醫學
醫學
醫學
醫學 博士學位
博士學位
博士學位
博士學位 論文
論文
論文
論文
Application of Cell-Derived
Extracellular Matrix (ECM) Scaffold
on Tissue Engineering Cartilage
亞
亞
亞
亞 洲
洲
洲 大
洲
大
大 學
大
學
學 校
學
校 大
校
校
大
大 學
大
學
學 院
學
院
院
院
醫
醫
醫
醫 學
學
學
學 科
科
科
科
金
金
金
金 成
成
成
成 哲
哲
哲
哲
Application of Cell-Derived
Extracellular Matrix (ECM) Scaffold
on Tissue Engineering Cartilage
by
Cheng Zhe Jin
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF PHILOSOPHY
Supervised by
Byoung-Hyun Min, M.D., Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
金成哲
金成哲
金成哲
金成哲의
의
의 醫學
의
醫學
醫學 博士學位
醫學
博士學位
博士學位
博士學位 論文
論文을
論文
論文
을
을
을 認准
認准
認准
認准함
함
함.
함
審査委員長
審査委員長
審査委員長
審査委員長 朴
朴
朴 基
朴
基
基 東
基
東
東
東 印
印
印
印
審査委員
審査委員
審査委員
審査委員 閔
閔
閔
閔 炳
炳
炳 顯
炳
顯
顯 印
顯
印
印
印
審査委員
審査委員
審査委員
審査委員 朱
朱
朱
朱 一
一
一 路
一
路
路 印
路
印
印
印
審査委員
審査委員
審査委員
審査委員 元
元
元
元 禮
禮
禮 淵
禮
淵
淵 印
淵
印
印
印
審査委員
審査委員
審査委員
審査委員 朴
朴
朴
朴 소
소
소 라
소
라
라 印
라
印
印
印
亞
亞
亞
亞 洲
洲
洲 大
洲
大
大 學
大
學
學
學 校
校 大
校
校
大
大 學
大
學
學 院
學
院
院
院
2007 年
年
年 6 月
年
月
月 22 日
月
日
日
日
감사의
감사의
감사의
감사의
글
글
글
글
드디어 힘겨운 박사과정을 마치게 되어 정말 기쁘게 생각합니다. 저는 나름대로 학위과정을 하면서 정말 보람차게 지냈다고 생각합니다. 그래서 여기서 그 소감과 감사하고자 하는 분들께 말씀 몇 마디를 드리고자 필을 들게 되었습니다. 하지만 막상 쓰자고 하니 정말 어떻게 시작하고 누구를 써야 할지 고민이 많습니다. 감사해야 할 사람이 너무 많아서 그런 것 같 습니다. 우선 먼저 저를 민병현, 박소라교수님과 만나게 계기를 마련해주시고, 지금은 돌아가신 저의 어머님께 감사의 말씀을 드리면서 이 기쁨을 같이 나누고 싶습니다. 저는 어머님이 하늘나라 어디선가 항상 저를 지켜보고 있을 것이라고 믿으면서 힘내어 공부해 왔습니다. 그리고 묵묵히 생활비 버느라, 아이들을 돌보느라 뒷바라지를 해온 아내에게 “미안해요, 수고했어 요, 사랑해요”라고 말을 전하고 싶습니다. 또 저를 하늘처럼 믿어준 예쁜 우리 큰딸 리엽이게도 “사랑한다”라고 말하고 싶습니다. 엽이가 매사에 열 심히 하여 커서 아빠처럼 훌륭한 사람이 될 수 있을 것으로 믿는다. 그리 고 우리 집 “순둥이” 막내 딸 리향이게도 “건강하게 태어나 줘서 감사해” 라고 말하고 싶습니다. 또 항상 저희들 생활을 도와주신 장모님께도 깊이 감사 드립니다. 여동생과 매부와 이 시각의 기쁨을 같이 하고 싶습니다. 우 리가족 모두들 사랑합니다. 뭐니뭐니해도 너무나도 부족한 저를 키워주시고 지도해주신 민병현 교 수님과 박소라교수님께 깊은 감사의 말씀을 드리면서 이 기쁨을 같이 나누 고 싶습니다. 정말 두 분의 교수님을 못 만났더라면, 두 분의 각별한 관심 과 사랑이 없었더라면 오늘의 저가 없을 것입니다. 여기서 저는 두 분께 이후에도 지금처럼 항상 매사에 열심히 할 것을 약속 드립니다. 이외에도 실험실의 연구교수님들, 연구원 선생님들, 선후배들, 그리고 아주대학교 정형외과 교실 교수님들, 레지던트들, 수술방 및 외래 간호사들 께 깊은 감사를 드리면서 이 기쁨을 같이 하고 싶습니다.
-ABSTRACT–
Application of Cell-Derived Extracellular Matrix (ECM)
Scaffold on Tissue Engineering Cartilage
In recent years, cartilage tissue engineering for reconstruction or repair of a cartilage injury has been emerged as alterative solutions based on the application of selected chondrocytes and scaffold. The purpose of present study is to evaluate the feasibility of novel cell-derived extracellular matrix (ECM) scaffold on cartilage tissue engineering in vitro and in vivo as nude mouse and rabbit model.
Chapter I: A porous cell-derived ECM scaffold was prepared with a freeze-drying
protocol using porcine chondrocytes. The ECM scaffold had highly uniform porous microstructure by scanning electron microscope (SEM). It showed an average pore diameter of 504±108um, a porosity of 90±10.4%, a surface area of 905±204 m2/g and a tensile strength of 0.34±0.09 MPa, respectively. In vitro study, Then, rabbit chondrocytes were seeded dynamically on the ECM scaffold and cultured for 2 days, 1, 2 and 4 weeks in vitro for analysis. The neocartilage-like tissue was observed after 1 week of culture, and the volume and compressive strength were significantly increased with culture time. The DNA, glycosaminoglycan (GAG) and collagen contents also increased gradually with time. Histological staining for GAG (Safranin O staining) and type II collagen (immunohistochemistry) showed sustained accumulation of the ECM
molecules along with time, which gradually and uniformly filled the porous space in the ECM scaffold.
Chapter II: In vivo study as nude mouse, cell-seeded ECM scaffold was cultured
for 2 days in vitro, and then implanted into the nude mouse subcutaneously. They were retrieved at 1, 2, and 3 weeks post-implantation. Under macroscopic analysis, the cartilage-like tissue formation matured by time and developed a smooth, white surface. And, the size of the neocartilage tissue increased slightly by the 3rd week and remained more stable. Total GAG content and the GAG/DNA ratio increased significantly by time in the chemical analysis. The histology exhibited a sustained accumulation of newly synthesized sulfated proteoglycans. Immunohistochemistry, Western blot, and RT-PCR clearly identified type II collagen at all time points. Compressive strength of in vivo neocartilage increased from 0.45±0.06MPa at 1 week to 1.18±0.17MPa at 3 weeks.
Chapter III: In vivo study as rabbit model, the knee defects were implanted with in
vitro cultured tissue engineering cartilage using ECM scaffold and allogenic rabbit chondrocytes as 2days, 2, and 4 weeks (experimental group 2, 3, and 4), respectively. The left knee defects were not implanted as control (group 1). The maturity of cultured implants was evaluated by histological, chemical and mechanical assay in chapter I. The repair examination was evaluated with macroscope and histological assay at 1 and 3 months post-surgery. After 1 month, fibro/hyalinecartilge was found on histological examination in the group 1, 2 and hyalinecartilage was found in group 3, 4. However, a mature matrix and a columnar organization of chondrocytes can be observed with
Saflanin-O staining in group 4 at 3 months. Moreover, the subchondral bone was well remodeled and the more type II collagen was expressed at that time in the group 4. Thus ICRS histological score were significantly increased in the group 4 at that time.
In conclusion, this study demonstrated that the novel cell-derived ECM scaffold could provide a promising environment for generating a high quality cartilage in vitro and in vivo as nude mouse. Moreover, the engineered cartilage using the cell-derived ECM scaffold and allogenic chondrocytes could regenerate the cartilage defects particularly when cultured mature cartilage provided better results.
Key words: cell-derived extracellular matrix (ECM) scaffold, chondrocyte, tissue engineering cartilage in vitro and in vivo
TABLE OF CONTENTS
ABSTRACT…….………i
TABLE OF CONTENTS……….iv
LIST OF FIGURES ………....vi
LIST OF TABLES ………viii
I. INTRODUCTION………...1
II. MATERIALS AND METHODS………...10
III. RESULTS……….21
IV. DISCUSSION………...42
V. CONCLUSION………..54
REFERENCES………...55
LIST OF FIGURES
INTRODUCTION
Fig. 1. Articular cartilage………1
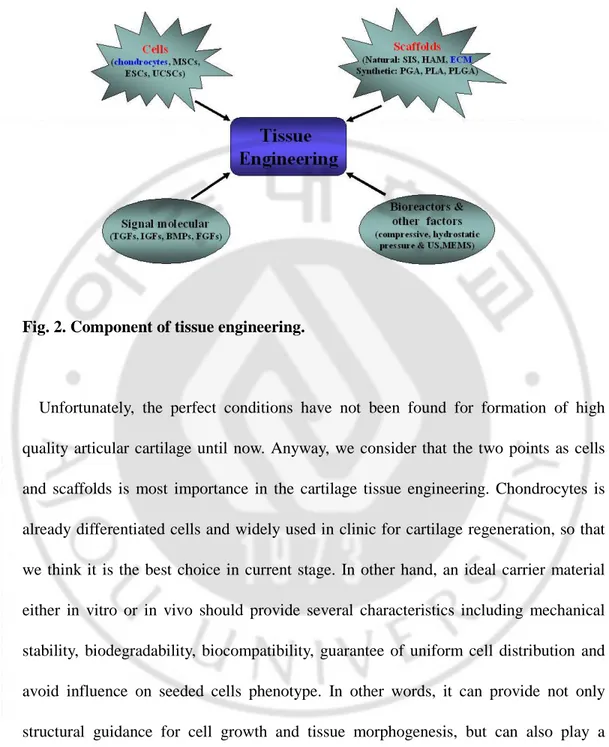
Fig. 2. Component of tissue engineering………3
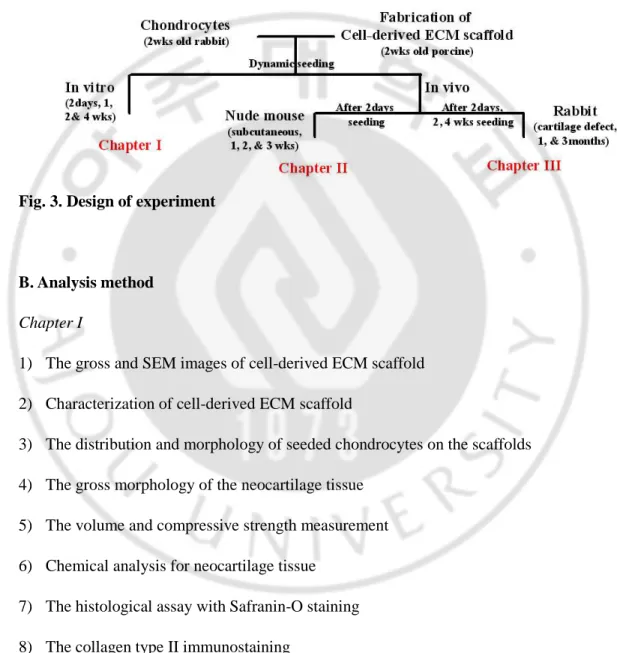
MATERIALS AND METHODS Fig. 3. Design of experiment………..10
CHAPTER I Fig. 4. The process of making cell-derived ECM scaffold………21
Fig. 5. The SEM image of cell-derived ECM scaffold………..22
Fig. 6. The distribution and morphology of seeded chondrocytes on scaffolds…23 Fig. 7. The gross morphology of the neocartilage tissue………...24
Fig. 8. The volume and compressive strength of neocartilage tissue……….25
Fig. 9. Chemical analysis for neocartilage tissue………..27
Fig.10. The neocartilage tissue with Safranin-O staining……….28
Fig.11. The neocartilage tissue with collagen type II immunostaining………….29
CHAPTER II Fig.12. The gross morphology of the neocartilage tissue………...31
Fig.13. The volume of neocartilage tissue………..31
Fig.14. The neocartilage tissue with Safranin-O and Alcian blue staining ………32
Fig.16. Chemical analysis for neocartilage tissue………34
Fig.17. The neocartilage tissue with collagen type II immunostaining …………...35
Fig.18. The western blot analysis of the neocartilage tissue ………36
Fig.19. The compressive strength of the neocartilage tissue……….37
CHAPTER III Fig.20. The gross finding of the cartilage defect with implants……….38
Fig.21. Safranin-O staining of repaired cartilage on defects………..40
Fig.22. The ICRS histological score………...40
LIST OF TABLES
MATERIALS AND METHODS
Table. 1. ICRS histological scoring system……….19 CHAPTER I
I. INTRODUCTION
General introduction
Normal articular cartilage is avascular, anuronal tissue. And it is complex and consists of chondrocytes and cartilage specific extracellular matrix (ECM) which is mainly composed by collagens and proteoglycans (Fig. 1). Articular cartilage in the knee plays a key role to absorb and distribute various mechanical loads in the joint. Unfortunately, once damaged adult human articular cartilage show a poor capacity for repair and regeneration. Explanation for this include that the limited potential for chondrocyte proliferation, the capacity chondrocytes to become catabolic in response to pathological mediators, and the avascular nature of the tissue. Until now, various surgical procedures such as debridgement, subchondral microfracture and drilling, auto or allogenous osteochondral transplantation and autologous chondrocytes implantation have been developed to help regeneration of damaged cartilage. In fact, so far no perfect methods and results have been found to substitute the shapely articular cartilage normally present in the joint.
Surface of cartilage Chondrocyte Collagen
Proteoglycan Proteoglycan Chodrocyte
(C) Molecular level of articular cartilage.
Recently, the tissue engineering technology has been a strong candidate as regeneration for damaged articular cartilage.
The tissue engineering technique first introduced in the late 1980s. It is a multidisciplinary research area that incorporates both biological and engineering principle for the purpose of generating new, living tissues to replace the diseased/damaged tissue and restore tissue/organ function. So far, tissue engineering technique was widely used in various filed of regenerative medicine. Among them, for successful articular cartilage tissue regeneration four tools are necessary (Fig. 2): i) tissue specific cells including stem cells (embryonic stem cells (ESC), mesenchymal stem cells (MSC), umbilical cord stem cells (UCSC)), chondrocytes, perichondrials cells, periosteum; ii) Biocompatible carrier scaffolds by which seeded cells are supported and can develop. It is including natural materials such as small intestinal submucosa (SIS), De-mineralized bone matrix (DBM), human amniotic membrane (HAM), fibrin, collagen, and synthetic materials such as polyglycolic acid (PGA), polylactic acid (PLA) and polylactic-glycolic acid (PLGA); iii) Signaling molecules including growth factors (tissue growth forming factors (TGFs), insulin-like growth factors (IGFs), bone morphogenetic proteins (BMPs), fibroblasts growth factors (FGFs)), cytokines, and non-proteinaceous chemical compounds; iv) Bioreactors including hydrodynamic, dynamic compressive loading, hydrostatic pressure et al.
Fig. 2. Component of tissue engineering.
Unfortunately, the perfect conditions have not been found for formation of high quality articular cartilage until now. Anyway, we consider that the two points as cells and scaffolds is most importance in the cartilage tissue engineering. Chondrocytes is already differentiated cells and widely used in clinic for cartilage regeneration, so that we think it is the best choice in current stage. In other hand, an ideal carrier material either in vitro or in vivo should provide several characteristics including mechanical stability, biodegradability, biocompatibility, guarantee of uniform cell distribution and avoid influence on seeded cells phenotype. In other words, it can provide not only structural guidance for cell growth and tissue morphogenesis, but can also play a functional role such as enhancing the cell attachment and the metabolism. We consider that this condition might be provided by the significance of extracellular matrix (ECM)
natural scaffolds. Because, the ECM scaffold as a nature’s natural scaffold, and it has high tissue compatibility and a potential to retain cytokines, growth factors, and other functional proteins. Therefore, the many ECM scaffolds such as SIS, HAM and DBM has been already noticed in the tissue-engineering field recently.
In present study, we made a novel cell-derived extracellular matrix (ECM) scaffold using porcine chondrocyte and freeze-drying technique, and evaluated the feasibility of the scaffold on cartilage tissue engineering in vitro and in vivo as nude mouse and rabbit model.
Special introduction
Chapter I
Articular cartilage in the knee plays a key role to absorb and distribute various mechanical loads in the joint. Once damaged by traumatic or pathological causes, the cartilage degenerates and often advances to osteoarthritis (OA) without appropriate cares, because it has very poor self-healing capability. Many researchers have investigated a tissue engineering approach using cultured cells and/or various scaffolds with the ultimate aim of promoting the regeneration of injured cartilage. The scaffolds used include both synthetic and natural polymers such as polylactic-co-glycolic acid (PLGA), polylactic acid (PLA), polyglycolic acid (PGA), fibrin gel, collagen, alginate, chitosan, hyaluronic acid (HA) and collagen-glycosaminoglycan (GAG) composite, etc (Nehrer et al 1997; Rotter et al 1998; Honda et al 2000; Lee et al 2000; Griogolo et al
2001; Liu et al 2004; Park et al 2005; Li and Zhang 2005). Although some successful results were reported using these scaffolds in vivo and in vitro environments, no satisfactory scaffold is currently available that can regenerate high quality cartilage tissue.
The significance of ECM scaffolds has been recently noticed in the tissue-engineering field. As a nature’s natural scaffold, the ECM scaffold has high tissue compatibility and a potential to retain cytokines, growth factors, and other functional proteins. Therefore, it can provide not only structural guidance for cell growth and tissue morphogenesis, but can also play a functional role such as enhancing the cell attachment and the metabolism. For these reasons, some ECM scaffolds have been already used successfully in preclinical animal studies and clinical applications. For example, the small intestinal submucosa (SIS) was used successfully for urinary tract, dura mater, and vascular reconstructions (Prevel et al 1994;Cobb et al 1996; Kropp et al 1996), and the human amniotic membrane (HAM) was used successfully for corneal and cartilage regeneration (De Rotth 1940; Lee and Tseng 1997; Jin et al 2007). These ECM scaffolds were derived from tissues and used directly or with some modifications. However, natural cartilage cannot be used as a scaffold on account of its peculiar dense structure and shape. This was the driving basis of our interest to make a cell-derived ECM scaffold using chondrocytes. It was hypothesized that a cell-derived ECM scaffold can provide a favorable 3-dimensional (3-D) environment to support the retention of chondrocytic phenotype, the synthesis of the ECM components, and the
formation of hyaline cartilage, and eventually to make a natural cartilage-like structure. The cell-derived ECM scaffold was made using porcine chondrocytes and evaluated as a potential scaffold for cartilage tissue engineering in vitro. Its cellular compatibility and construction of a neocartilage-like structure were analyzed together with its morphological, physical and chemical properties.
Chapter II
Adult articular cartilage has a very limited healing potential. Tissue engineered cartilage has been a strong candidate as a replacement material for articular cartilage defects. Cartilage tissue engineering requires a multidisciplinary combination of medium supplements, growth factors, cell sources, and scaffolds, among which scaffolds play a pivotal role. Previously, various scaffolds have been utilized in the forms of gels, sponges, fibers, and microspheres (Griogolo et al 2001; Chen et al 2003; Honda et al 2004; Kang et al 2005). Generally, scaffolds require a 3-dimensional (3D) structure, high porosity with an interconnection, balanced biodegradability, and biocompatibility. Although a perfect material for scaffolds was developed in the tissue engineering field, natural extracellular matrix (ECM) scaffolds has gained a prominent interest for long time. Because the ECM scaffolds could provide specific environments similar to natural tissues both structurally and functionally, the small intestine submucosa (SIS), urinary bladder (UBS), and human amniotic membrane (HAM) have been used for the reconstruction of vascular, bladder, tendon, and corneal tissues
(Badylak et al 1995; Kopp et al 1996; Lee and Tseng 1997; Piechota et al 1998;). In addition, a mixed scaffold of collagen and glycosaminoglycan (GAG) was also used recently (Lee et al 2000; O`Brien et al 2005; Zhong et al 2005).
In this report, we utilized a different strategy to make ECM scaffolds compared to the previously ones. Instead of using tissue itself as a scaffold, chondrocyte cells cultured in vitro was processed to form a scaffold that had the most similar structure to that of the natural cartilage. In our previous study, we had introduced a well-fabricated cartilage from porcine articular chondrocytes without any scaffolds (Park et al 2006). We developed this into a highly porous, sponge type, cell-derived ECM scaffold with a freezing and drying technique (Jin et al 2006). We hypothesized that this cell-derived ECM scaffold could be able to provide an ideal 3D environment forming a hyaline cartilage for the cartilage tissue engineering. This study was designed to observe the process of cartilage formation after inoculation of chondrocytes on the scaffold and to evaluate its resemblance to the natural cartilage in terms of morphology, chemistry, and mechanical strength.
Chapter III
Articular cartilage injury is hard to repair due to the poor self-healing capacity of the cartilage, which easily causes degeneration of intact cartilages at the border resulting in osteoarthritis (OA) (Mankin 1982). Many surgical methods such as debridgement (Insall 1974; Baumgaertner et al 1990), subchondral microfracture and drilling
(Mitchell and Shepard 1976) have been reported to help regeneration of damaged cartilage. However, the regenerating tissues usually changed to the fibro-cartilage losing biomechanical properties of normal articular cartilage.
The osteochondral grafts technique suffered from the incongruence of the joint surface between the graft and the host cartilage, and the limitation in the source of donor tissues (Garrett 1994). In 1994, Britberg et al reported excellent results with autologous chondrocytes transplantation (ACT) technique (Brittberg et al 1994). However, several questions still remain in this procedure overall such as the phenotypic changes in chondrocytes during in vitro culture, the possible leakage of the transplanted chondrocytes from the graft site, uneven distribution of chondrocytes and periosteal hypertrophy of them on the graft site, etc. These problems have led many researchers search for exogenous biocompatible materials to deliver chondrocytes to the lesion site for tissue engineering technique. Until now, a number of biocompatible materials have been used for cartilage repair including natural materials such as fibrin, collagen gels and sponges (Nehrer et al 1997; Lee et al 2000; Park et al 2005), and synthetic materials such as polyglycolic, polylactic acid and polylactic-glycolic acid (Rotter et al 1998; Honda et al 2000; Liu et al 2004). Unfortunately, however, no biocompatible materials were reported showing satisfactory results of cartilage regeneration in vivo.
In our previous study, the feasibility of a novel cell-derived ECM scaffold in cartilage tissue engineering was demonstrated in vitro and in vivo in nude mouse (Jin et al 2006; 2007). This study investigated the potential of the ECM scaffold to induce
cartilage regeneration in rabbit defect model in particular depending on their maturity in vitro. The cartilage is resilient load-bearing material to absorb mechanical shock and spread the applied load onto bone in the joint. So, the properties of tissue engineered cartilage before implantation such as the chemical composition and mechanical strength could be very important in the result of cartilage regeneration in vivo. For example, immature engineered cartilage is likely to integrate itself well with the surrounding tissues, but could be easily broken down by the mechanical load. In contrast, mature cartilage might be more resistant to the mechanical load, thereby more excellent in regenerating the cartilage in vivo. Tissue-engineered cartilages with different maturity were prepared in vitro using the ECM scaffold and allogenous rabbit chondrocytes, and implanted into the cartilage defect in the rabbit trochlear groove to evaluate their regenerative capacity in vivo.
II. MATERIALS AND METHODS
A. Design of experiment
Fig. 3. Design of experiment
B. Analysis method
Chapter I
1) The gross and SEM images of cell-derived ECM scaffold 2) Characterization of cell-derived ECM scaffold
3) The distribution and morphology of seeded chondrocytes on the scaffolds 4) The gross morphology of the neocartilage tissue
5) The volume and compressive strength measurement 6) Chemical analysis for neocartilage tissue
7) The histological assay with Safranin-O staining 8) The collagen type II immunostaining
Chapter II
1) The gross morphology of neocartilage tissue 2) The volume of neocartilage tissue
3) The histological assay with Safranin-O and Alcian-blue staining 4) The RT-PCR analysis
5) Chemical analysis for neocartilage tissue 6) The collagen type II immunostaining 7) The western blot analysis
8) The compressive strength measurement
Chapter III
1) The gross finding of the cartilage defect with implants 2) The Safranin-O staining of repaired cartilage on defects 3) The ICRS histological score
4) The collagen type II immunostaining of repaired cartilage
C. Preparation of cell-derived ECM scaffold
The construction of cell-derived ECM scaffold was described in our previous study (Jin et al 2007). Briefly, articular chondrocytes were acquired from the knees of 2-3 week-old porcine using a collagenase digestion method (Min et al 1998). The isolated chondrocytes were cultured at a density of 1.9 x 105 cells/cm2 in 100 mm dishes using
Dulbecco’s Modified Egle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% antibiotics-antimycotics and 50 µg/ml L-ascorbic acid for 3-4 days. The medium was then removed and the cell layer containing the ECM components was carefully detached from the bottom using 0.05% trypsin-EDTA (Gibco; Grand Island, NY, U.S.A.) and a wide-bore pipette. The cell/ECM membrane was transferred individually in a 50 ml conical tube containing 30 ml DMEM and 5% FBS. The tube was then centrifuged at 600 g for 20 mins to consolidate the membrane into a pellet-type construct. It was then incubated overnight at 37℃ and transferred to a six-well culture plate for extended culture. The constructs were allowed to grow into the cartilage-like tissue for 3 weeks with the culture medium (5 ml) changed three times a week (Park et al 2006). After 3 weeks, the neocartilage constructs were washed in phosphate-buffered saline (PBS) and subjected to the three cycles of freeze (-20°C) and thawing (room temperature) at 12 hr intervals. The constructs were then stored at -20°C for 1 day and freeze-dried for 48 hrs at -56°C under a pressure of 5 mTorr. A cylindrical form of cell-derived ECM scaffold was obtained by cutting the constructs using a biopsy punch (6 mm in diameter) and trimming the uncut area at the top and bottom layers by less than 1 mm using a clean razor blade (Fig. 4).
D. Characterization of cell-derived ECM scaffold
After fabrication, the color and size of the cell-derived ECM scaffolds were observed by gross images. The micro-structure of the ECM scaffold was analyzed by the images
obtained using a scanning electron microscope (SEM). Briefly, the cell-derived ECM scaffolds were fixed at 4℃ in 2.5% glutaraldehyde prepared in 0.1 M PBS for 2 hr and washed twice in PBS for 1 hr each. Then the fixed samples were dehydrated in a series of ethanol solutions (from 70% to 100%) and cut into pieces of 1~2 mm in size using a razor blade. The cross-sections were coated with gold under 50 mTorr and at 5 mA for 50 sec using a sputter coater (Sanyu Denshi, Tokyo, Japan). The samples were observed with a SEM (JSM-6400Fs; JEOL, Tokyo, Japan) operated at voltage of 5 kV. The cell-derived ECM scaffolds were analyzed by mercury intrusion porosimeter using an AutoPore II 9220 (Micromeritics Co. Ltd., USA) to determine pore size distributions, specific pore area, median pore diameter and porosity. The tensile strength of the cell-derived ECM scaffold (n=5, 6 mm in length, 3 mm in height and 3~5 mm in width, respectively) was measured at rupture point by Universal Testing Machine (Model H5K-T, H.T.E; Salfords, England) with free load of 0.01 N.
E. Seeding rabbit chondrocytes to the scaffold
The cell-derived ECM and PGA scaffolds (6 mm in diameter) were soaked in sterile 70% ethanol for l hr, washed several times in PBS, and immersed in DMEM overnight prior to cell seeding. The rabbit chondrocytes were obtained from New Zealand white rabbits (2 weeks old) and cultured in the same medium as above used for porcine chondrocytes (Min et al 1998). The cells were seeded at passage 1 dynamically in the scaffolds for 1.5 hr using a rotator at 3 x 106 cells/ml of density.
In chapter I: The chondrocytes-seeded scaffolds were cultured for 2 days, 1, 2, and 4 weeks in vitro for analysis with the medium changed 3 times a week. The distribution and morphology of chondrocytes in the scaffolds were observed by SEM as above at 1 day and 7 days culture. The PGA scaffold was used as control.
In chapter II: Specimens of the cell-ECM scaffold constructs were immersed briefly for 48 hrs in the culture media before the nude mice implantation. Under sterile conditions in a clean room, four constructs at a time subcutaneously were implanted in the backs of the eighteen nude mice. Six mice were sacrificed at each time point of 1, 2, and 3 weeks post-implantation. The cell-free scaffold was used as control.
In chapter III: The chondrocyte-seeded ECM scaffold was cultivated in 6-well plates for 2 days, 2, and 4 weeks in vitro before implantation. The empty group was used as control group.
F. The gross morphology and the volume changes of neocartilage tissues
The gross morphology was observed after culturing the chondrocytes-seeded cell-derived ECM and PGA scaffolds. The volume of neocartilage tissue was measured using a computer vision system developed in our laboratory (Choi et al 2006). Briefly, three images of anteroposterior, posteroanterior and lateral views of the neocartilage (256x256 pixels) were obtained on a white background and put into the computer vision system. The sequence of the image processing algorithm was divided into three stages
of image preparation, shape extraction, and surface area measurements. The volume was calculated using the base area, the topmost area, and the height.
G. Compressive strength of neocartilage tissue
The neocartilage tissues were subjected to the mechanical compressive strength test using a Universal Testing Machine (Model H5K-T, H.T.E; Salfords, England). The specimens (n=4) were cut into a uniform disk shape and then placed on a metal plate, where they were pressed at a crosshead speed of 1 mm/min at a free load of 0.001 N. The individual compressive strengths were calculated at 10% strain point (Jin et al 2007).
H. Chemical assays of neocartilage tissue
The DNA, GAG and collagen contents were determined by digesting dried samples in a papain solution (5 mM L-cysteine, 100 mM Na2HPO4, 5 mM EDTA, and 125
µg/ml papain type III, pH 6.4) at 60°C for 24 hr, followed by centrifugation at 12,000 g for 10 min. The supernatant was used for assays. The total DNA content was determined using quit-iT DNA assay Kit (Invitrogen Eugene, Oregon, USA,). The GAG content was measured by the dimethylmethylene blue (DMB) colorimetric assay (Shihabi and Dyer 1988) using chondroitin sulfate from the shark cartilage for a standard curve (Sigma, St Louis, MO, USA). The collagen content was measured by the analysis for hydroxyproline (Reddy and Enwemeka 1996). The collagen concentration
was calculated by comparing the data with the optical density of a standard solution of bovine collagen (0~10 µg/ml of tracheal cartilage; Sigma Chemical Co, St Louis, MO, USA).
I. Histology and immunohistochemistry
The neocartilage tissues were fixed with 4% formalin for at least 24 hr. The tissues were then embedded in paraffin and sectioned by 4 µm in thickness. The sections were stained with Safranin O/Fast green or Alcian-Blue stain. For immunohistochemical analysis of type II collagen, the sections were treated with 3% H2O2 for 5 min and reacted with 0.15% Triton X-100 to increase the tissue permeability. Once nonspecific binding was blocked with 1% bovine serum albumin (BSA), the sections were incubated for 1 hr with mouse anti-rabbit collagen type II antibody (1:200; Chemicon, Temecula, CA). Then they were incubated sequentially with biotinylated secondary antibody (1:200) for 1 hr and peroxidase-conjugated streptavidin solution for 30 min (DAKO, Carpentaria, CA, USA) at room temperature. The sections were finally counterstained with Mayer’s hematoxylin (Sigma, St Louis, MO, USA) and mounted with a mount solution prior to microscopic observation (Nikon E600, Tokyo, Japan).
J. RT-PCR analysis
The total RNA from each neocartilage was extracted with Trizol reagent (Gibco; Carlsbad, CA, U.S.A). Each RNA sample was reverse-transcribed with Superscript First
Standard Synthesis System (Gibco; Carlsbad, CA, U.S.A) to produce complementary DNAs (cDNAs). PCR reactions were preformed with specific primers for types I and II collagens, and cDNA samples. Glyceraldehyde-3-phsphatase dehydrogenase (GAPDH) was used as a housekeeping gene. The system was programmed to run for 35 cycles and the end products were separated with 1.5% agarose gel electrophoresis and visualized with ethidium bromide.
K. Western blot analysis
Synthesis of types II and I collagen in the neocartilage tissues was screened with Western blotting. Total proteins were extracted from the tissues with a lysis buffer of 40 mM Tris-HCl (pH 8.0), 120 mM NaCl, 0.5% Nonidet p-40 (NP-40), 2 µg/ml aprotinin, 2 µg/ml pestetin, 2 µg/ml leupetin, and 100 µg/ml phenylmethylsulfonyl fluoride (PMSF). Calibrated by the BCA method, equal amount of the proteins was loaded and separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred to a nitrocellulose membrane (Millipore; Bedford, MA, U.S.A.) with a transblot apparatus (Bio-Rad; Hercules, CA, U.S.A.). The blotting membrane was incubated first with a mouse anti-rabbit types II or I collagen monoclonal antibody (Chemicon; Temecula, CA, U.S.A.), diluted at 1:1000 ratio and then rinsed three times with Tris-buffered saline (TBS) containing 0.5% Tween 20. This was followed by incubation with a secondary antibody, peroxidase-labeled sheep anti-mouse IgG (Lockland; Gilbertsville, PA, U.S.A.). This was
visualized with an ECL kit (Amersham; Buckinghamshire, UK)
L. Experimental design and surgery in rabbit
The experimental protocol was approved by the Institutional Animal Experiment Committee. Eighteen New Zealand white rabbits were used in the study with average weight of 3.0~3.5 kg. The surgical procedures were performed under general anesthesia with katamin and lumpun (ratio 3.5:1.5), including limb preparation and draping. Both knee joints were operated in the same surgery. An arthrotomy was made through a midline longitudinal incision on a medial parapatellar with the patella dislocated laterally to expose the femoral condyles. To create an osteochondral defect, a 5 mm drill was used at the patella groove. There were total 36 condyles that were assigned to four groups including untreated control (group 1) and experimental groups implanted with engineered cartilages cultured in vitro for 2 days, 2 weeks and 4 weeks (groups 2, 3 and 4, respectively). The implants were inserted in the defects and press-fixed without any covers or suture materials. At 1 and 3 months after surgery, the rabbits were euthanized by over-dose injection of Pentobarbital to retrieve the femoral condyles.
M. Histological scoring (ICRS score) in rabbit
To evaluate the quality of the repaired articular cartilage in the defects, the modified version of the histological grading scale was used (Wakitani et al 1994). The scale consists of seven categories and assigns a score ranging from 0 to 18 points (Table 1).
The parameters included such as cell morphology, matrix staining (Safranin-O), structural integrity, surface regularity, thickness of cartilage, regenerated subchondral bone and integration with adjacent cartilage.
N. Statistical analysis
Statistical analysis of the experimental data was carried out using a one-way analysis of the variance (ANOVA) for multiple comparisons and a student t test (two-tail) for the pair wise comparisons. Statistical significance was assigned as *p<0.05, **p<0.01 and ***p<0.001, respectively.
III. RESULTS
Chapter I
A. The structure and physical property of cell-derived ECM scaffold
The cell-derived ECM scaffold was made using porcine chondrocytes as described above. According to the gross examination, the disc-shaped scaffold was approximately 6 mm in diameter and had a white macrostructure with uniform pores (Fig. 4). By SEM images, a highly uniform porous microstructure was observed with the peripheral region of the scaffold densely coated (Fig. 5, white arrowheads). The dense peripheral coat was trimmed off using a 6 mm biopsy punch, resulting in a final cell-derived ECM scaffold with a standardized dimension of 6 mm in diameter and 3 mm in thickness. The constructs had approximately 504±108㎛ of pore diameter, 90±10.4% porosity and 905±204 m2/g of surface area. The tensile strength was measured as 0.34±0.09 MPa (Table 2).
Fig. 4. The schematic illustration shows the process of making cell-derived ECM scaffold. Experimental details are described in Materials and Methods. A white
cylinder-shape of porous scaffold was finally produced with 6 mm in diameter and 2~3 mm in thickness. The scale bar is in the millimeter (mm) unit.
Table. 2. Physical properties of the cell-derived ECM scaffold.
Fig. 5. The SEM image of cell-derived ECM scaffold (x30). A cross section of the
cell-derived ECM scaffold was observed by scanning electron microscope (SEM). (A) The exterior of the scaffold was densely coated with micro-pores (white arrows). (B) A highly uniform porous microstructure was observed in the central region of the scaffold.
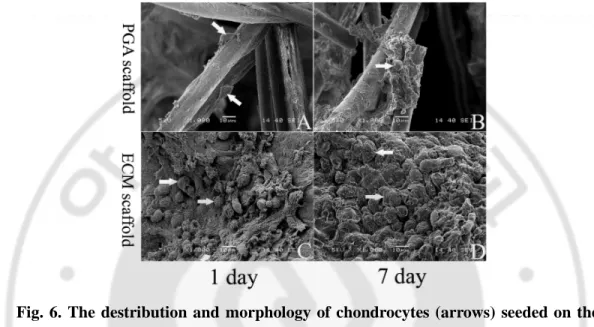
B. Distribution and morphology of seeded chondrocytes on scaffold
In the SEM images taken after 1 day of cell seeding, many chondrocytes were evenly distributed on the cell-derived ECM scaffold in a round shape, whereas a few cells were attached to the PGA scaffold in a spindle shape (Figs. 6A and 6C). After 7 days of
culture, the newly synthesized ECM was found in both scaffolds, but, more densely populated cells were observed in the cell-derived ECM scaffold (Figs. 6B and 6D).
Fig. 6. The destribution and morphology of chondrocytes (arrows) seeded on the PGA (A, B) and the cell-derived ECM scaffolds (C, D), respectively. The images
were taken by SEM at 1 (A, C) and 7 days (B, D) after culture in vitro (x1000).
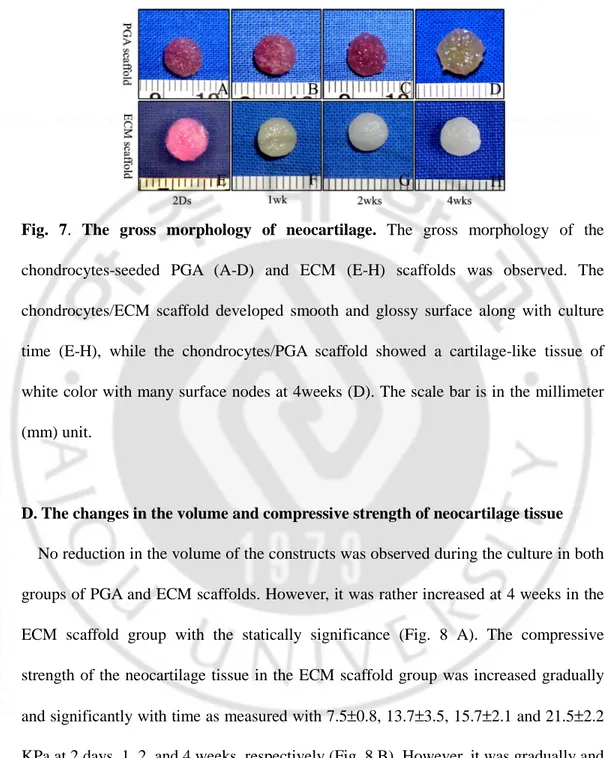
C. Gross morphology of neocartilage tissue
When the chondrocytes in the PGA and ECM scaffolds were cultured until 4 weeks, they appeared to mature over time to form a neocartilage-like tissue. The neocartilage constructs of the ECM scaffold was a silvery white cartilage-like morphology after 1 week of culture, and showed a smooth glossy surface gradually with time (Figs. 7E-H). In contrast, the constructs of PGA scaffolds was white in color and showed many surface nodes at 4 weeks (Figs. 7A-D).
Fig. 7. The gross morphology of neocartilage. The gross morphology of the
chondrocytes-seeded PGA (A-D) and ECM (E-H) scaffolds was observed. The chondrocytes/ECM scaffold developed smooth and glossy surface along with culture time (E-H), while the chondrocytes/PGA scaffold showed a cartilage-like tissue of white color with many surface nodes at 4weeks (D). The scale bar is in the millimeter (mm) unit.
D. The changes in the volume and compressive strength of neocartilage tissue
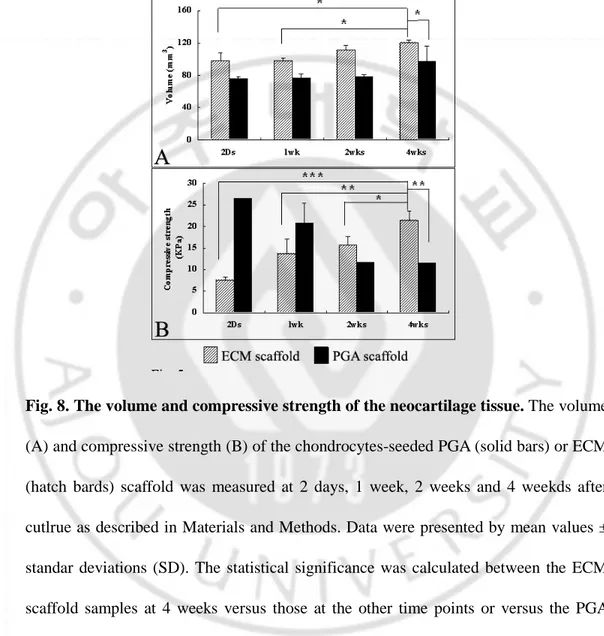
No reduction in the volume of the constructs was observed during the culture in both groups of PGA and ECM scaffolds. However, it was rather increased at 4 weeks in the ECM scaffold group with the statically significance (Fig. 8 A). The compressive strength of the neocartilage tissue in the ECM scaffold group was increased gradually and significantly with time as measured with 7.5±0.8, 13.7±3.5, 15.7±2.1 and 21.5±2.2 KPa at 2 days, 1, 2, and 4 weeks, respectively (Fig. 8 B). However, it was gradually and significantly decreased with time in the PGA scaffold group, which showed 26.5,
compressive strength of the ECM scaffold group was much lower than that of the PGA scaffold group at 2 days but became higher by more than 2 folds at 4 weeks.
Fig. 8. The volume and compressive strength of the neocartilage tissue. The volume
(A) and compressive strength (B) of the chondrocytes-seeded PGA (solid bars) or ECM (hatch bards) scaffold was measured at 2 days, 1 week, 2 weeks and 4 weekds after cutlrue as described in Materials and Methods. Data were presented by mean values ± standar deviations (SD). The statistical significance was calculated between the ECM scaffold samples at 4 weeks versus those at the other time points or versus the PGA scaffold samples at 4 weeks by Student-Newman-Keuls Multiple comparisons test (n=4; *p<0.05, **p<0.01, ***p<0.001).
E. Chemical composition of neocartilage tissue
The total contents of DNA, GAG and collagens were examined in the neocartilage-like tissues from the PGA and ECM scaffold groups during the culture (Fig. 9). The DNA content of the neocartilage-like tissue was gradually and significantly increased with time in both groups. The DNA content of ECM scaffold group increased more than 3 folds at 4 weeks, when compared with that of the ECM scaffold itself (Fig. 9A). The GAG content also increased with time in all groups. The amount of GAG was 276.5±20.6 µg in the ECM scaffold itself and 378.5±65.6, 396.7±128.9, 1302.8±65.4 and 1450±30 µg in the neocartilage tissue with the ECM scaffold at 2 days, 1, 2, and 4 weeks, respectively. The GAG content of the ECM scaffold group was significantly higher that that of the PGA scaffold group until 2 weeks, but they were quite similar at 4 weeks (Fig. 9B). Although the collagen content also increased gradually with time in both groups, the ECM scaffold group showed much higher values than the PGA group at all time points (Fig. 9 C). The collagen content in the ECM scaffold group was more than 4 folds of the initial amount at 4 weeks. The amount of newly synthesized GAG and collagen from the seeded cells was calculated to be about 1173.5 and 1474.2 µg, respectively, in the ECM scaffold group at 4 weeks.
Fig. 9. Chemical analysis for the neocartilage tissues. The neocartilage constructs
from the chondrocytes-seeded PGA (solid bars) or ECM (hatch bards) scaffold were measured for the contents of DNA (A), GAG (B) and collagen (C), respectively as described in Materials and Methods. The statistical significance was analyzed between the ECM scaffold samples at 4 weeks versus those at the other time points or versus the PGA scaffold samples at 4 weeks by Student-Newman-Keuls Multiple comparisons test (n=3; ***p<0.001).
F. Histological assay of the neocartilage tissue
The expression of sulfated proteoglycan and type II collagen was then examined by histological observation in the neocartilage-like tissue. The Safranin-O staining of samples confirmed the accumulation of sulfated proteoglycans, which filled gradually with time the porous space and uniformly distributed within the cell-derived ECM
group decreased slowly with time probably indicating biodegradation of the scaffold (Fig. 10M-P, black arrows). In the PGA scaffold group, the sulfated proteoglycan was accumulated only after 2 weeks and distributed at peripheral area only (Fig. 10A-H).
Immunohistochemical analysis also showed that the synthesis of type II collagen was uniformly observed in the pericellular and pore regions in the ECM scaffold group (Fig. 11G-11L). Just like the sulfated proteoglycans, the expression was collagen type II was gradually increased with time but they were accumulated only in the peripheral area starting after 2 weeks of culture in the PGA scaffold group (Fig. 11A-11F).
Fig. 10. Expression of sulfated proteoglycans was examined in the neocartilage-like tissues with Safranin O staining. The proteoglycans was gradually accumulated in
both of the PGA and ECM scaffolds with culture time, however the pattern of their distribution was significantly differanted between two groups. The magnification was
Fig. 11. Expression of type II collagen in the neocartilage-like tissues. The
neocartilage tissues from the PGA and ECM scaffolds were subjected to immunostaining for type II collagen along with culture time. As in the distribution of proteoglycan, the pattern of collagen II distribution was also significantly differanted between two groups. The magnification was x20 (A-C and G-I) and x200 (D-F and J-L), respectively.
Chapter II
A. Morphology, volume and histology of neocartilage tissue
When the chondrocyte-seeded ECM scaffold was allowed to grow for 1, 2, and 3 weeks in the nude mice, we observed a smooth, white-colored surface and a relatively hard cartilage-like tissue based on the pinch test. There was also no apparent change in tissue size in the experimental group 1 week after implantation (Fig. 12). In fact, the volumes of the neocartilage were 45.3 ± 3.6 mm3, 48.1 ± 6.3 mm3, and 52.2 ± 2.1 mm3 at the 1st, 2nd, and 3rd weeks, respectively, in the experimental group (Fig. 13). The average volume gradually increased, although there were no significant statistical differences among the specimens of the experimental group. Comparatively, it decreased significantly in the control group by the 3rd week of implantation. Histological staining with Safranin O (Fig. 14A-F) and Alcian blue (Fig. 14G-L) exhibited a sustained accumulation of the sulfated proteoglycan and, thus, gradually filled the pore spaces in the scaffold in the experimental group. Cartilaginous ECM appeared as early as the 1st week of implantation and it was more prominent at the peripheral region than at the core at the 3rd week of implantation. However, the positive staining was not observed in the pore space of the ECM scaffold, and fibrous tissue filled in the control group.
Fig. 12. The gross morphology of neocartilage tissues. The neocartilage tissue had a
white color and smooth surface, and was a relatively hard, cartilage-like tissue on the pinch test. It was notable that there was no apparent change in tissue size in the experimental group after 1 week of implantation.
Fig. 13. The volume of neocartilage tissues. The volume of neocartilage tissue
increased gradually and was about 72% of the initial volume at the 3rd week in the experimental group. Comparatively, it decreased continuously by time and significantly
Fig. 14. Histological stainings of neocartilage tissue. Histological stainings with
Safranin O (A-F) and Alcian blue (G-L) exhibited a sustained accumulation of the sulfated proteoglycan, and the pore space in the scaffold was thus gradually filled in the experimental group. However, the positive staining was not observed in the control group.
B. Analyzing phenotypes of chondrocytes: RT-PCR analysis
The biomaterial induced a comparable constant gene expression of types I and II collagen over a period 3 weeks in both groups. Type II collagen gene was expressed constantly in the experimental group throughout the experimental period; however, neither types I nor II collagen gene was expressed in the control group. And type I
collagen gene was also expressed in the experimental group, but it decreased gradually with time in the culture (Fig. 15).
Fig. 15. RT-PCR analysis. Type II collagen was expressed constantly in the
experimental group during the entire period, while the expression of type I collagen decreased gradually by time (lanes d-f). However, types I and II collagen genes were not expressed in the control group (lanes a-c). a and d, 1 week; b and e, 2 weeks; c and f, 3 weeks.
C. Chemical assay for neocartilage tissues
The water content was 89.4 ± 1.3%, 91.1 ± 0.5%, and 90.5 ± 0.8% in the control group and 89.1 ± 0.5%, 87.8 ± 1.1%, and 87.0 ± 0.4% in the experimental group at the 1st, 2nd, and 3rd weeks, respectively (n=4). Statistically, the water content was not significantly different in the control group; whereas, it decreased gradually in the experimental group during the study period (Fig. 16A). Measured from the papain-digested samples, the total GAG content was 62.6 ± 13.1 µg/mg, 56.3 ± 17.8 µg/mg, and 22.3 ± 7.4 µg/mg in the control group (dry weight) and 75.6 ± 19.8 µg/mg, 171.4 ± 15.3 µg/mg, and 201.3 ± 13.4 µg/mg in the experimental group at the 1st, 2nd, and 3rd
weeks, respectively (n=4). The GAG content decreased gradually in the control group; whereas, it increased steadily with time in the experimental group, reaching 66% of the native cartilage at the 3rd week (Fig. 16B). Compared with the GAG content, the total DNA content decreased significantly in both groups after the 2nd week of the study (Fig. 16C). The DNA-normalized GAG content increased significantly in the experimental group; whereas, it decreased continually in the control group over time (Fig. 16D).
Fig. 16. Chemical assay. The water content decreased gradually by time in the
experimental group; however, it was not significantly changed in the control group (A). The GAG content gradually decreased in the control group, whereas it increased gradually in the experimental group over time and reached about 66% of the native cartilage at the 3rd week (B). However, the total DNA content decreased significantly in both groups at the 2nd week post-implantation (C). The GAG content normalized by the DNA content increased significantly from 2 weeks in the experimental group; whereas, it decreased continually in the control group over time (D). *p<0.05, **p<0.01, and
D. Collagen analysis
Immunohistochemistry identified type II collagen synthesized in the neocartilage tissues. With the protein deposited in the pericellular and pore regions, it was apparent that more type II collagen accumulated over time in the experimental group (Fig. 17D-F). However, type II collagen was not detected in the control group (Fig. 17A-C).
Western blot analysis revealed that while type II collagen was clearly detected throughout the study period, it was especially prominent at the 3rd week in the experimental group; however, it was not expressed at any time in the control group (Fig. 18). On the contrary, type I collagen was not expressed at all in either group (data not show).
Fig. 17. Immunohistochemistry analysis. Immunohistochemistry identified type II
collagen synthesized in the neocartilage tissues in the experimental group (D-F). However, it was not detected in the control group (A-C).
Fig. 18. Western blot analysis. Western blot analysis revealed that type II collagen
was clearly detected throughout the experimental period, particularly prominent at 3 weeks, in the experimental group (lanes d-f). However, it was not expressed at any time points in the control group (lanes a-c). a and d, 1 week; b and e, 2 weeks; c and f, 3 weeks.
F. Compressive strength of neocartilage tissue
For the mechanical properties of the neocartilage, the specimens were tested for ultimate compressive strength at a point of 10% strain. The result was 0.45 ± 0.06 MPa, 0.8 ± 0.14 MPa, 1.18 ± 0.17 MPa (n=4) in the experimental group at the 1st, 2nd, and 3rd weeks, respectively (Fig. 19). This result demonstrated that the compressive strength was increased significantly along with the time points in the experimental group. However, it could not be measured in the control group because of its fragility.
Fig. 19. The compressive strength of the neocartilage tissue. The compressive
strength of the neocartilage tissue increased significantly by time in the experimental group. However, it could not be measured in the control group because of its fragility. **p<0.01 and ***p<0.001.
Chapter III
A. Gross finding of cartilage defects
The gross appearance of cartilage defects right after implantation of the engineered cartilages was shown in Fig. 20. The implants were stably inserted on the defect sites (Fig. 20A). At 1 month after surgery, the repaired defects in groups 3 and 4 showed a smooth and glistening appearance and continuity with the surrounding host cartilage tissue. In contrast, the defect was not repaired well in group 1 (control) and filled partially with fibrous tissues in group 2. At 3 months, the white and glistening appearance of repaired tissues was shown on defects in all groups. However, the smooth and hard surface of repaired tissue was observed in groups 2, 3 and 4 by forceps test, while the slightly rough surface with many fissures was shown in group 1 (Fig. 20B). A Initial B 1 month 3 months
Fig. 20. Gross findings of the cartilage defect with implants. (A) Gross image of the
engineered cartilages right after implantation in the defect site. (B) Gross image of the repaired cartilages at 1 and 3 months after implantation. The macroscopically smooth and glistening tissues were appearance with time.
B. Histological evaluation
In the Soffranin-O staining, the defect was partially filled with fibrous tissues in groups 1 and 2 at 1 month, which was not integrated with surrounding host cartilage and bone (Fig. 21A, B, E, F). In contrast, it was repaired to hyaline cartilage-like tissues at 1 month partially in group 3 and completely in group 4, respectively (Fig. 21C, D, G, H). The repaired tissues were integrated successfully to the host cartilage but the subchondral bone was not remodeled in all groups. At 3 months, the fibrous/hyaline cartilage was regenerated and partially integrated to the surrounding host cartilage in groups 1, 2 and 3 (Fig. 21I, J, K, M, N, O). Among them, the rough surface of repaired tissue was found in group 1 and the subchondral bone was partially remodeled in groups 1, 2 and 3. In contrast, the hyaline cartilage tissue with a mature matrix and a columnar organization of chondrocytes was observed in group 4. Moreover, the subchondral bone was well remodeled in the group (Fig. 21L, P).
The total ICRS histological score increased significantly along with time in all groups (Fig. 22). At 1 month, the ICRS score was higher in groups 3 and 4 than in groups 1 and 2, while it was higher in group 4 at 3 months.
1 month
3 months
Group 1 Group 2 Group 3 Group 4
Fig. 21. Histological assay of repaired cartilage on defects. Safranin-O staining of
repaired cartilages from each group at 1 and 3 months post-implantation. A, C, E, G, I, K, M and O: x40; B, D, F, H, J, L, N and P: x100.
Fig. 22. The ICRS histological score. The ICRS score was determined from the results
C. Expression of type II collagens
The immunohistochemistry identified the expression of type II collagen was gradually increasing along with time at the pericellular region in the repaired tissues of groups 2, 3 and 4 (Fig. 23). It was not much detected in group 1 both at 1 and 3 month. The most significant expression of type II collagen with dark brown color was observed in zonal-structure in group 4 at 3 months (Fig. 4H).
1 month
3 months
Group 1 Group 2 Group 3 Group 4 Fig.23. Immunohistochemical staining for repaired cartilage. Immunohistochemical
staining of type II collagen synthesized in the repaired tissues at 1 and 3 months after implantation (x200).
IV. DISCUSSION
Chapter I
The original concept of the current protocol was derived from a scaffold-free cartilage construct made from porcine articular chondrocytes and their high level production of ECM components (Park et al 2006). It was noticed that the scaffold-free cartilage construct could be re-formed into a porous ECM scaffold by freeze-drying process, by virtue of its high water content (~95%) than that of the normal articular cartilage (~85%) (Simon). As shown in the SEM images, a highly porous micro-structure was formed in the cell-derived ECM scaffold, probably comparable to that of PGA scaffold that is used most widely for cell adhesion and growth. Therefore, the cell-derived ECM scaffold might provide a favorable environment for chondrocytes functionally and structurally.
The compatibility of the cell-derived ECM scaffold to chondrocytes was evident from the efficient adhesion and round shape morphology of cells on the ECM scaffold from the early stage. Besides, the rapid increase in the DNA content comparable to that in the PGA scaffold also indicates that the cell-derived ECM scaffold supports well the growth of chondrocytes. The gradual increase in the GAG and collagen contents with time suggests that it provides a favorable environment for the expression of chondrogenic phenotypes. In addition, the synthesized ECMs were uniformly distributed in the cell-derived ECM scaffold, whereas they were observed mainly in the
peripheral area in the PGA scaffold as already shown in many previous studies (Mahnoudifar and Dorn 2005; Mahnoudifar and Dorn 2005; Hu and Athanasiou 2005). It is speculated that various biological elements resident on the cell-derived ECM scaffolds might have played an important role in this process, such as cytokines, growth factors and potent functional proteins (Badylak 2005). The dynamic interaction between the ECM components and intracellular cytoskeleton is believed to be important in the chondrocyte functions (Hering 1999; Takahashi et al 2003).
One of the most noticeable findings in the study was that the volume of the constructs did not decrease significantly but rather increased at 4 weeks of culture in the cell-derived ECM scaffold. It has long been a greatest challenge to overcome the shrinkage of tissue engineered cartilage. The chondrocytes-seeded type II collagen matrix in vitro was reported to shrink continuously to approximately 50% of its original volume within 4 weeks (Lee et al 2000). In addition, a composite of collagen type I gel and chondrocytes decreased in volume to 50~60% of its initial size after 12 days of culture (Badylak). Natural scaffolds such as fibrin/hyaluronic acid also experienced the same problem of shrinkage in size (Park et al 2005). The contraction of both cell-seeded scaffolds and in vivo implants occurs frequently, which makes the implants unfit for a defect site. Such contraction may even cause the loosening of implants, ultimately leading to separation from the surrounding host tissue. The increase rather than decrease in the construct volume observed in this study was probably caused by more active synthesis of ECM components such as GAG and type II collagen than the
degradation rate of the scaffold. This probably also explain why the compressive strength was gradually increased with culture time in the cell-derived ECM scaffold. In contrast, the compressive strength was gradually decreased with time in PGA scaffold, although the synthesized ECM was increased. Thus, we consider that the physical property of neocartilage is related with the amount and distribution of newly synthesized ECM, and the balance between degradation and synthesis rates of ECM as well. The thickness of the scaffold walls was maintained intact until 2 weeks when it began to degrade, as shown in the histological results. Considering that ECM is degraded by degradation enzymes released by chondrocytes, the cell-derived ECM scaffold can be maintained until the chondrocytes fully expand its number. At this moment they progressively switch their phenotype to synthesis their ECM together with releasing degradation enzyme. Chemical analysis can explain this with showing that GAG synthesis increased rapidly from 2 week of culture on. It is likely that degradation was not dominant until the construct was sufficiently filled with newly synthesized ECM. In contrast, PGA degradation was caused by hydrolysis of water, not by a proteolytic enzyme from chondrocytes. Mechanical study supported this evidence. ECM construct never decreased its initial mechanical strength, rather increased with time, comparing that PGA construct was getting rapidly weaker regardless of ECM synthesis.
Regarding the size measurements, a new technique was developed using a computer-assisted image analyzing system in our previous report (Choi et al 2006). Compared
with the conventional 2-dimensional (2-D) method, which simply measures the diameter of the constructs (Lee et al 2000; Park et al 2005; Galois et al 2006), the new technique allows volume measurements 3-dimensionally. These results were validated using a hydrostatic weighing apparatus, which produced a margin of error of 0.62~2.2% (data not shown). Therefore, this method could provide a more accurate measure of the changes in the volume of neoconstructs.
In conclusion, the cell-derived ECM scaffold has a highly porous structure that was suitable for chondrocyte attachment and proliferation. As a cartilage-derived scaffold, its degradation was balanced well by the synthesis of new ECM. In addition, it has the potential to enhance the metabolic activity of chondrocytes as GAG and collagen synthesis. All these favorable influences allowed the construct to not only maintain its size but also produce efficiently cartilage-like structure. These results strongly suggest the importance of the cell-derived ECM scaffold as a novel and promising biomaterial for cartilage tissue engineering.
Chapter II
There have been many reports on the methods of using ECM from the allogenous or xenogenous tissues as a scaffold to produce other organs by tissue engineering (Badylak 2004; Gilbert 2006). However these techniques cannot be applied to the cartilaginous tissue, because the natural cartilage is too compact to provide enough spaces for seeding chondrocytes. In addition, it can cause size and/or shape mismatch, leading to a
step-off in the area of the implantation due to the differences in the surface configurations between the host and donor joints. In order to facilitate the seeding of chondrocytes and maintaining the contents of the cartilaginous ECM, we adopted cell cultivation as a gathering method of ECM. In a previous study, we presented a method for the fabrication of a cell-derived ECM scaffold, and seeding on it of isolated chondrocytes in vitro (Jin et al 2006). Based on this work, we evaluated its feasibility, not only as a cell carrier but also as a structural matrix for cartilage tissue engineering, by implanting the cell-derived ECM scaffold in vivo with chondrocytes. In this study, the cartilaginous tissue was obtained successfully over a short-term period of 3 weeks, by means of a morphological assay, and its biochemical and mechanical properties were evaluated. The experimental specimens retrieved at the 3rd week of post-implantation resembled, in general, normal cartilage.
The most striking feature in this study was that it maintained its volume during cultivation in vivo, even though there was an initial reduction. The shrinkage of tissue-engineered cartilage during cultivation occurs frequently, both in vitro and in vivo. Theoretically, the reduction of size can make implants unfit for a defect site, and consequently cause loosening of implants and separation from the surrounding host tissue. Lee et al reported that when a cell-seeded type II collagen matrix was used in vitro, it decreased in size continuously until it reached approximately 50% of its original diameter by 4 weeks (Lee et al 200). Galois reported that the collagen type I gel and chondrocyte composites reached 50~60% of their initial size by the 12th day of