality rate to lower than 10%, except for the 120 minute ischemia group.
Figure. 5 Changes of body weights according to ischemic time. There was no difference in body weight between the vehicle-treated and GTP-treated groups at each ischemic time
vehicle GTP
4. 4. 4.
4. The effects of GTP pretreatment on renal function The effects of GTP pretreatment on renal function The effects of GTP pretreatment on renal function The effects of GTP pretreatment on renal function
When the rabbits underwent 90 minutes of ischemia, followed by 24 hours of reperfusion, creatinine levels in the blood and BUN were increased up to 3.8 ± 1.2 mg/dl and 31.3 ± 5.1mg/dl, which were 4.3 and 1.7 times higher than the levels of the control group, respectively (Figure. 6). In contrast, these phenomena were significantly (P < 0.05) prevented by GTP (200μg/kg) treatment before renal I/R, which maintained SCr and BUN levels close that of the controls.
Figure 6. The effects of green tea polyphenol (GTP) pretreatment on renal function. Serum creatinine (SCr) level
*
#*
#*
vehicle GTP Fig. 6A*
#*
vehicle GTP Fig. 6B(A) and blood urea nitrogen (BUN) level (B) are expressed as milligrams per deciliter. The bars represent the mean ± SD. The data was analyzed by a Tukey HSD test. The values marked with asterisks are significantly different from sham-operated groups (P < 0.05). The values marked with sharps are significantly different from GTP-treated groups (P < 0.05).
5. 5. 5.
5. The effects of GTP pretreatment on renal morphology The effects of GTP pretreatment on renal morphology The effects of GTP pretreatment on renal morphology The effects of GTP pretreatment on renal morphology A major contributor to the development and progression of I/R-induced injury is the death (deletion) of tubular epithelial cells by necrosis and apoptosis11
. The control kidneys of sham-operated rabbits showed normal-looking tubular epithelial cells and glomeruli without apparent necrosis (Figure. 7A). H&E stained sections of the ischemic kidney showed the most prominent changes in the proximal tubules after 90 minutes of ischemia (Figure 7, 8). These alterations were characterized by extensive tubular epithelial necrosis, the sloughing of epithelial cells into the tubular lumen, luminal
calcification and glomerular collapse (Figure. 8). Many of the tubules were dilated, leading to increased amounts of PAS positive materials in the tubular lumen (Figure. 10). Some tubules showed the complete loss of the lining cells, whereas others showed single-cell necrosis with nuclear pyknosis and cytoplasmic eosinophilia. The changes were more pronounced in the medulla. 60 minutes of renal ischemia followed by 24 hours of reperfusion led to glomerular collapse and extensive tubular necrosis, as well (Figure. 7). Although the damage was severe in vehicle-treated or GTP-treated groups, the ischemic kidneys from rabbits that received GTP showed a much lesser degree of an ischemic injury induced epithelial damages (Figure. 7, 8) compared with the kidneys obtained from saline-treated groups. These results suggest that GTP pretreatment may result in architectural and cytologic preservation.
Histological examinations by PAS staining confirmed significant improvement in renal morphology in the GTP-treated rabbits (Figure. 9, 10). This effect was especially
remarkable in the medulla, an area that is more susceptible to oxidative damage. Glomerular collapse, an index of hypoperfusion, and platelet clots in the capillary tuft were also considerably reduced in the GTP-treated groups (Figure. 9, 10). The tubular injury score of the vehicle-administered kidneys after I/R injury was significantly (P < 0.05) increased in an ischemic time-dependent manner, while GTP pretreatment resulted in an appreciable reduction of the tubular necrosis score (Figure. 11).
Figure 7. The renal histology of sham-operated kidney (A), vehicle-treated kidney (B, C) and GTP-treated kidney (D, E) after 60 minutes(B, D) and 90 minutes(C, E) of ischemia, followed by 24 hours of reperfusion. The photographs shown in this figure are representative of 6 independent rabbits showing similar results. The arrows represent the glomerular collapse (Hematoxylin-eosin stain, Original magnification × 100).
60 min
90 min
(A)
(B)
(D)
(C)
(E)
Figure 8. The renal histology of vehicle-treated kidney (A) and GTP-treated kidney (B) after 90 minutes of ischemia, followed by 24 hours of reperfusion. Collapsed glomeruli, luminal calcification, and sloughing of epithelial cells into the tubular lumen were observed in the vehicle-treated kidney (A). But, GTP-treated kidney (B) demonstrated intact tubular lumen and glomerulus. (H & E stain, Original magnification × 100).
A
Figure 9. The PAS stain of sham-operated kidney (A), vehicle-treated kidney (B, C) and GTP-vehicle-treated kidney (D, E) after 60 minutes(B, D) and 90 minutes(C, E) of ischemia, followed by 24 hours of reperfusion. The arrows represent intraluminal calcification and the asterisks (*) in (B) represent PAS positive materials in the tubular lumens (PAS stain, Original magnification ×
(B)
(D)
(A)
*
*
*
*
60 min 90 min(E)
(C)
Figure 10. The renal histology of vehicle-treated kidney (A) and GTP-treated kidney (B) with PAS stain after 90 minutes of ischemia, followed by 24 hours of reperfusion. Collapsed glomeruli, luminal calcification, and disfigurations of basement membrane on the tubules (A). But, GTP-treated kidney (B) was observed some intact basement membrane and glomeruli.
A
Figure 11. The tubular injury score of vehicle-treated and GTP-treated kidneys in rabbits after IR. The bars represent the mean ± SD. The data was analyzed by a Tukey HSD test. The values marked with asterisks are significantly different from sham-operated groups (P < 0.05). The values marked with sharps are significantly different from GTP-treated groups (P < 0.05).
IV. IV. IV.
IV. DISCUSSION DISCUSSION DISCUSSION DISCUSSION
0 1 2 3 4 5
T
u
b
u
la
r
in
ju
ry
s
c
o
re
control 3 6 9 12Ischemic time (min)
vehicle GTP
*
*
*
*
# #*
*
In vitro study, the injurious effects of ROS, such as H2O2
and Xn/XO, on the viability and morphology of the HUMVECs during the culture period were examined using FCM analysis. It was shown that the GTP could act as a biological antioxidant in a cell culture experimental system and protect the ECs from oxidative stress-induced toxicity. The GTP pretreatment and it’s protective effects against ROS-induced oxidative stress might have important implications for the successful protection of vascular cells or tissues such as kidney. The viability of ECs is essential in the prediction of the post-operative function and durability of a transplanted vessel.
SCr level has been commonly used as an indirect marker of post-ischemic renal function in rats and mice subjected to renal I/R injury. Typically, in the experimental models of renal I/R injury, the SCr level is markedly increased at 24 - 48 hours after ischemia and then declines over the next several days, although not necessarily to normal, control levels25. In this study, the glomerular filtration rate was also
measured using BUN in rabbits with renal I/R injury.
Importantly, values of BUN in the control rabbits subjected only to sham surgery were very similar to the respective values in anesthetized, normal rabbits reported by other investigators26, 27. The extent to which BUN was increased in
I/R-injured rabbits after 90 minutes of ischemia was consistent with elevated post-ischemic BUN previously found in several studies of rabbits with renal I/R injury. Interestingly, BUN in rabbits subjected to 90 or 120 minutes of ischemia did not fully recover and remained at about 60% of the control value. In contrast, BUN in GTP-treated rabbits subjected to 60 or 90 minutes of renal ischemia was essentially the same as that in the control rabbits. The increased BUN in I/R-injured rabbits was not a result of either lower blood pressure or reduced body weight. These results demonstrated that GTP, as an antioxidant agent, could have maintained or reduced SCr and BUN levels in the I/R-injured rabbits, compared to the vehicle-treated groups (Figure. 6).
The maintained levels of SCr and BUN might be closely related to the preserved renal morphology (Figures. 7 - 10). The histological results partially account for these protective effects of GTP. The preservation of proximal tubules and glomeruli might contribute to the protective properties attributed to GTP and, therefore, to its beneficial effects in post-transplantation patency. Catechin and its derivatives are the major antioxidant and polyphenolic compounds contained in green tea28. However, catechin alone cannot be responsible
for the renal protective activity exerted by GTP. The GTP used in this study was a mixture of seven polyphenolic components. A concentration of 20 μg/ml produced significant protection in renal morphology as well as tubular epithelial cell viability after 24 hours of reperfusion following 90 minutes of ischemia. Other studies reported that when rats were administered with catechin prior to renal failure induced by 45 minutes of bilateral ischemia followed by 24 hours of reperfusion, the catechin pretreatment could markedly attenuate renal dysfunction and morphological alterations,
reduce elevated TBARS levels and restore the depleted renal antioxidant enzymes22
. Although the animal model and the level of I/R damage used in this study were different from those of a similar previous I/R-induced kidney study, both renal function and morphology were maintained close to the control levels by GTP administered prior to 90 minutes of ischemia. These results suggest that the vehicle-treated kidneys suffer from oxidative stress during reperfusion period, but the treated kidneys do not, due to GTP-mediated suppression of ROS-induced injury.
The phenomena that exogenous GTP can protect cell membranes may be related to the intrinsic characteristics of polyphenolic compounds, which readily penetrate cell membranes due to their amphipathic properties. The compound is easily adsorbed by lipid bilayers, extracellular matrices and various cell membrane receptors29.31
. The adsorption of GTP to such proteins is rapid, and their desorption rates are low. Consequently, the renal cells and tissues could be protected from I/R-induced injuries due to
the adsorption of GTP to various membrane proteins and lipids. Furthermore, our earlier studies have already shown that GTP was significantly effective in protecting rat calvarial osteoblasts32
, human microvascular endothelial cells33
and human saphenous vein34 from ROS-induced oxidative stress
in a similar manner. Consequently, the rabbit kidney could be preserved by the adsorption of GTP to collagenous or elastic fibers within the renal tissues, leading to the prevention of tubular epithelial necrosis and proximal tubule dilation as well as the inhibition in glomerular collapse. It appears that the polyphenolic compounds not only have protective effects on the renal tissues, but also act as antioxidants on the renal cells.
Studies in several models of inflammatory renal disease have demonstrated that sensitized T cells were an important component of the renal lesions24
. Specifically, CD8+ T cells have been identified as important modulators during experimental renal IR injury35, 36
. However, renal IR injury has a complex pathophysiology with involvement of multiple
processes, including cytokines, neutrophils, complement, growth factors, apoptosis, and deranged vascular reactivity. It is likely that T cells are working in conjunction with other factors, rather than working alone in mediating tubular toxicity.
V. V. V.
V. CONCLUSION CONCLUSION CONCLUSION CONCLUSION
In vitro experimental results demonstrate that GTP can act as a biological antioxidant in a cell culture experimental model and prevent oxidative stress-induced cytotoxicity in endothelial cells. And in vivo results showed that pretreatment with 200 μg/kg of GTP could reduce the mortality of I/R rabbits and significantly improve renal function as revealed by reduction of SCr and BUN. Histologic analyses confirmed these findings. I/R rabbits treated with GTP showed a marked reduction in necrosis and sloughing of the proximal tubules. In addition, we observed a reduction of necrotic areas in the medulla and of obstructing PAS-stained casts in the tubular lumen. There was less collapse of the glomerular tufts and a reduction of intracapillary platelets clots in damaged proximal tubular cells, suggesting decreased vasoconstriction. These properties of GTP, coupled with its apparent low toxicity, make GTP an ideal candidate for use as a therapeutic agent to increase the success of kidney transplantation.
RE RE RE
REFERENCES FERENCES FERENCES FERENCES
1. Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol 1996;271:F477-F488.
2. Weight SC, Bell PR, Nicholson ML. Renal ischaemia-reperfusion injury. Br J Surg 1996;83:162-170.
3. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 2004;364814-827.
4. Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int 2004;66:523-527.
5. Lloberas N, Torras J, Herrero-Fresneda I, et al. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J 2002;16:908-910.
6. Fukai M, Hayashi T, Yokota R, et al. Lipid peroxidation during ischemia depends on ischemia time in warm ischemia and reperfusion of rat liver. Free Radic Biol Med
2005;38:1372-1381.
7. Naskalski JW, Bartosz G. Oxidative modifications of protein structures. Adv Clin Chem 2000;35:161-253.
8. Elliott RM, Astley SB, Southon S, Archer DB. Measurement of cellular repair activities for oxidative DNA damage. Free Radic Biol Med 2000;28:1438-1446.
9. Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair 2004;3:1109-1115.
10.Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol 2003;23:511-521.
11.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 2003;284:F608-F627.
12.Seo MY, Lee SM. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J Hepatol 2002;36:72-77.
13.Kadkhodaee M, Aryamanesh S, Faghihi M, Zahmatkesh M. Protection of rat renal vitamin E levels by
ischemic-preconditioning. BMC Nephrol 2004;5:6.
14.Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation 1998;66:147-152. 15.Giovannini L, Migliori M, Longoni BM, et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol 2001;37:262-270. 16.Hyon S.H, Kim D.H. Long-term preservation of rat pancreatic islets under physiological conditions. J Biotechnol 2001;85:241-246.
17.Fiorini RN, Donovan JL, Rodwell D, et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl 2005;11:298-308.
18.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 2004;10:55-62.
19.Muia C, Mazzon E, Di Paola R, et al. Green tea polyphenol extract attenuates ischemia/reperfusion injury of the gut. Naunyn Schmiedebergs Arch Pharmacol 2005;371:364-374. 20.Ikeguchi R, Kakinoki R, Matsumoto T, Hyon S.H, Nakamura T. Peripheral nerve allografts stored in green tea polyphenol solution. Transplantation 2005;79:688-695.
21. Yokozawa T, Rhyu DY, Cho EJ, Aoyagi K. Protective activity of (-)-epicatechin 3-O-gallate against peroxynitrite-mediated renal damage. Free Radic Res 2003;37:561-571. 22.Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia -reperfusion-induced renal injury in rats. Pharmacol Rep 2005;57:70-76.
23.Rabb H, Ramirez G, Saba SR, et al. Renal ischemic-reperfusion injury in L-selectin deficient mice. Am J Physiol 1996;271:F408-F413.
24.Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens 2003;12:85-90.
limitations of serum creatinine as a measure of renal function in experimental renal ischemia-reperfusion injury. Transplantation 2002;73:1841-1844.
26.Tanaka T, Kita T, Liu R, Tanaka N. Protective effect of peptide leukotriene antagonist on renal failure induced by a tourniquet in rabbits. Forensic Sci Int 1995;71:57-64.
27.Subramanian S, Bowyer MW, Egan JC, Knolmayer TJ. Attenuation of renal ischemia-reperfusion injury with selectin inhibition in a rabbit model. Am J Surg 1999;178:573-576. 28.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med 1992;21:334-350.
29.Rabb H, Daniels F, O’Donnell M, et al. Pathophysiological role of T lymphocytes in renal ischemia.reperfusion injury in mice. Am J Physiol Renal Physiol 2000;279:F525-F531. 30.Wang Y, Wang YP, Tay YC, Harris DC. Role of CD8+ cells in the progression of murine adriamycin nephropathy. Kidney Int 2001;59:941-949.
31.Nakayama T, Hashimoto T, Kajiya K, Kumazawa S. Affinity of polyphenols for lipid bilayers. Biofactors
2000;1-4:147-151.
32.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol 2004;11:380-381.
33.Han D.W, Park YH, Kim JK, et al. Long-term preservation of human saphenous vein by green tea polyphenol under physiological conditions. Tissue Eng 2005;11:1054-1064. 34.Park YH, Han D.W, Suh H, et al. Protective effects of green tea polyphenol against reactive oxygen species-induced oxidative stress in cultured rat calvarial osteoblast. Cell Biol Toxicol 2003;19:325-337.
35.Rah DK, Han D.W, Baek HS, Hyon S.H, Park J.C. Prevention of reactive oxygen species-induced oxidative stress in human microvascular endothelial cells by green tea polyphenol. Toxicol Lett 2005;155:269-275.
36.Han D.W, Suh H, Park YH, Cho BK, Hyon S.H, Park J.C. Preservation of human saphenous vein against reactive oxygen species-induced oxidative stress by green tea polyphenol pretreatment. Artif Organs 2003;27:1137-142.
국문요약 국문요약 국문요약 국문요약
허혈이
허혈이
허혈이
허혈이 유발된
유발된
유발된
유발된 가토신장의
가토신장의
가토신장의
가토신장의 보존에
보존에
보존에
보존에
polyphenol
polyphenol
polyphenol
polyphenol이
이
이 미치는
이
미치는
미치는
미치는 영향
영향
영향
영향
<지도교수 박 병윤> 연세대학교 대학원 의학과 임 중 혁 허혈 현상은 신장이식 후 급성 및 만성 거부반응의 위험성을 증가시키며, 급성 신장 거부반응에서 유발요인의 하나로서 작용 한다. 허혈 이후의 재관류는 신장 이식 시 허혈이 유발된 신장 조직의 생존에 필수적이지만 이 또한 신 손상을 유발하는데 이 러한 신장의 허혈 재관류 손상은 신장손상의 병인에 영향을 미 친다. 이때 유해산소군(Reactive oxygen species, ROS)와 reactive nitrosative species가 주요한 인자로 작용한다. 최근 연구에 의하면 녹차 성분의 polyphenol(GTP)은 심근과 간을 허 혈 재관류 손상으로부터 보호한다고 밝혀졌다. 하지만 신장에 대 한 GTP의 보호 효과에 관해서는 간과 되어 왔다. 본 연구는 인 간 미세혈관 내피세포 (HUMVECs)에서 유해산소군의 손상에 대한 GTP의 보호 역할과 가토 신장에 있어서 허헐 재관류 손상으 로부터 신장 세포를 보호할 수 있는 GTP의 능력에 대하여 알아 보았다.
시험관 연구에서는 10Mm의 H2O2를 첨부하거나 xanthine
(250uM) 존재 하에 10 U/I xanthine oxidase (XO)를 반응시킴 으로써 배양된 인체혈관 내피세포의 산화적 스트레스를 유도하 였을 때 유해산소군의 세포독성 정도와 GTP의 전처치가 혈관내 피 손상을 억제할 수 있는지를 알아보았다. In vivo 연구에서는 가토 신장을 이용하여 실험군을 3개로 나누어서 ( 1. 샴 대조군 (sham-operated), 2. 생리 식염수로 관류 시킨 후 허헐 재관류 손상을 유도한 군, 그리고 3. GTP의 전처치 후 허헐 재관류 손 상을 유도한 군) 실험하였다. 각 실험군은 6 마리 가토가 포함되 어 있었으며 각각 30, 60, 90 그리고 120분의 허헐을 유도한 다 음 24시간의 재관류를 받았다. GTP(200ug/kg)의 전처치는 허헐 재관류 실험 시작 45분전에 시행하였다. 두 물질(H2O2, xanthine+xanthine oxidase)에 의한 산화적 스 트레스는 인체혈관 내피세포의 생존에 의미 있는 감소를 나타냈 다(각각 68% 와 71%). 이러한 산화적 스트레스에 의한 세포 독 성을 광학현미경으로 관찰하였을 때 형태의 변화와 괴사 조직의
탈락이 유의하게 증가되는 것을 관찰할 수 있었다. 10 ug/ml GTP를 내피세포에 1시간 동안 전처치함으로써 H2O2에 의한 유
도된 변화를 유의하게 예방할 수 있었다. 같은 GTP 농도 하에 서 XO에 의해 유도된 산화적 스트레스에도 세포 생존력과 형태 는 상당히 유지되었다. In vivo 연구에서는, 혈청 creatinine(SCr) 과 urea nitrogen(BUN) 농도가 대조군과 생리 식염수로 전처치한 군보다 실험군에서 유의한 감소를 보였는데 이러한 GTP의 전처치가 90분의 허헐 후 발생하는 신장 손상의 의미 있는 감소로 이어지는 결과를 나타냈다. 상기 결과는 조직 학적 검사로 확인하였으며 GTP의 전처치는 허헐과 재관류에 의 한 근위 세관(proximal tubule)의 괴사와 탈락을 방지하는 것으 로 나타났다. 뿐만 아니라 허헐 재관류에 의해 손상된 가토에서 수질의 괴사 부위의 감소와 사구체 허탈의 감소도 관찰되었다. 결론적으로 본 연구를 통하여 녹차 polyphenol은 항산화제로 서 인체혈관내피세포의 산화적 스트레스에 대한 보호작용을 하 며 허헐 재관류 손상에 있어서 산화적 스트레스로 유발되는 유 해산소군에 의한 손상을 예방함으로써 신장 손상을 감소시키며 신이식에서 급성 거부반응을 막을 수 있는 항산화제의 한가지로 사용할 수 있을 것이다.
핵심 되는 말: 녹차 polyphenol, 유해산소군, 항산화제, 허혈 재 관류 손상