저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in Neuroscience
To evaluate the therapeutic effects of
genetically modified mesenchymal stem
cells in a chronic stroke animal model
by
Subash Marasini
Major in Neuroscience
Department of Biomedical Sciences
Ajou University Graduate School
To evaluate the therapeutic effects of
genetically modified mesenchymal stem
cells in a chronic stroke animal model
by
Subash Marasini
A Dissertation submitted to The Graduate School of
Ajou University in partial Fulfillment of the Requirements
for the Degree of Ph.D. of Biomedical Sciences
Supervised by
Haeyoung Suh-Kim, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
Ajou University, Graduate School
This certifies that the dissertation of Subash
Marasini is approved
SUPERVISORY COMMITTEE
Thesis Defense Committee Chair
Byung Gon Kim…………
Member
Eun Joo Baik…………..
Member
Sung-Rae Cho ………
Member
Sung-Soo Kim……….
Member
Haeyoung Suh-Kim…….
The Graduate School, Ajou University
2019.12.23
-ABSTRACT-
To evaluate the therapeutic effects of genetically modified mesenchymal stem cells in a chronic stroke animal model
Stroke is the major cause of death and disability worldwide. Despite numerous clinical and preclinical studies in the stroke arena, therapeutic approaches to treat chronic stroke are not properly established. We previously reported that in the acute phase of stroke, mesenchymal stem cells overexpressing Neurogenin 1 (MSCs/Ngn1) showed enhanced therapeutic efficacy compared to naïve mesenchymal stem cells (MSCs). In this study, we found that MSCs/Ngn1 were unable to exert similar beneficial effects when transplanted in the chronic phase. Thus, we genetically modified MSCs/Ngn1 cells using adenovirus encoding hepatocyte growth factor, HGF (MSCs/Ngn1+HGF) and transplanted in the chronic stroke brain. Transplantation of MSCs/Ngn1+HGF cells in chronic stroke significantly improved the behavioral deficits and brain tissue integrity when assessed with sensory-motor function tests and MRI, respectively. Immunohistochemical analyses indicated that the transplantation of MSCs/Ngn1+HGF cells rejuvenated the microenvironment of the damaged brain tissue. To explore the mechanism of post-stroke neurogenesis, we utilized Nestin-GFP mice, a
transgenic mouse model where neural stem/progenitor cells can be detected as GFP. Here, we report that in normal adult Nestin-GFP mice, GFP transgene labeled mostly GFAP+ type B neural stem cells in SVZ and NG2 proteoglycan expressing progenitors in striatum and cortex. In a chronic stroke model of Nestin-GFP mice, mesenchymal stem cells co-expressing Ngn1 and HGF enhanced the proliferation and neuronal differentiation of these NestGFP/NG2+ cells in the injured brain. This result was also observed in the in-vitro culture of cortex derived Nestin-GFP/NG2+ cells. Furthermore, this enhanced neurogenesis was associated with subsequent improvement in behavior and brain tissue integrity.
Our genetically modified MSCs, MSCs/Ngn1+HGF cells satisfied the STEP3 guidelines suggested for preclinical studies by FDA and NIH (2014), might provide a potential therapeutic strategy for the treatment of chronic stroke.
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF FIGURES ... vii
LIST OF ABBREVIATIONS ... xi
I. INTRODUCTION ... 1
II. MATERIALS AND METHODS ... 8
1. Study approval... 8
2. Animals... 8
3. Tamoxifen injection ... 9
4. Tissue preparation ... 9
5. Assessment of Cre induction efficiency ... 9
6. Neurosphere Culture ... 10
7. Mesenchymal stem cell culture and genetic modification ... 10
9. Induction of multi-lineage Differentiation ... 13
10. Senescence associated β-galactosidase assay ... 15
11. Transgene stability under growth-restrictive conditions ... 15
12. Quantitative RT-PCR analysis ... 16
13. Western blotting ... 16
14. Immunocytochemistry ... 17
15. In-vitro Angiogenesis assay... 18
16. In-vitro NSC Proliferation assay ... 18
17. In-vitro Neuronal differentiation assay ... 19
18. Animal models and cell transplantation ... 19
19. Behavior assessment ... 21
20. Measurement of infarct volume (MRI) ... 24
21. Histological analysis ... 26
22. Statistical analysis ... 28
III. RESULTS ... 29
Part A. Chronic stroke animal models and therapeutic effects of MSC/Ngn1 cells in chronic stroke ... 29
1. Characterization of chronic stroke animal models ... 29
Part B. Adenoviral mediated therapeutic genes transfer in MSCs ... 42
1. MSC transduction with adenoviral vector expressing GFP ... 42
2. Transgene expression and growth kinetics of Ad-GFP-transduced MSCs ... 42
3. Cell surface marker expression and multi-lineage differentiation ... 45
4. Long-term transgene expression under growth-restricted conditions ... 48
5. Generation of MSC and MSC/Ngn1 cells overexpressing HGF... 48
6. Cell surface marker expression and multilineage differentiation of Adeno- HGF transduced MSC and MSC/Ngn1 cells ... 51
Part C. Therapeutic effects of HGF overexpressing MSC/Ngn1 cells in chronic stroke ... 54
1. Improved functional recovery and tissue integrity with MSCs/Ngn1+HGF ... 54
2. Augmentation of neuro-regenerative mechanisms by MSC/Ngn1+HGF ... 57
3. Neuronal differentiation of MSC/Ngn1+HGF cells in chronic stroke ... 63
Part D. Chronic stroke model of Nestin-GFP transgenic mice as a tool for studying non-canonical neurogenesis ... 65
1. Characterization of Nestin-GFP cells in adult mouse brain ... 65
2. Characterization of Cortical Nestin-GFP cells in-vitro ... 69
3. Enhanced proliferation and neuronal differentiation of adult brain parenchymal Nestin-GFP progenitors by MSC/Ngn1+HGF cells in chronic stroke model of Nestin-GFP mice ... 71
Part E: NG2CreERTM::RosaFloxedTdTomato double transgenic reporter mice as a Model System to study parenchymal neurogenesis from NG2 cells in chronic stroke ... 77
1. Characteristics of NG2 positive progenitor cells in the parenchyma /
non-canonical niche of the normal brain ... 77 2. In-vivo differentiation of NG2-TdTomato cells in adult mouse brain
parenchyma ... 83
3. Characterization of NG2-TdTomato cells in vitro ... 85
Part F: Role of conditioned media from HGF overexpressing MSCs on in-vitro model of Neurogenesis and angiogenesis ... 88
1. Effects of Conditioned media from HGF overexpressing MSCs on proliferation and neuronal differentiation of NG2 cells ... 88 2. Effects of conditioned media from HGF overexpressing MSCs on in-vitro
angiogenesis of bEND.3 cells ... 93
IV. DISCUSSION & CONCLUSION ... 95
LIST OF FIGURES
PART A
Fig. 1. Induction of middle cerebral artery occlusion (MCAo) Rat and mouse
models ... 31
Fig. 2. A general overview of a brain tissue structure of chronic stroke animal models ... 32
Fig. 3. Temporal changes in neural cells in acute (Day 3) and chronic stroke (Day 30) ... 35
Fig. 4. Characterization of Glial Scar in chronic stroke ... 36
Fig. 5. Behavior assessment in chronic stroke animal models ... 39
Fig. 6. Ineffectiveness of MSC/Ngn1 cells in chronic stroke ... 41
PART B Fig. 7. Adenoviral gene transduction of mesenchymal stem cells (MSCs) ... 43
Fig. 8. Transgene expression in Ad-GFP MSCs ... 44
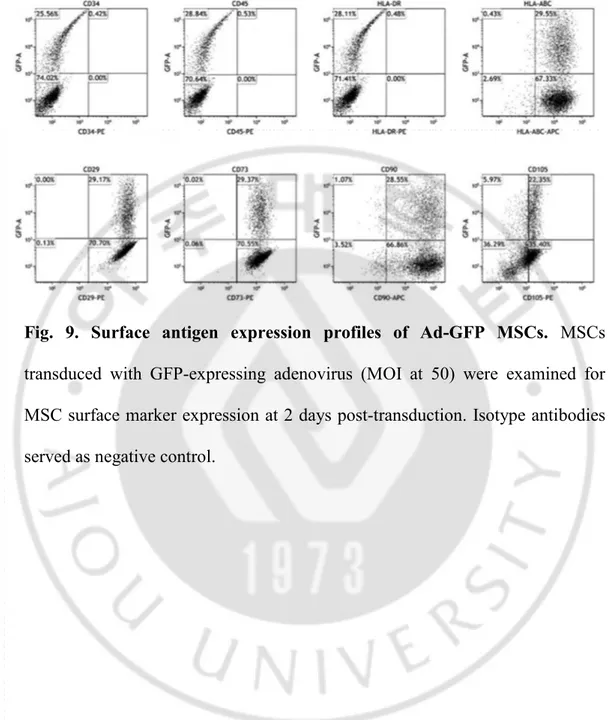
Fig. 9. Surface antigen expression profiles of Ad-GFP MSCs ... 46
Fig. 10. Mesodermal differentiation potential of Ad-GFP MSCs ... 47
Fig. 11. Transgene stability in Ad-GFP MSCs under non-dividing culture conditions ... 49
Fig. 12. Generation of MSC and MSC/Ngn1 cells overexpressing Hepatocyte growth factor, HGF ... 50 Fig. 13. Surface antigen expression profiles of Adeo-HGF transduced MSCs and
MSC/Ngn1 cells ... 52 Fig. 14. Mesodermal differentiation potential of Ad-HGF transduced MSCs and MSC/Ngn1 cells ... 53
PART C
Fig. 15. Enhanced functional recovery in the chronic stroke animals treated with MSC/Ngn1+HGF cells ... 55 Fig. 16. Improved brain tissue integrity of chronic stroke animals treted with
MSC/Ngn1+HGF cells ... 56 Fig. 17. Enhanced angiogenesis by MSCs and MSC/Ngn1 cells overexpressing
HGF ... 59 Fig. 18. Reduced GFAP immunoreactivity in chronic stroke brain by MSC/Ngn1
cells overexpressing HGF ... 60 Fig. 19. Reduced IBA1 immunoreactivity in chronic stroke brain by
mesenchymal stem cells ... 61 Fig. 20. Expression of TGF-β, a key player of immune suppression by HGF
overexpressing MSC and MSC/Ngn1 cells ... 62 Fig. 21. Trans-differentiation of grafted MSC/Ngn1+HGF into neuronal cell 64
PART D
Fig. 22. Nestin-GFP transgene reveals NG2+ oligodendrocyte progenitor cells (OPCs) in non-Canonical NSC niche of adult mouse brain ... 67 Fig. 23. Adult Non-SVZ derived Nestin-GFP cells form neurospheres in-vitro 70 Fig. 24. MSC/Ngn1+HGF treatment during chronic stroke increases Neural
progenitor cells in the ipsilateral striatum ... 73 Fig. 25. MSC/Ngn1+HGF cell treatment during chronic stroke increases the
proliferation of Nestin-GFP+ cells in the ipsilateral striatum ... 74 Fig. 26. MSC/Ngn1+HGF cell treatment during chronic stroke increases the
proliferation of DCx+ neuroblasts in the ipsilateral striatum ... 75 Fig. 27. MSC/Ngn1+HGF cells enhance the neuronal differentiation of
Nestin-GFP+ cells in the ipsilateral striatum ... 76
PART E
Fig. 28. NG2-Cre targets Nestin-GFP cells in Non-canonical stem cell niche, i.e, cortex and striatum... 79 Fig. 29. Cre recombination efficiency in Double transgenic mice ... 81 Fig. 30. NG2 Progenitors Give Rise to Oligodendrocytes in adult brain Cortex
and striatum... 84 Fig. 31. NG2-TdTomato form neurospheres in-vitro ... 86 Fig. 32. NG2-TdTomato cells express progenitor markers in-vitro ... 87
PART F
Fig. 33. Conditioned media from HGF over-expressing MSC and MSC/Ngn1 cells enhance the proliferation of NG2-TdTomato cells in vitro ... 89 Fig. 34. Conditioned media from HGF over-expressing MSC and MSC/Ngn1
cells enhances the neuronal differentiation of NG2-TdTomato cells in vitro ... 91 Fig. 35. HGF over-expressing MSC and MSC/Ngn1 cells enhance in-vitro
LIST OF ABBREVIATIONS
bEND.3 cells: Immortalized mouse brain endothelial cells bFGF: Basic fibroblast growth factor
BrdU: 5-bromo-2'-deoxyuridine CCA: Common carotid artery CM: Conditioned media
CSPG: Chondroitin sulfate proteoglycans DCx: Doublecortin
DMEM: Dulbecco’s Modified Eagle’s medium ECA: External carotid artery
EGF: Epidermal growth factor
FACS: Fluorescence-activated cell sorting FBS: Fetal bovine serum
GAPDH: Glyceraldehyde 3-phosphate dehydrogenase GFAP: Glial fibrillary acidic protein
GFP: Green florescence protein HGF: Heptocyte growth factor ICA: Internal carotid artery
MFI: Mean Florescence intensity MOI: Multiplicity of infection MRI: Magnetic resonance imaging MSC: Mesenchymal stem cells NeuN: Neuronal nuclei
NG2: Neuron-glia antigen 2 Ngn1: Neurogenin 1
NSC: Neural stem cells
NSS: Neurological Severity Scale
OLIG2: Oligodendrocyte transcription factor 2 OPC: Oligodendrocyte precursor cells
PBS: Phosphate-buffered saline
RT-PCR: Reverse transcription polymerase chain reaction SGZ: Sub-granular zone
STEPS III: Stem cell Therapeutics as an Emerging Paradigm for Stroke III SVZ: Sub-ventricular zone
INTRODUCTION
In the chronic phase of an injury, stroke survivors are left with fixed anatomical and functional deficits for which few therapeutic options exist. One reason for limited recovery from stroke could be attributed to the development of a glial scar at the border between normal and ischemic brain tissue (Fawcett and Asher 1999, Yiu and He 2006). Despite extensive studies in stroke arena, very little progress has been achieved to ameliorate the persisting behavioral impairments. Preclinical studies have reported stem cell therapy as a possible therapeutic regimen for stroke, but with very poor clinical translation (Borlongan 2009, Chopp, Steinberg et al. 2009, Savitz, Cramer et al. 2014). At the present time, there are limited numbers of preclinical models of chronic stroke and insufficient understanding of its pathophysiology to provide definitive guidelines for its treatment.
Stem Cells as an Emerging Paradigm in Stroke III (STEPS III) guideline recommends focussing on more advanced stages of clinical testing, as well as the testing of cell therapies in a broader stroke population including chronic stroke(Savitz, Cramer et al. 2014). It has encouraged researchers to work in preclinical models of chronic stroke to reveal its complex science which will aid in the development of potential treatment.
Ample preclinical evidence is available that animal or human-derived Mesenchymal stem cells (MSCs) are safe and efficacious in the preclinical model of neurological disorders like acute stroke, amyotrophic lateral sclerosis and spinal cord injury (Lindvall and Kokaia 2006, Uccelli, Milanese et al. 2012, Forostyak, Jendelova et al. 2013, Yoo, Chang et al. 2013). It has also been shown that to enhance their therapeutic efficacy MSCs can be genetically modified to overexpress therapeutic genes while maintaining their stemness (Kim, Yoo et al. 2008, Chan-Il, Young-Don et al. 2013). In our previous report, neural induction with neurogenin1 was sufficient to achieve a significant enhancement in the therapeutic efficacy of MSCs in stroke when the cells were transplanted in the acute phase (Kim, Yoo et al. 2008). However, very less is known about the efficacy of Mesenchymal stem cell-based therapy in chronic stroke. The mechanism of action of the stem cell therapy involving neuroprotection and immunomodulation might be operational only in the acute to subacute stages. Furthermore, the development of inhibitory glial scar developing at the border between normal and ischemia brain further complicates the pathophysiology and treatment strategy of chronic stroke (Fawcett and Asher 1999, Rhodes and Fawcett 2004, Yiu and He 2006, Huang, Wu et al. 2014).
Recent studies have revealed that the modification of brain tissue microenvironment to aid in neuroplasticity is the key strategy for the improvement of behavioral recovery in chronic stroke. Enzymatic dissolution of
the post-ischemic glial scar was associated with axonal sprouting and subsequent behavioral improvement in chronic stroke rats (Hill, Jin et al. 2012, Soleman, Yip et al. 2012, Gherardini, Gennaro et al. 2015). Also, it has been well reported that augmentation of angio-neurogenesis in the ischemic brain is associated with increased neuroplasticity and behavioral improvement (Taguchi, Soma et al. 2004, Shyu, Lin et al. 2006, Piao, Gonzalez-Toledo et al. 2009).
After a stroke, neural stem cells (NSC) increase their proliferation and newly formed neuroblasts migrate towards the site of injury. Experimental findings have raised the possibility that functional improvement after stroke may be induced through neuronal replacement by endogenous NSCs(Marlier, Verteneuil et al. 2015). The production of new neurons in the adult injured brain takes place in areas called classical neurogenic niches, the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG). However, recently it has been shown that adult neurogenesis could also take place in other brain areas, along the ventricular system (Lin, Cai et al. 2015) and from the glial cells like pericytes, oligodendrocyte precursors, and astrocytes following brain injury (Nakagomi, Molnar et al. 2011, Robel, Berninger et al. 2011, Heinrich, Bergami et al. 2014, Torper, Ottosson et al. 2015). However, the process of forming new neurons after the injury is not efficient and finding ways to improve it may help with recovery after stroke injury. The role of classical neural stem cells residing in the subventricular zone
(SVZ) and sub-granular zone (SGZ), is well established after stroke injury. However, the role of the largest pool of local progenitors also known as “NG2” progenitors located throughout the grey and white matter of the adult mouse brain(Nishiyama 2001, Belachew, Chittajallu et al. 2003), in post-stroke neurogenesis, mostly in chronic phase is still unknown. NG2 progenitors are also known as oligodendrocyte progenitor cells as these cells predominantly generate myelinating oligodendrocytes in physiological as well as pathological conditions (Watanabe, Toyama et al. 2002, Islam, Tatsumi et al. 2009, Kang, Fukaya et al. 2010, Valny, Honsa et al. 2018). However, these cells have also been reported to have multi-potential differential potential generating protoplasmic astrocyte (Tatsumi, Takebayashi et al. 2008, Zhu, Bergles et al. 2008, Zhu, Hill et al. 2008, Guo, Ma et al. 2009, Tripathi, Rivers et al. 2010, Chung, Guo et al. 2013, Huang, Zhao et al. 2014), projecting neurons in the piriform cortex (Rivers, Young et al. 2008, Guo, Maeda et al. 2010) and interneurons in the olfactory bulb (Belachew, Chittajallu et al. 2003, Aguirre and Gallo 2004, Tsoa, Coskun et al. 2014) of both injured and normal rodent brain but with several controversial results. Moreover, it is still not known whether this reported multi-lineage differentiation potential of these NG2 progenitors is augmented in chronic stroke brain with some therapeutic interventions.
We hypothesize that Mesenchymal stem cells overexpressing several beneficial therapeutic genes, act to enhance endogenous repair mechanisms like
local endogenous neurogenesis, angiogenesis, synaptogenesis and brain plasticity in chronic stroke brain which is otherwise are dropped to the normal state after a period of initial activation following stroke. The enhancement of neurogenesis in the chronic stroke brain is shown to be directly associated with the improvement of behavior functions.
Hepatocyte growth factor (HGF), also known as scatter factor (SF) is a pleiotropic factor acting in various organs like the liver, heart, and brain (Nakamura, Nawa et al. 1984, Nakamura, Nishizawa et al. 1989, Tashiro, Hagiya et al. 1990, Maina and Klein 1999). HGF and its receptor c-MET are highly upregulated in the chronic phase of stroke indicating its potential role in brain repair mechanisms (Honda, Kagoshima et al. 1995, Achim, Katyal et al. 1997, Sun, Funakoshi et al. 2002, Nagayama, Nagayama et al. 2004). HGF is also a known niche signal for neural stem cell (NSCs) amplification and self-renewable and its delivery during the acute phase of stroke provides long term neuroprotection via mechanisms involving NSCs proliferation and differentiation (Nicoleau, Benzakour et al. 2009, Doeppner, Kaltwasser et al. 2011). Preclinical stroke studies utilizing recombinant HGF protein or HGF gene therapy have shown the potent neuro-restorative effects like neurogenesis, angiogenesis, synaptogenesis and most importantly anti-fibrosis(Shimamura, Sato et al. 2006, Doeppner, Kaltwasser et al. 2011, Shang, Deguchi et al. 2011). Meanwhile, HGF overexpressing MSCs have been shown to exert superior
therapeutic effects than naïve MSCs in various disorders of cardiac, hepatic and neural origin (Bai, Lennon et al. 2012, Jeong, Kwon et al. 2012, Lu, Zhao et al. 2013, Kim, Kim et al. 2014). In the field of therapeutic angiogenesis to treat hindlimb or myocardial ischemia, HGF has been identified as a novel target (Morishita, Nakamura et al. 1999, Kim, Yoo et al. 2008, Yuan, Zhao et al. 2008). Furthermore, in-vitro studies show that HGF has more potent pro-angiogenic activity than the most commonly used proangiogenic factor, Vascular endothelial growth factor (VEGF) (Nakamura, Morishita et al. 1996, Makarevich, Tsokolaeva et al. 2012).
With all these viewpoints, we hypothesized that HGF might play a central role in neuro-restoration and enhance behavior in chronic stroke. To confirm this, we transferred the human HGF gene into our neurally induced MSCs (MSC/Ngn1) using an adenoviral vector (MSC/Ngn1+HGF cells) and transplanted into the brain of chronic stroke animal models. We demonstrated that overexpression of HGF in MSC/Ngn1 cells significantly enhanced their therapeutic efficacy in chronic stroke as depicted by enhanced behavioral recovery, preservation of tissue integrity and upregulation of mechanisms for neuro-restoration. i.e, anti-fibrosis, angiogenesis and neurogenesis. Additionally, in a chronic model of Nestin-GFP mice (Yamaguchi, Saito et al. 2000), where neural stem can be visualized with green fluorescence, HGF overexpressing MSC/Ngn1 cells robustly increased the number of GFP+ neural stem cells in the
ischemic striatum.
To establish the role of these NG2+ cells in post-stroke neurogenesis, we induced middle cerebral artery occlusion (MCAo) in NG2Cre reporter mice and assessed the fate of NG2-TdTomato cells in acute and chronic phase. In the chronic stroke model of NG2Cre reporter mice, MSC/Ngn1+HGF cells were injected into peri-infarct striatum. The assessment of kinetics and fate of TdTomato+ cells will provide direct evidence whether NG2 cells become multipotent and generate new functional neurons in the chronic stroke brain and is highly enhanced by MSC/Ngn1+HGF cells.
MATERIALS AND METHODS
1.
Study approvalAll experimental protocols using MSCs were approved by the Institutional Review Board of the Ajou University Hospital (AJIRB-GEN-SMP-11-187) and all animal protocols were approved by the Institutional Animal Care and Use Committee of the Ajou University School of Medicine (2015-0012).
2. Animals
B6; BAkik/J (NG2-creERTM), B6.Cg-Gt(ROSA)26Sortm14.CAG-tdTomato/Hze/J (iTdTomato), mice were purchased from Jackson Laboratory. Nestin-GFP transgenic mice (Yamaguchi, Saito et al. 2000) were obtained from the University of Tokyo. All mice were maintained in pathogen-free conditions under a 12:12-h light/dark cycle and fed ad libitum. All experiments were carried out using littermates. For stroke studies, 3-5-month-old mice and male Sprague Dawley rats weighing 250–270 g (Koatech, Pyeongtaek, Korea) were used. For assessing the spatiotemporal characteristics of Nestin-GFP and NG2-TdTomato cells, transgenic mice at P10, P30, and P90 were used. NG2creERTM and Rosa26TdTomato mice were maintained as homozygotes, and double-heterozygous transgenic NG2Cre-TdTomato reporter mice were used for the experiments.
3. Tamoxifen injection
Cre activity in postnatal NG2Cre: TdTomato reporter double-transgenic and Nestin-GFP:NG2Cre:: TdTomato triple transgenic mice was induced by intraperitoneal injection of Tamoxifen (Sigma). A 10 mg/ml stock solution was prepared by dissolving tamoxifen in corn oil. Postnatal day P30, P60, and P90 mice were injected with 100mg/kg/day intraperitoneally for five consecutive days. P10 mice were injected with 50 mg/kg/day intraperitoneally for five consecutive days. Control animals were injected with the same volume of vehicle.
4. Tissue preparation
Mice were deeply anesthetized with CO2 gas and killed by perfusion through the heart, first with 0.9% saline (wt/vol), and then with 10% formalin in PBS. Brains were removed and immersed in 10% formalin overnight at 4 °C. Tissue was cryo-protected overnight at 4 °C in 20% sucrose (wt/vol) in PBS, embedded in Tissue-Tek OCT compound, frozen on isopentane and stored at – 72 °C. Cryo-sections (30µm) were collected onto silane-coated slides.
5. Assessment of Cre induction efficiency
Cre induction efficiency was obtained 1 day after the last tamoxifen injection by dividing the number of TdTomato+Olig2+ or TdTomato+NG2+
cells by the total number of NG2+ cells in randomly selected fields of defined areas in cortex and striatum in parasagittal sections.
6. Neurosphere Culture
Neurosphere culture was carried out using the cells from the cortex of P90 Nestin-GFP mice or total brain cells of NG2-TdTomato mice induced with tamoxifen (100 mg/Kg body weight) for five consecutive days, starting on P30. Brain tissues were carefully dissociated into single-cell suspension using Miltenyi Biotech’s Adult brain dissociation kit following the manufacturer’s protocol. Cells were suspended in PBS and proceeded for FACS using BD FACSVantage (BD Bioscience, San Jose, CA, USA). After FACS, cells were rinsed twice with PBS solution and resuspended in Neural stem cell-like medium, i.e., DMEM/F12 (1:1, vol/vol) supplemented with 1% N2, 1% B27 (GIBCO BRL/Life Technologies), 20 ng/ml EGF, and 10 ng/ ml FGF2 (Peprotech). For suspension cultures, FACS-sorted cells were seeded onto uncoated 24-well plates (5 x104cells/well) and grown in NSC medium with daily addition of growth factors at the concentrations listed above.
7. Mesenchymal stem cell culture and genetic modification
Human MSCs were isolated from bone marrow aspirates and cultured in vitro, as previously described (Kim, Choi et al. 2005, Marasini, Chang et al.
2017). Briefly, mono-nucleated adherent cells were collected and maintained in Dulbecco’s modified Eagle’s medium (Welgene, Gyeongsan, South Korea)
supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Queensland, Australia) 100 U/ml penicillin, 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) and 10 ng/ml basic fibroblast growth factor (PeproTech, Rocky Hill, NJ, USA). MSCs/Ngn1 were prepared using a retroviral vector encoding Ngn1 as previously described (Kim, Yoo et al. 2008). Replication-defective recombinant human adenovirus type 5 harboring a GFP reporter under the control of an elongation factor 1 alpha-promoter (Ad-GFP) was purchased from Vector Biolabs (USA. For transduction experiments, MSCs were plated at a density of 5 X 104 cells per 100 mm dish in the presence of standard MSC growth media. After reaching 70- 80% confluency, cells were transduced with adenovirus in 6 ml of growth media at 0, 1, 10, 25, 50, 100, and 200 MOI. After a 2 h incubation at 37℃, the transduction media was replaced with standard MSC growth media. Cells were then analyzed for GFP expression at 48 h post-infection by fluorescence microscopy using an Olympus 1X71 microscope (Olympus Optical Co. Ltd., Japan) at 200X magnification and flow cytometry with a BD FACS Vantage (BD Biosciences, USA). For flow cytometry, 10,000 events were acquired for each condition and the data analyzed using with Cell Quest Pro software (BD Biosciences). To assess GFP stability and cell proliferation after long-term culture, cells were cultured with a standard
conditions (plating MSCs at density of 1 X 105 cells per 100 mm dish and sub-culturing upon 70-80% confluency) for 2 subsequent passages. At the end of each passages, cell proliferation was examined by trypan blue exclusion assay. In addition, the percentage of GFP-positive cells and the mean fluorescence intensity (MFI) was analyzed by flow cytometry.
MSC+HGF and MSC/Ngn1+HGF cells were prepared by the transduction of MSCs and MSC/Ngn1 cells with a Replication-defective recombinant human adenovirus type 5 harboring a human HGF. For transduction, MSCs were plated at a density of 5 x 104 cells per 100 mm dish in the presence of standard MSC growth media. After reaching 70- 80% confluency, cells were transduced with adenovirus in 6 ml of growth media 200 MOI. After a 2 h incubation at 37℃, the transduction media was replaced with standard MSC growth media. For, cell transplantation experiments, cells were harvested with 1 ml of 0.25% Trypsin-EDTA (Invitrogen, Carlsbad, CA, USA) and washed with PBS three times and suspended in PBS at a concentration of 1x106 Cells in 10µl PBS. For assessing HGF expression by immunocytochemistry and western blotting, Cells were cultured for 48 h post-infection at 37℃.
8. Surface antigen analysis
were harvested with 0.25% Trypsin-EDTA and were resuspended in PBS containing 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA). The cells were stained with fluorochrome-conjugated antibodies against CD90, CD45, CD29, CD73, HLA-DR, CD34, CD105 or isotype controls (Biolegend, San Diego, CA, USA) for 15 min at 22ºC. After washing with PBS containing 1% BSA, the cells were analyzed with an Attune NxT Acoustic Focusing Cytometer (Invitrogen, Carlsbad, CA, USA). All assays included unstained and isotype controls.
9. Induction of multi-lineage Differentiation:
MSCs, MSC+Ad-GFP, MSC/Ngn1, MSC+HGF, and MSC/Ngn1+HGF cells were harvested on day 2 after transduction and were used for differentiation assay. All differentiation assays included non-induction media (MSC growth media) controls. Osteogenic, adipogenic, and chondrogenic differentiation were performed as previously described elsewhere (Pittenger, Mackay et al. 1999, Park, Chang et al. 2013) with a slight modification. Briefly, cells were plated at a density of 4 ×104 cells per 4 well plate in normal MSC culture medium and grown to confluence. The culture medium was replaced with adipogenic differentiation media (StemPro® Adipogenesis Differentiation Kit; Gibco, Cat: A10070-01), or osteogenic medium (StemPro® Osteogenesis Differentiation Kit; Gibco, Cat: A10072-01) for 12 days. Adipogenic
differentiation was verified by accumulation of lipid droplets stained with Oil Red O and osteogenic differentiation and the associated accumulation of extracellular calcium crystals was assessed by staining with Alizarin Red S. Chondrogenic differentiation was induced by cultivating 3×105 cells in pellets in a chondrogenic differentiation medium (StemPro® Chondrogenesis Differentiation Kit; Gibco, Cat: A10071-01 ) for 4 weeks. Paraffin embedded chondrogenic differentiated MSCs and Adeno-GFP-transduced MSCs pellets were sectioned at 5 µm thickness and mounted to coated slides for further GFP immunohistochemistry and Alcian blue staining procedures. Briefly, the sections were incubated with 1% H2O2 in PBS for 20 min, blocked with 10%
normal goat serum for 1 h at room temperature, and then probed with a chicken anti-GFP antibody (1: 500, Abcam, UK) at 4℃ overnight. After washing the primary antibody, the sections were incubated with biotinylated goat anti-chicken secondary antibody (1:200, Vector Laboratories) for 1 h and avidinperoxidase conjugate (ABC Kit, Vector Laboratories, USA) solution for 30 min. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.0).
Sections were subsequently incubated with Alcian blue staining solution for 45 min at room temperature. Stained images were acquired using a Zeiss Axiophot microscope (Carl Zeiss, Germany).
10. Senescence associated β-galactosidase assay
MSCs transduced with Ad-GFP at 50 MOI were cultured for 1 passage under standard conditions and then plated at 1 X 105 cells per well in 6-well plates. The next day, cells were washed with phosphate-buffered saline (PBS), fixed in 10% formalin for 10 min at room temperature, rinsed twice with PBS, and incubated with senescence-associated β-galactosidase staining solution containing 5 mM potassium ferricyanide (K3[Fe(CN)6]), 5 mM potassium ferrocyanide (K4[Fe(CN)6]) , 2 mM MgCl2 in 0.2M citric acid/PBS (pH6.0), and 0.5 mg/mL X-gal solution. Cells were incubated in a 37℃ incubator for 12-16 h. X-gal staining was observed under phase contrast microscopy at 200X and merged with corresponding fluorescence images to identify double-positive cells.
11. Transgene stability under growth-restrictive conditions
Ad-GFP MSCs at 50 MOI were cultured under growthrestrictive conditions (i.e. high-density plating without passaging). Briefly, cells were harvested 2 days post-transduction with EDTA/trypsin and counted by trypan blue exclusion method. A total of 1.5 X 106 live cells were plated in 100-mm
dish and cultured for 30 days at 37℃ without passaging. Media was changed every 2 days. The stability of transgene expression was analyzed 30 days after plating by assessing the intensity and frequency of GFP expression by florescence microscopy and compared to cells after 1 day of high-density plating.
12. Quantitative RT-PCR analysis
Total RNA was extracted from MSC, MSC/Ngn1, MSC+HGF and MSC/Ngn1+HGF cells using RNAzol B (Tel-Test; Friendswood, TX, USA), and cDNA was synthesized from 1 μg total RNA using the First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s recommendations. Real-time PCR was performed to quantify the expression of chemokine receptors using StepOnePlus Real-Time PCR system (Applied Biosystems). Each 20 µl reaction mixture contained 5 pmol of each primer, 10 µl Power SYBR Green PCR Master Mix (Applied Biosystems), and 100 ng cDNA. The PCR protocol consisted of 2 min of denaturation at 95ºC followed by 40 cycles of 95ºC for 15 sec, 65ºC for 30 sec, and 72ºC for 30 sec. The primer sequences utilized are summarized in Table 1.
Table 1. Primers for qRT-PCR Gene Primer Sequence
HGF Foward : CAATAGCATGTCAAGTGGAG Reverse : CTGTGTTCGTGTGGTATCAT Ngn1 Foward : GCTCTCTGACCCCAGTAGC Reverse : GCGTTGTGTGGAGCAAGTC TGF-β Forward : GGCCAGATCCTGTCCAAGC Reverse : GTGGGTTTCCACCATTAGCAC GAPDH Forward : GTCTCCTCTGACTTCAACAGCG
Reverse : ACCACCCTGTTGCTGTAGCCAA
13. Western blotting
100mm dish were lysed with RIPA buffer (50 mM Tris; pH 7.4, 1 M NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate) using a standard protocol and subjected to polyacrylamide gel electrophoresis. After transferring to the PVDF membrane, the protein was identified with anti-HGF antibody (1∶500, R&D systems). Immunoreactivity was visualized using horseradish peroxidase-conjugated anti-goat IgG antibody (1∶50,000 Zymed, San Francisco, CA) and the SuperSignal Chemiluminescence Substrate kit (PIERCE, Rockford, IL). To confirm equal loading, blots were stripped and probed against α-actin antibody (1∶1000, Sigma).
14. Immunocytochemistry
Cultured cells on coverslips were fixed with 10% Formalin for 10 min at room temperature, washed with PBS, and incubated with blocking solution for 1 h at room temperature before exposure overnight at 4 °C to primary antibodies diluted in the same solution. The primary antibodies included HGF, NG2, Nestin, Tuj1, GFAP, Olig2, ki67, BrdU. The cells were washed with PBS containing 0.1% Triton X-100 and then were incubated for 1 h at room temperature with secondary antibodies labeled with Alexa Fluor 488 or 568. Nuclei were stained with Hoechst 33258. The coverslips were mounted with Vectashield mounting medium (Vector Laboratories), and images were acquired with a Fluorescence scanner, Axioscan.Z1.
15. In-vitro Angiogenesis assay
96 well plates were coated with 50 ul of BD matrigel. 50 ul of conditioned media from MSC, MSC/Ngn1, MSC+HGF, and MSC/Ngn1+HGF cell culture was added into the well. 2x104 mouse brain endothelial cells, bEND.3 cells suspended in 100ul of MSC culture media was added into the well. The plate was incubated for 4 hrs at 37ºC and light microscopic images were acquired by Olympus microscope at 200X magnification. 10 random fields per well were selected for analysis. The experiment was done in duplet and repeated three times. The tube formation assay was analyzed by using the Angiogenesis analyzer program of ImageJ.
16. In-vitro NSC Proliferation assay
Secondary and tertiary neurospheres were dissociated into single cells and plated into 0.1 mg/ml PDL coated cover glasses. Conditioned media (in Serum-free media) from MSC, MSC/Ngn1, MSC+HGF, and MSC/Ngn1+HGF cell culture was obtained 48 hours of confluent culture. The culture was incubated for 16 hours in conditioned media and was fixed with 10% formalin for assessment of proliferation by Ki67 immunocytochemistry. Fresh MSC culture media was used as a control.
17. In-vitro Neuronal differentiation assay.
Secondary and tertiary neurospheres were dissociated into single cells and plated into 0.1 mg/ml PDL coated cover glasses. Conditioned media (in 10% Serum containing media) from MSC, MSC/Ngn1, MSC+HGF, and MSC/Ngn1+HGF cell culture was obtained 48 hours of confluent culture. The culture was incubated for 3 days in conditioned media and was fixed with 10% formalin for assessment of neuronal differentiation by Tuj1 immunocytochemistry. Fresh MSC culture media containing 10% serum was used as control.
18. Animal models and cell transplantation. 18.1. Rat model
Transient focal ischemia was induced by intraluminal filament occlusion of the middle cerebral artery (MCAo) according to a modified procedure originally described by Longa et al(Longa, Weinstein et al. 1989).
Male Sprague-Dawley rats (250 g) were anesthetized with intraperitoneal (i.p.) administration of ketamine (75 mg/kg) and xylazine hydrochloride (5 mg/kg).
The right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed. A 4-0 monofilament nylon suture with a silicon-coated tip (Doccol Co., Sharon, MA, USA) was advanced from the ECA lumen into the ICA until it blocked the bifurcating origin of the MCA.
Two hours after MCAo, animals were re-anesthetized and reperfusion was performed by withdrawing the suture until the tip cleared the lumen of the ECA.
Rectal temperature was maintained at 37°C throughout the surgical procedure, using an electronic temperature controller linked to a heating pad (FHC, Bowdoinham, ME, http://www.fh-co.com). Animals showing similar scores (e.g.,
remaining on the Rotarod [Ugo Basile, Comerio, Italy,
http://www.ugobasile.com] for more than 300 seconds during pre-training but less than 10 seconds after surgery) and comparable infarct volumes in magnetic resonance imaging (MRI) were selected and randomly grouped. This way, we were able to scrupulously control the quality of animals and minimize variations
among experimental groups. One month after MCAo, 1x106 cells in a total fluid
volume of 10ul were intracranially transplanted into the striatum (anteroposterior [AP], 0.5; mediolateral [ML], 2.5; dorsoventral [DV], 5.0) and cortex (AP, 0.5; ML, 2.0; DV, 2.5) in the ipsilateral hemisphere (5 ul per site).
18.2. Mouse model
Adult (3-5 months old) Nestin-GFP and NG2-CreERTM/Nestin-GFP/Rosa-TdTomato double transgenic mice were used for assessing neurogenesis in local brain micro-environment. Transient middle cerebral artery occlusion (MCAo) was induced by an intraluminal suture method. Briefly, animals were anesthetized with isoflurane (3% for induction and 2% for maintenance) in a mixture of N2O: O2 (70:30) while being intubated and
mechanically ventilated. Rectal temperature was maintained at 37℃ throughout the surgical procedure. MCAo surgery and post-surgical care were performed as described in our previous report (Park, Marasini et al. 2014). After 60 minutes of occlusion, the nylon suture was gently removed from MCA to re-perfuse the MCA territory. One month after MCAo, 3x105 cells in a total fluid volume of 6ul PBS were intra-cranially transplanted into the striatum (anteroposterior [AP],
0.5; mediolateral [ML], 2.5; dorso-ventral [DV], 3.0.
19. Behavior assessment:
19.1 Behavirol Assement of the rat stroke model
Assessment of neurological severity test, Adhesive Removal Test and
Rotarod Test were performed as described earlier (Kim, Yoo et al. 2008, Park, Marasini et al. 2014). For the Adhesive Removal Tests, an adhesive tape of 10 mm x10 mm was placed on the dorsal paw of each forelimb, and the time to remove each tape from the dorsal paw was measured. For the Rotarod Test, experimental animals were tested for their ability to run on a rotating cylinder that was accelerated from 4 to 40 rpm for 5 minutes. Two weeks before stroke induction, only animals capable of removing the adhesive tape within 10 seconds and remaining on the Rota-rod cylinder for more than 300 seconds were selected and included in the experiment. Data are presented as the mean latency to fall calculated from three trials. For the adhesive removal test, two square dots of adhesive-patch (100 mm2) were used as bilateral tactile stimuli
occupying the distal-radial region on the wrist of each forelimb. The time taken for each animal to remove the adhesive dot was recorded with a cut-off time of 300 sec. Data are presented as the average time to remove the patch from three trials.
19.2 Behavior assessment of the mouse stroke model, behavioral tests were
carried out on 2nd, 4th, 7th, 14th, 21st and 28th day after MCA occlusion. Rotarod test evaluates the balance and coordination function (Terborg, Bramer et al. 2004). Prior to surgery, the mice were trained for balancing on the rotating drum (Acceler Rota-Rod 7650, UGO BASILE, Varese, Italy) for 5 days (3 trials per day) (Rogers, Campbell et al. 1997). The rotarod was accelerated from 4 to 40 r.p.m. for 250 sec as a preoperative baseline. Animals not achieving the baseline criteria were excluded from the subsequent study. The latency before falling off the accelerating drum was recorded with a maximum of 250 seconds. The longest latency from three consecutive trials for each testing day was extracted for data analysis using Sigma plot software (Systat Software Inc, San Jose, CA, USA).
Corner test is used to detect unilateral abnormalities of sensory and motor functions in the stroke model(Zhang, Schallert et al. 2002). A corner was made by placing two wooden cardboards (30 cm × 20 cm × 1 cm) at an angle of 30 degree. The mouse was made to enter the corner upon its placement at midway to the corner. As the mouse reached deep into the corner, both sides of
the vibrissae were stimulated together. Upon the stimulation of vibrissae, the mouse reared forward and upward and finally turned back towards the open end. The direction towards which the mouse turned was recorded. A total of 10 trials were recorded per each animal pre-operatively and on indicated days. Sham animals did not show any preference in the direction while the ischemic mice showed marked preference in turning towards the non-impaired side (right turn). The percentage of the right turn was analyzed as the indicator of the deficit.
Pole test is a simple behavior test used to assess motor dysfunction after stroke (Bouet, Freret et al. 2007). Mice were placed in the top of a 60 cm vertical pole with a diameter of 1 cm. The pole was placed in the home cage so that mice might prefer to descend to the floor of the cage. The recording was started when the animal began the turning movement. The time to turn completely downward (Tturn) and total time to descend to the floor (Ttotal) were
recorded. When the animal paused while descending, the trial was repeated. When the animal could not turn but instead descended with a lateral body position, then Ttotal was attributed to Tturn. When the animal fell off the pole
immediately, the maximum scores for Tturn (10 sec) and Ttotal (15 sec) were
assigned. The test was repeated for 3 trials per animal in each setting and the average Tturn and Ttotal were used for data analysis.
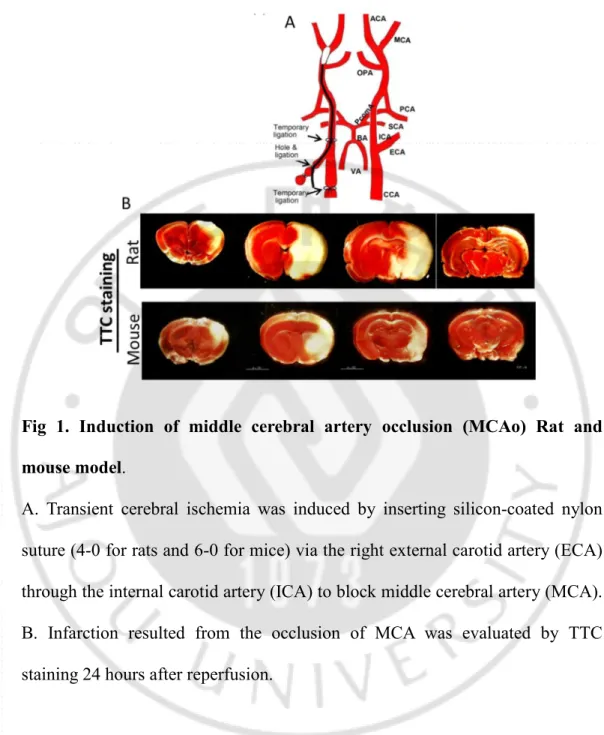
For the NSS test, a 0-5 grading scale was used, with a slight modification performed by Bederson et al. (Bederson, Pitts et al. 1986). Normal
mice were scored 0, while dead mice or mice unresponsive to stimulation were scored 5 as shown in Table 2.
Table 2. Neurological Severity Scale (NSS). Score Left forepaw
disability Body swing Rightward rotation Depressed level of consciousness Unresponsive to stimulation or death 0 - - - - - 1 + - - - - 2 + + - - - 3 + + + - - 4 + - - + - 5 + - - - +
20. Measurement of infarct volume (MRI)
MRI scanning in rat stroke models was performed at Ajou University Hospital (Suwon, Korea) using a 3.0 Tesla whole-body MRI scanner (Achieva 3.0T X-Series Qasar Dual, Philips Healthcare, Amsterdam, Netherlands) equipped with a gradient system capable of 35 milliteslas/m. A fast-spin echo imaging sequence was used to acquire T2-weighted anatomical images of the rat brain in vivo using the following parameters: repetition time, 3,000 milliseconds (msec); effective echo time, 120 msec; field of view, 55 x 55 mm2; image matrix, 256 × 256; slice thickness, 1.5 mm; flip angle, 90°; pixel size, 0.21 x 0.21 mm2.
A 300-mm diameter quadrature 16-ring birdcage coil arrangement was used for radiofrequency excitation, and a 40-mm diameter saddle coil was used for signal detection A total of 15 slices were scanned to cover the whole rat brain. For each
slice, the ischemic area from each T2-weighted image was marked manually and calculated using Osiris software (University of Geneva, Geneva, Switzerland). Relative infarct volume (RIV) was normalized as described by Kim et al. (Kim, Yoo et al. 2008) and Neumann-Haefelin et al. (Neumann-Haefelin, Kastrup et al. 2000) using the equation RIV = (LT – (RT – RI)) x d, where LT and RT represented the areas of the left and right hemispheres in mm2, respectively, RI was the infarcted area in mm2, and d was the slice thickness (1.5 mm). RIV was
expressed as a percentage of the right hemispheric volume. For mouse stroke models, MRI was performed using a 4.7-T animal MRI scanner (Biospec 47/40; Bruker, Karlsruhe, Germany) located in the Korea Basic Science Institute (Ochang, Korea). The animal was placed on a non-magnetic holder equipped with a nose cone for the administration of anesthetic gas containing 2% isoflurane in 70% N2O and 30% O2. T2-weighted images were obtained using
the following parameters: repetition time (TR), 5,000 msec; echo time (TE), 90 msec; average, 4; acquisition matrix, 256 × 256; 8 slices with 1-mm thickness; flip angle, 180°. T2*-weighted multi-slice images were acquired using the following parameters: TR, 561 msec; TE, 20 msec; average, 4; acquisition matrix, 256 × 256; 15 slices with 1-mm thickness; flip angle, 30°. The images were analyzed with Para-Vision Acquisition 5.1 (National Instruments, Austin, TX, USA).
21. Histological analysis 21.1 TTC staining
To confirm the proper MCA occlusion, animals were deeply anesthetized with a high dose of isoflurane gas 24 h after the MCAo surgery and were decapitated. The brains were extracted rapidly and coronally sliced into 2 mm sections on an ice-cold mouse mold (Leica Biosystems, Buffalo Grove, IL, USA). Each section was incubated in 2% of 2,3,5 triphenyl-tetrazolium chloride (TTC) in phosphate-buffered saline, pH 7.4 (PBS) for 10~12 min at 37oC in the dark. After the TTC solution was drained off, slices were fixed with 10% formalin in PBS. The unstained area in the section was regarded as an infarct. The images of slices were acquired with a stereoscopic microscope (SZX2-ILLB, Olympus Co. Tokyo, Japan)
21.2 Cresyl violet staining.
Animals were intra-cardially perfused with 10% formalin. Brains were carefully extracted and further fixed in 10% formalin overnight. After embedding in a paraffin block, five-micrometer coronal sections were mounted on slides and proceeded for cresyl violet staining as reported earlier(Tureyen, Vemuganti et al. 2004). Coronal sections representing the striatal region were used for analysis.
21.3 Immunohistochemistry
For immunohistochemical analysis, animals were anesthetized with ketamine (100 mg/kg) /xylazine (10 mg/kg) (Yuhan Co. Ltd., Seoul, Korea), perfused transcardially with ice-cold saline, and then fixed with 10% neutral buffered formalin (NBF). The brains were post-fixed in 10% NBF and embedded in paraffin. The paraffin blocks were serially sectioned to produce 5-mm thick sections, which were then deparaffinized and placed in boiled citrate buffer (pH 6.0) for 10 min. After blocking in 10% normal serum, the sections were incubated with antibodies against neuronal nuclei (NeuN; 1:500, Merck Millipore, Burlington, MA, USA), microtubule-associated protein 2 (MAP2; 1:500, Sigma-Aldrich, St. Louis, MO, USA), human mitochondrial antigen (1:100, Merck Millipore), IBA1(1:3000, WAKO), GFAP(1:500, Sigma), Tuj1 (1:500, Biolegend), APC-CC1(1:200, Abcam), CSPG (1:200, sigma), Pdgfrb (1:200, Abcam), Olig2 (1:200,EMD millipore), NG2 (1:200, EMD Millipore), Nestin (1:200, EMD Millipore) GFP (1:500, Abcam, Cambridge, UK), Tomato-lectin (1:500, Sigma), and ED1 (1:200, AbD Serotec, Kidlington, UK) at 4°C overnight. Antibody reactions were visualized using an ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. Alternatively, brains were removed after perfusion and cryoprotected in 30% sucrose in 0.1 M phosphate buffer (pH 7.4) overnight. The cryopreserved brains were sectioned to produce 30 μm-thick sections which
were then incubated with Alexa Fluor 488- or 568-conjugated anti-IgG secondary antibodies (Life Technologies, Carlsbad, CA, USA). For quantification of Lectin-, GFAP-, IBA1-, ED1-, GFP-, DCx-positive cells in the ischemic penumbra, 5 mm coronal sections from the ischemic core (Antero-Posterior, + 1.2 mm to −0.8 mm from the bregma) were prepared from three animals. Both light microscopic and fluorescence images were acquired using an AxioScan.Z1 slide scanner (Zeiss, Jena, Germany), and the total number of immunoreactive cells in 1 mm2 region of interest in the peri-infarct area of the
cortex and striatum were counted using ZEN software (Blue Edition, Zeiss). To trace TdTomato and GFP-positive cells in TdTomato and TdTomato-GFP reporter mice, 30 mm parasagittal sections were scanned with an AxioScan.Z1 equipped with an HBO lamp (HXP 120V, LEJ, Jena, Germany). The characteristics of TdTomato and GFP-positive cells in terms of their co-expression of various markers used were analyzed in the cortex, Striatum, sub-ventricular Zone (SVZ) and Sub-granular Zone of the dentate gyrus.
22. Statistical analysis
Statistical analyses were carried out using Sigmaplot (Systat Software Inc, San Jose, CA, USA). Data were analyzed by Student’s t test or one-way analysis of variance (ANOVA). Significant differences were further evaluated using Tukey’s honest significant difference post-hoc test. A p-value ˂ 0.05 was considered statistically significant. All data are expressed as mean ± S.E.
RESULTS
Part A. Chronic stroke animal models and therapeutic effects of MSC/Ngn1 cells in chronic stroke.
1. Characterization of chronic stroke animal models.
To generate a chronic stroke model of rat and mice, the intraluminal
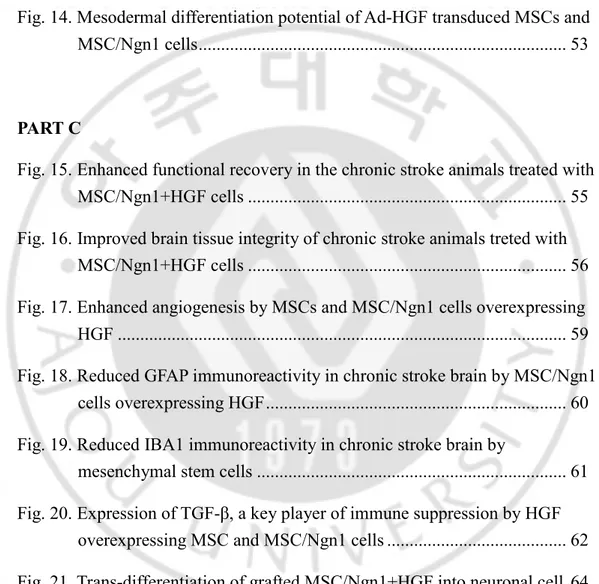
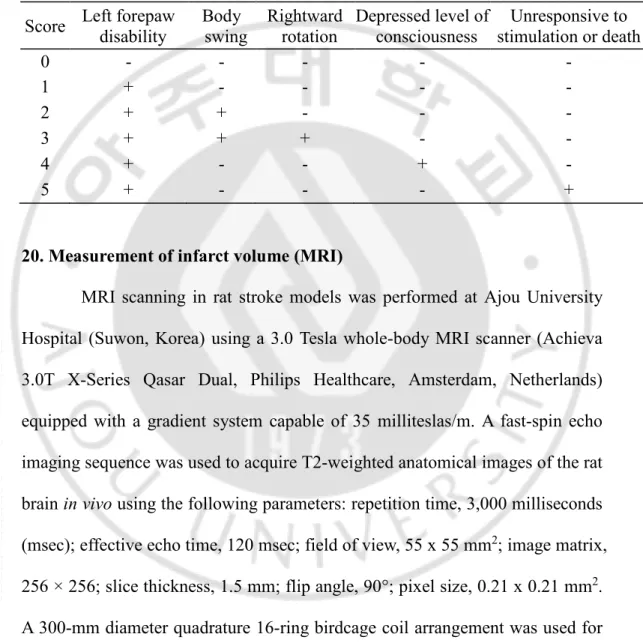
transient middle cerebral artery occlusion method was carried out in rats and mice (Fig 1A). Twenty four hours after reperfusion, TTC staining showed that both rat and mouse stroke models developed clear infarction (Non-stained area) in the ipsilateral striatum and dorsolateral cortex verifying proper occlusion of the Middle cerebral artery (Fig 1B) We also assessed the structural integrity of the ischemic brain over the same 28-day period using MRI. The hypointense areas in the ipsilateral hemisphere were evident at the acute phase of both rat and mouse models. In the chronic phase, rat MCAo showed an infarct cavity as shown by hypointense areas in the ipsilateral hemisphere (Fig 2A). However, In a mouse model of chronic stroke, MRI did not show any hypointense areas suggesting that the infarct cavity was not formed in a chronic stroke mouse model (Fig 2B). Sham-operated animals did not show any hypointense areas in the brain. Further, we evaluated the tissue integrity of chronic stroke animal models by Hematoxylin & Eosin and Cresyl-violet staining at 1 month after ischemia-reperfusion injury and compared with the histology of acute stroke i.e.,
3 days after reperfusion and sham-operated animals (Fig 2 C, D). Hematoxylin & Eosin and Cresyl violet staining of coronal brain sections at the level of striatum grossly depicts the edematous ipsilateral hemisphere with the faintly stained ischemic area in acute stroke while atrophied ipsilateral hemisphere in chronic stroke. The tissue integrity of the chronic stroke rat brain was worse than a chronic stroke mouse brain. Furthermore, the presence of cells bearing pycnotic nuclei and vacuolated cytoplasm were readily present in the ischemic area of the acute stroke brain. In the chronic stroke brain, cells bearing non-neuronal morphology occupied the corresponding ischemic core region.
Fig 1. Induction of middle cerebral artery occlusion (MCAo) Rat and mouse model.
A. Transient cerebral ischemia was induced by inserting silicon-coated nylon suture (4-0 for rats and 6-0 for mice) via the right external carotid artery (ECA) through the internal carotid artery (ICA) to block middle cerebral artery (MCA). B. Infarction resulted from the occlusion of MCA was evaluated by TTC staining 24 hours after reperfusion.
Fig 2. A general overview of a brain tissue structure of chronic stroke animal models. The changes in brain tissue integrity were monitored by MRI, H&E staining and cresyl violet staining from acute (day 3) to chronic phase of stroke (Day 30) in both rat and mouse model of stroke. MCA occlusion resulted in infarction of striatum and dorsolateral cortex of both rat and mouse brain in the acute phase of the stroke. In the chronic phase, rat MCAo resulted in the infarct cavity while chronic mouse MCAo resulted in atrophied ipsilateral brain hemisphere (A, B). Hematoxylin & Eosin and Cresyl violet staining showed significant loss of neural cells in acute stroke. Cells with Pycnotic nuclei and vacuolated cytoplasm are evident in ischemic core and penumbra regions. In the chronic phase, the infiltration of glial cells (non-neuronal morphology) is evident in ischemic core and penumbra regions of the ischemic brain (C, D).
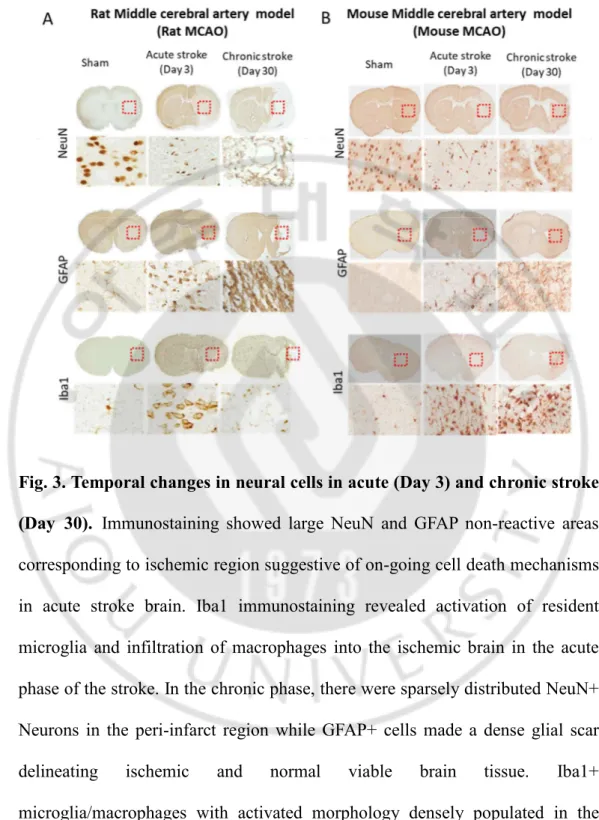
Immunostaining against the neuronal marker, NeuN, and astroglial marker, GFAP showed large NeuN and GFAP non-reactive areas corresponding to ischemic region suggestive of on-going cell death mechanisms in acute stroke brain. In the chronic phase, there were sparsely distributed NeuN+ Neurons in the peri-infarct region while GFAP+ cells made a glial scar delineating ischemic and normal viable tissue. Iba1+ microglia/macrophages were readily observed in the ischemic region of acute stroke brain and persisted in the chronic phase of the stroke (Fig 3 A, B). In the chronic phase of ischemic stroke, there is the presence of growth inhibitory glial scar forming a fine border between ischemic core and normal viable brain tissue (4 A). This glial scar primarily consists of reactive astrocytes, activated macrophages/microglia, and extracellular matrix molecules, predominantly chondroitin sulfate proteoglycans (CSPGs). We found that, in the brain of chronic stroke brain, GFAP positive astrocytes became hypertrophied and elongated their processes from penumbra into the infarct core (4 B). These astrocytes strongly upregulated GFAP protein, a hallmark of astrogliosis responding to ischemic stroke. We were also able to observe that the pronounced change in astrocytic morphology and GFAP expression in reactive astrocytes was also accompanied by the upregulation of intermediate filament protein, Nestin. Double immune-histochemical studies showed that GFAP-positive reactive astrocytes in the glial scar expressed a high amount of extracellular matrix-like Neurocan and CSPGs, but did not overlap with ED1
positive activated microglia/macrophages. GFAP and ED1 positive cells along with extracellular matrix molecules neurocan and CSPGs formed a layer around the ischemic lesion, suggesting the presence of inhibitory glial scar in the chronic stroke rat brain. The glial scar is positioned in such a way that it forms a border around the injury site and acts as a neuroprotective barrier to evading inflammatory cells.
Fig. 3. Temporal changes in neural cells in acute (Day 3) and chronic stroke (Day 30). Immunostaining showed large NeuN and GFAP non-reactive areas
corresponding to ischemic region suggestive of on-going cell death mechanisms in acute stroke brain. Iba1 immunostaining revealed activation of resident microglia and infiltration of macrophages into the ischemic brain in the acute phase of the stroke. In the chronic phase, there were sparsely distributed NeuN+ Neurons in the peri-infarct region while GFAP+ cells made a dense glial scar delineating ischemic and normal viable brain tissue. Iba1+ microglia/macrophages with activated morphology densely populated in the ischemic brain in the chronic phase of the stroke.
Fig. 4.Characterization of Glial Scar in chronic stroke. A. Low magnification
image of a chronic stroke brain stained with GFAP & MAP2 and Region of interest for characterizing glial Scar. B. In the chronic phase of ischemic stroke, there is the presence of growth inhibitory glial scar forming a fine border between ischemic core and normal viable brain tissue. This glial scar primarily consists of reactive astrocytes (GFAP/Nestin+), activated macrophages/microglia (Iba1/ED1+) and extracellular matrix molecules, predominantly chondroitin sulfate proteoglycans (CSPGs). Note GFAP positive astrocytes became hypertrophied and elongated their processes from penumbra
into the infarct core.
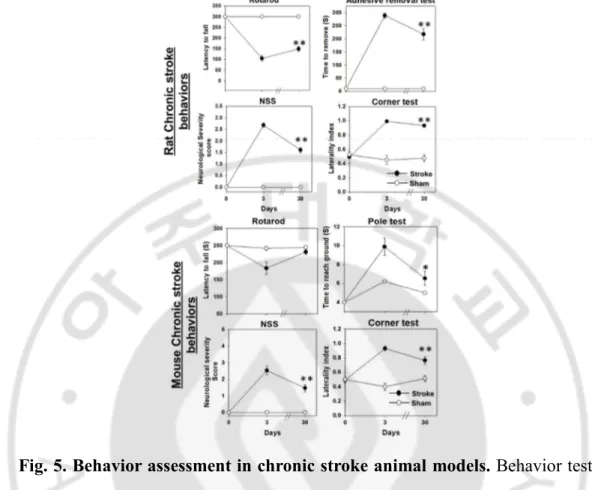
Overall, the MCAo mice exhibited poor performance in behavioral tests during the first week after ischemia, but their performance improved spontaneously over the time. The MCAo animals consistently showed higher NSS score compared to the sham group through the testing period (Fig. 5A) In corner tests, normal or sham-operated mice displayed similar tendency in right and left turning behavior when the animals reached the corner. However, the MCAo animals showed the right-sided bias in turning back since the animals had the paresis in the left side (Fig. 5 B). The laterality index, the indicator of right turning preference, was significantly increased in MCAo animals (p<0.05). Within the span of 28 days the right turning bias in the ischemic group was partially reduced owing to spontaneous improvement in their sensory-motor function on the left side of the body. The sham-operated animals turned both the directions with almost equal probability, over the entire period of behavioral testing. In the pole test before the surgery, when the mice were placed on the top of the pole facing their head upward, mice in both the sham and MCAo groups could turn their head completely vertically downward within baseline value of 1 second and reached the floor within 4 sec, when they were placed on the top of the pole facing their head upward.Ttotal, the time taken to reach the floor, was significantly increased after MCAo (Fig. 5C). Although the MCAo mice showed profound improvement in the motor function (bradykinesia) over the time, the
sham- operated animals always took lesser time to reach the floor over the testing span of 1 month. In the rotarod test, the MCAo mice remained on the rotarod for lesser time than did the sham-operated mice for the first week, but the difference became non-significant between the groups after a week (Fig. 5D)
Fig. 5. Behavior assessment in chronic stroke animal models. Behavior tests
were performed on acute i.e, day 3 and chronic i.e, day 30 after the MCAo surgery. All data are presented as means±S.E. Both Rat and mouse MCAo animals showed severe behavioral deficits in neurological severity scores and corner test compared to sham operated animals in acute as well as chronic phase. Rotarod and adhesive removal test were sensitive to detect behavioral deficits in chronic stroke rat models. Rotarod test was not sensitive to detect behavioral deficits in chronic phase of chronic stroke mouse model. The latency to reach the floor from the top of the pole was increased in the mouse MCAo animals compared to the sham group. (t-test, *p<0.05, ** p<0.01)
2. Therapeutic effects of MSC/Ngn1 cells in chronic stroke
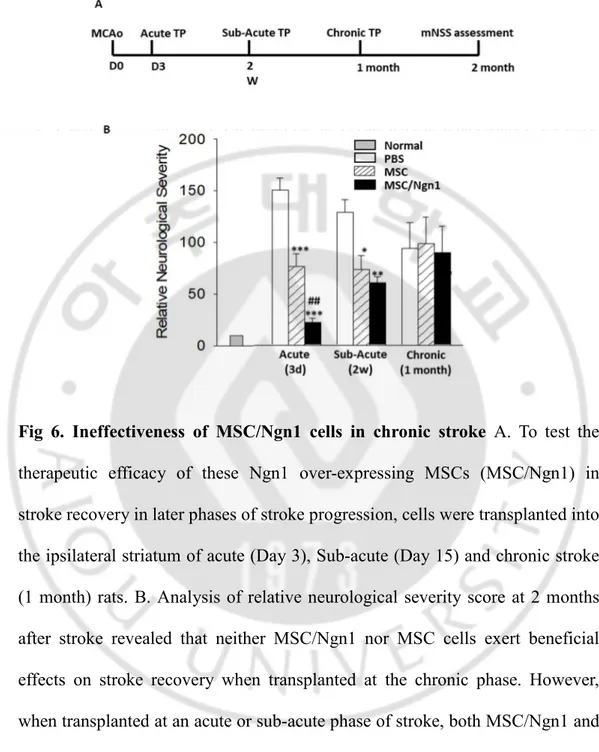
We previously reported that Ngn1 overexpressing MSCs exerted higher therapeutic effects than naïve MSCs in acute stroke animal models. To assess the role of these MSC/Ngn1 cells in stroke recovery in later phases of stroke progression, cells were transplanted into the brain of Sub-acute (Day 15) and chronic stroke (1 month) rats. Analysis of relative neurological severity score at 8 weeks after stroke revealed that neither MSC/Ngn1 nor MSC cells exert beneficial effects on stroke recovery when treated at the chronic phase (Fig 6). However, when treated at the acute or sub-acute phase of stroke, both MSC/Ngn1 and MSC cells reduced relative neurological severity compared to PBS vehicle with visibly higher recovery in MSC/Ngn1 group.
Fig 6. Ineffectiveness of MSC/Ngn1 cells in chronic stroke A. To test the
therapeutic efficacy of these Ngn1 over-expressing MSCs (MSC/Ngn1) in stroke recovery in later phases of stroke progression, cells were transplanted into the ipsilateral striatum of acute (Day 3), Sub-acute (Day 15) and chronic stroke (1 month) rats. B. Analysis of relative neurological severity score at 2 months after stroke revealed that neither MSC/Ngn1 nor MSC cells exert beneficial effects on stroke recovery when transplanted at the chronic phase. However, when transplanted at an acute or sub-acute phase of stroke, both MSC/Ngn1 and MSC cells reduced relative neurological severity compared to PBS vehicle with visibly higher recovery in MSC/Ngn1 group.
Part B: Adenoviral mediated therapeutic genes transfer in MSCs. 1. MSC transduction with adenoviral vector expressing GFP.
To investigate adenovirus transduction efficiency, MSCs were transduced with Ad-GFP at various MOIs for 2 h and then examined at 48 h post-infection by fluorescence microscopy and flow cytometry. Results demonstrate a linear increase in the frequency of GFP-positive cells with increasing MOI with no GFP signal detected at 0 MOI and about 80% GFP positive cells at 200 MOI. The histogram (Fig. 7A) and bar diagram (Fig. 7C) show the frequency of GFP-positive cells at the indicated transduction conditions.
2. Transgene expression and growth kinetics of Ad-GFP-transduced MSCs
To assess the effect of adenoviral transduction on the proliferation of cells, Ad-GFP-transduced MSCs were cultured under standard conditions for 2 passages. Cell counting studies revealed the linear decrease in the total cell numbers with increasing MOI after 12 days in culture (Figs. 8A and 8B). The number of GFP-positive cells and mean fluorescence intensity were also significantly decreased in subsequent passage (Figs. 8C and 8D). MSCs retained a normal fibroblastic morphology at 2 days post-transduction that gradually changed to a flattened, oval, and enlarged shape, mostly in GFP-positive cells (arrows in Fig. 8A). Senescence-associated beta-galactosidase (SA-beta-gal) staining confirmed that these morphological changes were the result of cell senescence (Fig. 8F).
Fig. 7. Adenoviral gene transduction of mesenchymal stem cells (MSCs).
(A) Fluorescence-activated cellsorting analysis of MSCs transduced with GFPexpressing adenovirus (1-200 multiplicity of infection [MOI]) and untransduced controls. (B) Microscopy images of GFP expression in MSCs transduced at 200 MOI. Scale bar = 50 µm. (C) The percentage of GFP positive cells with increasing MOI.
Fig. 8. Transgene expression in Ad-GFP MSCs. (A) Representative
fluorescence images of transduced cells at 2 and 12 days post-transduction. GFP-positive cells display a larger cell size with flattened morphology (arrows). Scale bars = 50 µm (B-D) The total number of cells, frequency of GFP-positive cells, and mean GFP intensity were measured using flow cytometry. (E) Senescence-associated β- galactosidase activity was examined in transduced MSCs (MOI 50) and cultured for one passage. Arrows indicated positive X-gal staining. Scale bars = 50 µm