Anti-inflammatory Role of IL-15R Up-regulated
α
by Poly I:C in Herpes Simplex Virus-induced
Behcet's Disease-like Mouse Model
by
Ju Young Choi
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Anti-inflammatory Role of IL-15R Up-regulated
α
by Poly I:C in Herpes Simplex Virus-induced
Behcet's Disease-like Mouse Model
by
Ju Young Choi
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Master of Biomedical Sciences
Supervised by
Seong Hyang Sohn, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Ju Young Choi is approved.
SUPERVISORY COMMITTEE
Seonghyang Sohn
Eun-So Lee
Sun Park
The Graduate School, Ajou University
December, 20th, 2011
감사의 글
설렘 반 두려움 반으로 시작되었던 4학기의 대학원 생활을 이 한 권의 논문 으로 마무리하며 그 동안 저에게 힘이 되어주셨던 많은 분들께 감사의 마음을 전해드리고자 짧게 나마 글을 남깁니다. 먼저, 여러모로 부족했던 저에게 많은 가르침을 주시고 격려와 지도로 이 논 문이 나올 수 있도록 이끌어주신 손성향 교수님께 깊이 감사드립니다. 연구와 강의로 바쁘신 가운데에도 제 학위논문 심사를 흔쾌히 맡아주시고 많 은 조언을 해주신 이은소 교수님과 박선 교수님께 감사드립니다. 학부시절 많은 가르침을 주시고, 대학원 진학을 결심하도록 이끌어주신 박경 숙 교수님께 감사드립니다. 실험의 기초부터 꼼꼼히 가르쳐주신 분순 언니와 주아 언니 감사합니다. 권 혁제 선생님의 배려에 감사합니다. Mostafa Anower, we can’t even make perfect communication, thank you for your consideration. flow cytometry 실험에 많은 도움을 주신 나도영 선생님 감사하고, 예비 엄마가 된 것을 축하합니다. 여러 조언을 주신 황은주 선생님 감사합니다. 대학원 면접 때 처음 만나 어려운 일이 있을 때 도움이 되어준 화니언니 고마워요. 대학교 때부터 같은 길을 걷고 있어 서로 응원해주며 힘이 되어준 수림이, 항상 밝은 모습으로 응원해준 은서 모두 고마 워. 같은 길을 걷고 있는 수경언니 졸업 때까지 화이팅! 논문 작업 때 많은 힘 이 되어준 준원오빠 고마워요. 항상‘넌 잘 할 수 있어’라며 격려와 용기 되는 말을 아낌없이 해주었던 이세상에 하나뿐인 자매 선영언니, 고마워. 마지막으로, 저의 잦은 짜증에도 불구하고 인내와 한 없는 사랑, 끊임없는 기도로 저를 응원해주신 너무나도 사랑하는 부모님께 진심으로 감사드립니다. 지나고 보면 짧았던 2년 간의 석사 생활을 마무리하고 새로운 시작을 준비하 는 지금, 이 모든 분들의 고마움을 마음 속 깊이 가지고서 앞으로 매 순간 최선 을 다하며 살아가겠습니다. 미처 이름을 하나하나 언급해가며 감사의 말을 전하 지 못한 분들께는 죄송하게 생각하며, “모두들, 진심으로 감사드립니다.”
- ABSTRACT -
Anti-inflammatory Role of IL-15Rα Up-regulated by Poly I:C
in Herpes Simplex Virus-induced Behcet’s Disease Mouse Model
Behcet’s disease (BD) is a chronic, multi-systemic disorder that has arthritic, intestinal, mucocutaneous, ocular, vascular, and central nervous system affects. Interleukin-15 receptor alpha (IL-15Rα) forms stable complex with IL-15 on the cell surface of activated monocytes and mediates the proliferation of memory CD8+ T cells. Recent studies informed that Polyinosinic:polycytidylic acid (Poly I:C) is an immunostimulant which boosts the generation of memory T cells though induction of IL-15 and IL-15Rα. We found that the frequencies of IL-15Rα were significantly lower in HSV-induced BD mouse model than control mice. Therefore, this study was conducted to determine whether Poly I:C supplementation could induce memory cells through up-regulation of IL-15Rα, and reduce inflammation in HSV-induced BD symptoms. To evaluate whether the expression of IL-15Rα could be increased by poly I:C in vivo, Poly I:C was administered into normal and BD mice. Then, Poly I:C administered BD mice were subsequently observed the change of symptoms until 17 days after the last injection. The frequencies of IL-15Rα and memory CD8+ T cells were significantly up-regulated in Poly I:C injected mice. Poly I:C improved the symptoms and decreased severity score through down-regulation of pro-inflammatory cytokines and elevated regulatory and memory T cells. In conclusion, these results suggest that IL-15Rα is an important factor for the regulation of symptoms in HSV-induced BD mice.
Key words: Bechet’s disease, IL-15Rα, Poly I:C, memory CD8+T cells, Herpes Simplex Virus, mouse model
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· Ⅲ LIST OF FIGURE ··· ⅴ LIST OF TABLES ··· ⅶ Ⅰ. INTRODUCTION ··· 1Ⅱ. MATERIALS AND METHODS ··· 3
A. Animal experiments ··· 3
B. BD symptoms in a mouse model ··· 3
C. In vivo administration of Poly I:C ··· 4
D. Flow cytometric analysis of cell surface staining ··· 4
E. ELISA ··· 5
F. Reverse transcription PCR (RT-PCR) ··· 5
G. Statistical analysis ··· 6
Ⅲ. RESULTS ··· 7
A. The frequencies of IL-15Rα expressing cells in BD mice compared with BDN mice ··· 9
B. Poly I:C up-regulates expression of IL-15Rα in PBMC of normal mice ··· 12
C. Poly I:C ameliorates HSV-induced BD symptoms ··· 16
D. Poly I:C up-regulates the frequencies of IL-15Rα but not IL-7Rα (CD127) in BD mice ··· 18
E. Poly I:C induces CD8+CD44+ memory T cells in BD mice ··· 20
F. Poly I:C up-regulate CD122(IL-15/2Rβ) and CD8+CD122+ T cells in BD mice ··· 24
G. Poly I:C down-regulates IL-23 receptor and IL-17A in BD mice ··· 28
Ⅳ. DISCUSSION ··· 30
Ⅴ. CONCLUSION ··· 34
REFERENCES ··· 36
LIST OF FIGURES
Fig. 1. The frequencies of IL-15Rα expression in PBMC, splenocytes and lymph node cells of BDN and BD mice ··· 8
Fig. 2. The frequencies of IL-15Rα expression were up-regulated by Poly I:C
injection in a dose dependent manner in PBMC of normal mice ··· 10
Fig. 3. The change of symptoms in BD mice after injection of Poly I:C ··· 13
Fig. 4. The expression of IL-15Rα, IL-7Rα in PBMC, splenocytes and lymph node cells isolated at 2 and 17 days after the last injection of Poly I:C in BD
mice ··· 18
Fig. 5. The frequencies of memory T cells in PBMC, splenocytes and lymph node cells isolated at 2 and 17 days after the last injection of Poly I:C in
BD mice ··· 22
Fig. 6. The frequencies of CD122(IL-15/2Rβ) and CD8+CD122+ T cells in lymph nodes cells isolated at 2 and 17 days after the last injection of Poly I:C into BD mice ··· 26
Fig. 7. mRNA expression of IL-23 receptor and serum protein levels of IL-17A in Poly I:C injected BD mice ··· 29
LIST OF TABLE
Ⅰ. INTRODUCTION
Behcet’s disease (BD) is a chronic, multi-systemic disorder that has arthritic, intestinal, mucocutaneous, ocular, vascular, and central nervous system affects. BD takes a chronic course with periodic exacerbations and progressive deterioration (Shimizu, et al., 1978). The etiology of BD is unclear, but viral infection has long been postulated as one of the main factors. Since Hulusi Behcet first proposed a viral etiology, the viral hypothesis has been verified by detection of the virus in the saliva, intestinal ulcers, and genital ulcers of patients with BD (Behcet, 1937; Lee, et al., 1996; Bang, et al., 1997; Lee, et al., 1997). Subsequent to these findings, inoculation of the earlobes of ICR mice with herpes simplex virus (HSV) was found to result in the development of BD symptoms (Sohn, et al., 1998). Manifestations in mice after HSV inoculation include multiple symptoms such as oral ulcers, genital ulcers, skin ulcers, eye symptoms, intestinal ulcers, arthritis, and neural involvement, as well as skin crusting (Sohn, et al., 2001).
In BD patients, the frequencies of CD45RO+ cells were lower than disease control group has been reported (Takase, et al., 2006). By this time, there was no report related to the improvement accompanies regulation of memory T cells in BD patients except up-regulation of CD4+CD45RO+ cells after Thalidomide treatment (Direskeneli, et al., 2008). Interleukin-15 (IL-15) is a pleiotropic cytokine that plays important roles in both innate and adaptive immunity. It is associated with a range of immunopathology, including rheumatoid arthritis and allograft rejection. IL-15 exerts its effects through binding to a
membrane receptor which consists of a high affinity binding α-chain (IL-15Rα), and the β-chain, common receptor subunit IL-2R also known as CD122, and the common γ subunit of different type I cytokine receptors (γC chain, CD132) (Tagaya, et al., 1996; Waldmann, et al.,
1998; Wei, et al., 2001). Increased IL-15 production was associated with proliferation and survival of memory-phenotype CD8+ T cells (Judge, et al., 2002) and Interleukin-15 receptor alpha (IL-15Rα) supports memory T cells in vivo (Burkett, et al., 2003). Polyinosinic:polycytidylic acid (Poly I:C) is a mismatched double-stranded RNA with one strand being a polymer of inosinic acid, and the other is a polymer of cytidylic acid. Poly I:C, immunostimulant, induces IL-15 and IL-15Rα (Lodolce, et al., 2001) which is essential for binding IL-15 (Lorenzen, et al., 2006) and boosts the generation of memory T cells (Wang, et al., 2010).
We previously observed the frequencies of CD8+CD44+ and CD8+CD62L- memory T cells in BD mice were significantly lower than BDN mice. In this study, we found that the frequencies of IL-15Rα were significantly lower in HSV-induced BD mouse model than control mice. Therefore, we investigated to determine whether Poly I:C supplementation could induce memory cells through up-regulation of IL-15Rα, and reduce inflammation in HSV-induced BD symptoms.
Ⅱ. MATERALS AND METHODS
A. Animal experiments
ICR male mice (4 to 5 weeks old) were infected with HSV type 1 (1x106 pfu/mL, F strain) grown in Vero cells, as previously described (Sohn, et al., 1998). Virus inoculation was conducted twice at an interval of 10-days and after second inoculation of HSV, mice were observed from 4 weeks to 12 months. Animals were handled in accordance with a protocol approved by the animal care committee of Ajou University School of Medicine.
B. BD symptoms in a mouse model
Manifestations in mice after HSV inoculation involved multiple symptoms. Of the total number of HSV-infected mice, 15% developed BD symptoms. Symptoms in human patients including oral ulceration, genital ulceration, erythema, skin pustules, skin ulcerations, joint-arthritis, diarrhea, red eye (right, left), reduced vision (right, left), loss of balance, discoloration, and swelling of the face were selected and analyzed as BD symptoms. Oral, genital, and other skin ulcers and eye symptoms were classified as major symptoms. Arthritis, gastrointestinal ulcers, and neurological involvement were identified as minor symptoms. Mice with ≥ 1 major and 1 minor symptoms were classified as having BD. The score of each symptom was one and the sum of the symptoms were used to determine the severity of BD. The disappearance of symptoms or a decrease in the lesion size of more than 20% was classified as improvement. The severity of BD was followed by determination of
the modified Behcet’s disease activity index as outlined in the BD Activity Form (www.behcet.ws/pdf/BehcetsDiseaseActivity-Form.pdf). As a control group, HSV was inoculated, but asymptomatic healthy mice were used as BD Normal (BDN) as previously described.
C. In vivo administration of Poly I:C
Polyinosinic-Polycytidylic acid (Poly I:C) (Sigma Chemicals, St. Louis, Mo, USA) was resuspended in physiological phosphate buffered saline (PBS) at a stock concentration of 10 mg/ml, aliquoted, and stored in freezer (-20°C) prior to use. 5 week aged male normal mice were randomly placed into the following treatment groups: PBS, Poly I:C 0.05, 0.2, 1 µg/g body weight. Normal mice were injected i.p. with Poly I:C or PBS for 2 times at 3-days interval. BD mice were injected i.p. with Poly I:C or PBS for 4 times at 3-days interval. Two days or seventeen days after the last administration of Poly I:C or PBS, peripheral blood mononuclear cells (PBMC), spleen cells, and lymph node cells were isolated for flow cytometric analysis and the sera were collected for ELISA.
D. Flow cytometric analysis of cell surface staining
Mouse peripheral blood mononuclear cells (PBMC), spleen cells, and lymph node cells were washed with phosphate buffered saline (PBS), after which 1x106 cells were incubated with 0.25 µg of APC-labeled anti-IL-15Rα (R&D system), FITC-labeled anti-CD4, CD122, PerCP-Cy5.5-labeled CD8, PE-labeled IL-7Rα, CD44, anti-CD62L (eBiosciences) for 30 min at 4°C. The stained cells were then washed with PBS and
analyzed by using a flow cytometer (FACSAria III; Becton Dickinson, San Jose, CA, USA) with X10,000 gated lymph node cells.
E. Enzyme-linked immunosorbent assay (ELISA)
Serum was analyzed by commercial ELISA kits for the detection of mouse IL-15 (eBioscience, San Diego, CA) and manual ELISA using monoclonal anti-mouse IL-17 antibody, biotinylated anti-mouse IL-17 antibody (R&D System, Minneapolis, MN), recombinant murine IL-17 (Peprotech, Rocky Hill, NJ), and was conducted according to the manufacturer’s recommendation. The means and standard deviations were calculated using ELISA values determined for each well. The ELISA values measured using a model 680 microplate reader (Bio-Rad, Hercules, CA,USA) and determined the absorbance at 450 nm.
F. Reverse transcription PCR (RT-PCR)
Total RNA was isolated with TRIzol (Life Technologies, Helgerman, CT), according to the manufacturer’s recommendations. An amount of 1 µg of total RNA was used as template for cDNA synthesis with AccuPowerTM RT PreMix for RT-PCR kit (Bioneer, Alameda, CA). The cDNA was amplified by PCR with the following primers: β-actin, sense: 5'-TGGAATCCTGTGGCATCCATGAAAC-3', antisense: 5'-TAAAACGCAGCTCAGTAA CAGTCCG-3'; mIL-23R (Kamiya, et al., 2007), forward primer: 5'-GCACTGCCGACCAA GGAATC-3' and reverse primer: 5'-GAGTTCTCCATGCCTAGGGA-3'. Amplified PCR products were visualized on 1.6-1.7% agarose gels.
H. Statistical analysis
All data shown represent the mean ± SE. Statistical differences between the experimental groups were determined using the Student’s t-test and Bonferroni correction. Statistical analysis was conducted using MedCalc® version 9.3.0.0.
Ⅲ. RESULTS
A. The frequencies of IL-15Rα expressing cells in BD mice compared with BDN
mice
The frequencies of IL-15Rα were analyzed in PBMC, splenocytes, and lymph node (LN) cells of BD and BDN mice (BD normal, HSV was inoculated but no symptomatic mice) by FACS analysis. The frequencies of IL-15Rα-expressing cells in PBMC from BD mice (n=11) were lower than BDN mice (n=10)(10.19 ± 3.22 vs. 13.58 ± 7.23%, p=0.03) (Fig. 1A). The expression pattern of frequencies was similar in splenocytes (n=3)(4.63 ± 0.12 vs. 5.7 ± 0.61%, p=0.04) (Fig. 1B). Whereas in lymph node cells, the frequencies were not significantly different between BD (n=8) and BDN mice (n=10)(11.55 ± 5.84 vs. 11.09 ± 5.66%, p=0.9) (Fig. 1C). The frequencies of IL-15Rα-expressing cells in PBMC and splenocytes from BD mice were significantly lower than BDN mice.
Fig. 1. The expression of IL-15R
and BDN mice. The data show the frequencies of IL cytometry in cells isolated from
BDN mice.
15Rα in PBMC, splenocytes and lymph node cells
The data show the frequencies of IL-15Rα were analyzed by Flow PBMC (A), spleens (B), and lymph nodes (C) from
lymph node cells from BD were analyzed by Flow (C) from BD and
B. Poly I:C up-regulates expression of IL-15Rα in normal mice
To determine whether the expression of IL-15Rα can be regulated by Poly I:C, Poly I:C or PBS (control) was injected into normal mice. At 2 days after the second injection of Poly I:C or PBS, the cells were isolated from blood of mice. Then, the frequencies of IL-15Rα were measured by flow cytometry (Fig. 2A). The frequencies of IL-IL-15Rα expression on PBMC from normal mice following Poly I:C 0.05 µg/g (n=2)(6.9 ±0.85), Poly I:C 0.2 µg/g (n=4)(8.3 ±2.45), Poly I:C 1 µg/g (n=2)(7.7 ±2.83), and PBS (n=3)(4.3 ±0.79) (Fig. 2B). The frequencies of IL-15Rα on PBMC from 0.2 µg/g of Poly I:C treated mice were significantly increased than that found in PBMC from PBS administered control mice (p=0.04). 0.2 µg/g of Poly I:C most efficiently up-regulated the expression of IL-15Rα. Therefore, we chose to apply 0.2 µg/g of Poly I:C for further experiments. In addition, the frequencies of IL-15Rα expression on splenocytes and lymph node cells from 0.2 µg/g of Poly I:C treated mice (n=5) were also up-regulated than that found from PBS administered control mice (n=3)(3.6 ±1.92 vs. 5.9 ±1.90%, p=0.1; 5.2 ±1.01 vs. 6.7 ±1.45%, p=0.2) but data was not significant.
Fig. 2. The expression of IL-15Rα were up-regulated by Poly I:C injection in a dose dependent manner in normal mice. 5 weeks aged male normal mice were randomly placed into the following treatment groups: PBS, Poly I:C 0.05, 0.2, 1 µg/g body weight for each group. (A) The mice were injected i.p. with PBS or Poly I:C for 2 times at 3-days interval. Two days after the last injection, the mice were sacrificed and PBMC were isolated, and the expression of IL-15Rα was analyzed by Flow cytometry. (B) The data show the frequency of IL-15Rα in cells isolated from PBMC, spleens, and lymph nodes after Poly I:C or PBS injection.
C. Poly I:C ameliorates HSV-induced BD symptoms
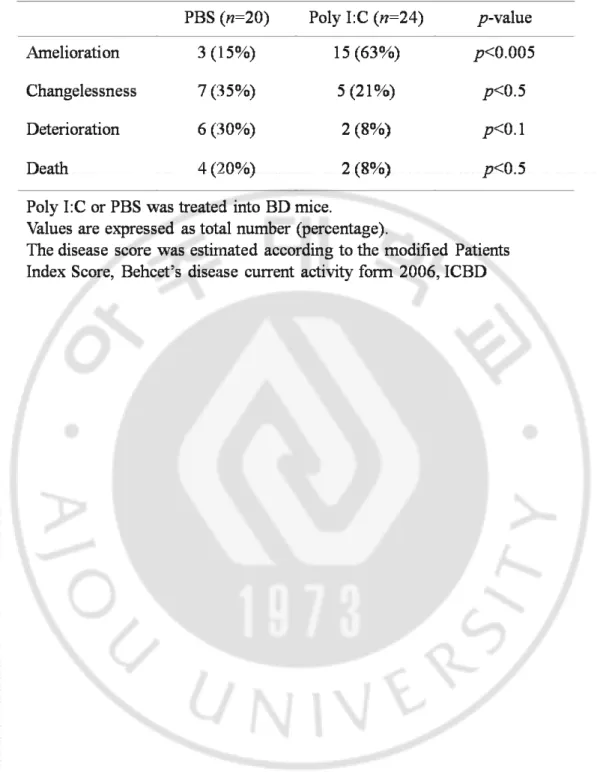
To determine whether Poly I:C can improve the BD symptoms, we conducted intra-peritoneal injection of Poly I:C or PBS into BD mice for four times with three days interval (Fig. 3A). Before and 2 weeks after the last injection, the changes of BD symptoms in BD mice were photographed. The cutaneous symptoms were improved in Poly I:C treated BD mice compared to PBS injected BD mice (Fig. 3B). In Poly I:C injected BD mice, BD symptoms were ameliorated in 15 of 24 BD mice (63%) or not changed in 5 of 24 BD mice (21%) whereas deteriorated in 2 of 24 BD mice (8%) or died in 2 of 24 BD mice (8%). However, in PBS injected BD mice, the BD symptoms were ameliorated in 3 of 20 BD mice (15%) or not changed in 7 of 20 BD mice (35%) whereas deteriorated in 6 of 20 BD mice (30%) or died in 4 of 20 BD mice (20%) (Table 1). Poly I:C administered BD mice with cutaneous symptoms showed diminished lesion size by 20% to 100% of the total area. The change of BD symptoms in BD mice was observed and scored according to the severity score of BD patients, which is outlined in the BD Current Activity Form. The scoring was followed by the changes of superficial symptoms because it is impossible to communicate with mice. In Poly I:C injected group, the severity score was decreased from 2.57 ±0.66 to 1.98 ±1.19 at 2 weeks after the first injection (n=22). Whereas, in the PBS injected group, the severity score was increased from 2.94 ±0.87 to 3.07 ±0.98 at 2 weeks after the first injection (n=14). At 2 weeks after the first injection, the severity score significantly lower in Poly I:C injected BD mice (n=22) than PBS injected BD mice (n=14)(1.98 ± 1.19 vs. 3.07 ±0.98, p=0.006) (Fig. 3C).
Fig. 3. The change of symptoms in BD mice after injection of Poly I:C. (A) The used experimental schedule throughout this study. Poly I:C or PBS was injected i.p. into BD mice for 4 times at 3-days interval, and the symptoms were photographed and the severity score was analyzed. (B) The symptoms of BD mice were compared before and after injected with Poly I:C or PBS. (C) The severity score of Poly I:C or PBS injected BD mice. The disease score was estimated according to the Patients Index Score, Behcet’s disease current activity form 2006, ICBD.
Table 1. The change of symptoms in BD mice after injection of Poly I:C. Table 1. The change of symptoms in BD mice after injection of Poly I:C.
D. Poly I:C up-regulates the frequencies of IL-15Rα but not IL-7Rα (CD127) in
BD mice.
To confirm the frequencies of IL-15Rα in Poly I:C injected BD mice, the cells were isolated from PBMC, spleen, and lymph nodes at 2 and 17 days after injection. As has been reported Poly I:C elicited a brief phase of T cell proliferation peaking at day 2 post-injection and was followed for 7 to 32 days (Zhang, et al., 1998; West, et al., 2011). At 2 days after the last injection, the frequencies of IL-15Rα from Poly I:C injected group were significantly increased than PBS injected group in PBMC (16.8±2.6 vs. 9.2±3.5%, p<0.001), spleen (12.9±2.8 vs. 4.6±0.1%, p=0.001), and lymph nodes (21.8±2.3 vs. 9.6±6.0%, p<0.001). At 17 days after the last injection, the frequencies of IL-15Rα from Poly I:C injected group was significantly increased than PBS injected group in PBMC (27.4 ±9.8 vs. 9.2 ±3.5%, p<0.0001) but recovered in spleen (5.2 ±2.1 vs. 4.6 ±0.1%, p=0.7) and lymph node (11.0 ±11.0 vs. 9.6 ±6.0%, p=0.7) (Fig. 4A). In PBMC of Poly I:C injected group, the up-regulated expression of IL-15Rα were sustained until 17 days (at 17 days vs. at 2 days, 27.4 ±9.8 vs. 16.8 ±2.6%, p=0.045). In spleen and lymph nodes of Poly I:C injected group, up-regulated IL-15Rα was decreased to control level at 17 days.
The frequencies of IL-7Rα expression in spleen cells were isolated from BD mice (n=5) were significantly lower than BDN mice (n=5)(28.5±3.8 vs. 20.0±1.4%, p=0.002) but not different in PBMC (42.6±12.5 vs. 37.8±7.8%, p=0.5) and lymph nodes cells (33.4±11.2 vs. 39.0±5.7%, p=0.3) between BD mice and BDN mice. Actually, IL-15 and IL-7 cytokines were involved with the generation and maintenance of memory CD8+ T cells (Kimberly, et al., 2003). These cytokines interact with IL-15Rα and IL-7Rα respectively, as has been
reported, the down-regulation of IL-15Rα and IL-7Rα expression is a contributing factor for the poor survival of memory CD8+ T cells in the airways (Shen, et al., 2008). To determine whether the injection of Poly I:C into BD mice can induce not only IL-15Rα but also IL-7Rα, we analyzed the frequencies of IL-7Rα in the same method. At 2 days after the last injection, the frequencies of IL-7Rα from Poly I:C injected group were not different compared with PBS injected group in PBMC (Poly I:C vs. PBS, 41.9 ±18.1 vs. 45.7 ±20.0%, p=0.7), spleen (26.1 ±6.4 vs. 28.0 ±12.9%, p=0.8), and lymph nodes (45.6 ±12.6 vs. 41.8 ±14.8%, p=0.6). At 17 days after the last injection, the frequencies of IL-7Rα from Poly I:C injected group were also not different compared with PBS injected group in PBMC (Poly I:C vs. PBS, 51.0 ±20.4 vs. 45.7 ±20.0%, p=0.6), spleen (23.4 ±14.0 vs. 28.0 ±12.9%, p=0.5), and lymph nodes (47.5 ±12.3 vs. 41.8 ±14.8%, p=0.4) (Fig. 4B). Poly I:C elevates the level of IL-15Rα but not IL-7Rα in BD mice. We next investigated the serum level of IL-7 and IL-15 by ELISA to determine whether the serum level of IL-7 and IL-15 was changed in BD mice after Poly I:C injection. Serum levels of IL-7 were 46.08±38.67 pg/ml in BDN (n=12) and 32.84±10.41 pg/ml in BD (n=13) by ELISA. The level of IL-7 was not different between BD mice and BDN mice (p=0.2). Serum IL-7 levels were elevated after injection of Poly I:C in BD mice at 2 days after the last injection (Poly I:C (n=6) vs. PBS (n=8))(48.8 ±18.22 vs. 34.33 ±13.12 pg/ml, p=0.1) and at 17 days after the last injection (Poly I:C (n=6) vs. PBS (n=8))(54.75 ±15.89 vs. 34.33 ±13.12 pg/ml, p=0.02) (Fig. 4C). Although the frequency of IL-7Rα were not affected, the serum level of IL-7 significantly up-regulated by Poly I:C injection in BD mice. The serum level of 15 was not analyzed because reliable mouse
IL-Fig. 4. The expression of IL-15Rα, IL-7Rα in PBMC, splenocytes and lymph node cells isolated at 2 and 17 days after the last injection of Poly I:C in BD mice. The cells were isolated from PBMC, spleen and lymph nodes at 2 and 17 days after the last injection of Poly I:C or PBS. The expression of IL-15Rα and IL-7Rα were measured by Flow cytometry. The data show the frequencies of IL-15Rα (A) and IL-7Rα (B) in PBMC, splenocytes, and lymph node cells from PBS or Poly I:C injected BD mice. (C) The serum levels of IL-7 were measured by ELISA in BDN, Poly I:C or PBS injected BD mice.
E. Poly I:C induces CD8+CD44+ memory T cells in BD mice
There has been reported, IL-15Rα on bone marrow derived cells trans-present IL-15 and it mediates the basal proliferation of memory CD8+ T cells (Schluns, et al., 2004). We previously observed the frequencies of CD8+CD44+ and CD8+CD62L- memory T cells isolated from spleen were significantly lower in BD mice compared to BDN mice (CD8+CD44+, p=0.022; CD8+CD62L-, p=0.04). Therefore, we injected Poly I:C into BD mice intra-peritoneally to determine whether the expression of memory T cells can be affected by injection of Poly I:C in BD mice. At 2 and 17 days after the last injection, the cells were isolated from blood, spleens and lymph nodes of BD mice and then the frequencies of memory cell types were measured by flow cytometry. At 2 days after the last injection, the frequency of CD4+CD44+ memory T cells in Poly I:C injected BD mice significantly increased than PBS injected BD mice in spleen (Poly I:C vs. PBS, 24.1 ±5.9 vs. 15.9 ±8.4%, p=0.03) and lymph node (44.7 ±6.8 vs. 33.7 ±10.9%, p=0.02) but was not significant at 17 days after the last injection in PBMC (Poly I:C vs. PBS, 34.2 ±16.3 vs. 22.8 ±16.6%, p=0.1), spleen (26.6 ±14.1 vs. 15.9 ±8.4%, p=0.07), and lymph nodes (41.5 ±12.6 vs. 33.7 ±10.9%, p=0.5) (Fig. 5A). Whereas, the frequencies of CD8+CD44+ memory T cells were not different between Poly I:C and PBS injected group at 2 days after the last injection in PBMC (Poly I:C vs. PBS, 9.4 ±2.4 vs. 7.2 ±4.0%), spleen (3.8 ±1.2 vs. 3.4 ±2.0%) and lymph node (7.8 ±2.6 vs. 10.5 ±5.5%). At 17 days after the last injection, the frequencies of CD8+CD44+ memory T cells from Poly I:C injected group were significantly increased than PBS injected group in PBMC (Poly I:C vs. PBS, 18.6 ±9.6 vs. 7.2 ±4.0%, p=0.003) and spleen (7.2 ±1.5 vs. 3.4 ±2.0%, p=0.0008) though there was no difference in
lymph node (14.0 ±9.2 vs. 10.5 ±5.5%, p=0.3) (Fig. 5B). The injection of Poly I:C significantly elevated CD8+CD44+ memory T cells in BD mice. We conducted to determine whether injection of Poly I:C in BD mice can induce not only CD8+CD44+ memory T cells but also CD4+CD62L- or CD8+CD62L- memory T cells. CD4+CD62L- and CD8+CD62L- memory T cells were measured in the same method. The frequencies of CD4+CD62L- memory T cells were not different between Poly I:C and PBS injected group in PBMC, spleen and lymph nodes (Fig. 5C). Whereas, the frequencies of CD8+CD62L- memory T cells significantly increased in Poly I:C injected group than PBS injected group at 2 days (Poly I:C vs. PBS, 8.8 ±2.4 vs. 2.4 ±1.6%, p=0.0004) and 17 days (10.9 ±3.9 vs. 2.4 ±1.6%, p=0.0006) after the last injection in PBMC but there was no difference in spleen and lymph nodes (Fig. 5D).
Fig. 5. The frequencies of memory T cells in PBMC, splenocytes and lymph node cells isolated at 2 and 17 days after the last injection of Poly I:C in BD mice. The cells were isolated from PBMC, spleen and lymph nodes at 2 and 17 days after the last injection of Poly I:C. The cells were stained for memory T cell markers and analyzed by Flow cytometry. The data show the frequencies of CD4+CD44+ (A), CD8+CD44+ (B), CD4+CD62L- (C), CD8+CD62L- (D) in PBMC, splenocytes, and lymph node cells after Poly I:C injection.
F. Poly I:C up-regulate CD122(IL-15/2Rβ) and CD8+CD122+ T cells in BD
mice
IL-15 binding to IL-15Rα likely leads to recruitment of CD122(IL-15/2Rβ) and common γ chain, and initiation of signaling cascades from these shared chains (Bamford, et al., 1994). We next investigated the frequency of CD122-expressing cells in Poly I:C injected BD mice to determine whether the expression of CD122 that demanded to signaling cascades of IL-15-15Rα complexes could be induced by Poly I:C.
The frequencies of CD122-expressing cells in PBMC (8.4±7.6 vs. 15.3±9.7%, p=0.2) and lymph nodes cells (4.6±2.0 vs. 5.7±1.1%, p=0.3) did little different between BD mice and BDN mice. At 2 days after the last injection, the frequencies of CD122-expressing cells were not different between Poly I:C and PBS injected BD mice in PBMC (Poly I:C vs. PBS, 12.1±4.8 vs. 8.4±7.6%) and lymph nodes (7.3±4.4 vs. 4.6±2.0%). At 17 days after the last injection, the frequencies of CD122-expressing cells from Poly I:C injected group were significantly elevated than PBS injected group in PBMC (Poly I:C vs. PBS, 25.9±12.0 vs. 8.4±7.6%, p=0.01) and lymph nodes (7.8±3.3 vs. 4.6±2.0%, p=0.05) (Fig. 6A). In result, the frequencies of CD122-expressing cells were significantly up-regulated in lymph nodes cells isolated form Poly I:C injected BD mice at 17 days after the last injection.
CD8+CD122+ T cells are newly identified regulatory T cells (Saitoh, et al., 2007)and reported the effect which involved anti-inflammatory responses (Rifa'i, et al., 2008) in the recovery phase of EAE mouse model (Lee, et al., 2008). The frequencies of CD8+CD122+ T cells in lymph nodes cells of BD mice (n=6) were significantly lower than BDN mice (n=5)(1.5±0.7 vs. 3.8±1.6%, p=0.009) but not different in PBMC between BD mice and
BDN mice (4.0±2.4 vs. 5.7±2.6%, p=0.4). At 2 days after the last injection, the frequencies of CD8+CD122+ cells were not different between Poly I:C and PBS injected BD mice in PBMC (Poly I:C vs. PBS, 3.9±3.1 vs. 6.1±1.3%) and lymph nodes (4.0±2.9 vs. 3.3±0.1%, p=0.07). At 17 days after the last injection, the frequencies of CD8+CD122+ cells from Poly I:C injected group (n=4) were significantly increased than PBS injected group (n=6) in lymph node (3.6±1.2 vs. 1.5±0.7%, p=0.008) though there was no difference in PBMC (5.8±1.0 vs. 4.0±2.4%, p=0.2) (Fig. 6B). In the result, the frequencies of CD8+CD122+ regulatory T cells were significantly higher in lymph nodes cells isolated from BDN mice compared with BD mice. Administration of Poly I:C into BD mice up-regulated the frequencies of CD8+CD122+ regulatory T cells in lymph nodes cells.
Fig. 6. The frequencies of CD122(IL-15/2Rβ) and CD8+CD122+ T cells in lymph node cells isolated at 2 and 17 days after the last injection of Poly I:C into BD mice. The cells were isolated from lymph nodes at 2 or 17 days after the last injection of Poly I:C. The frequencies of CD122- and CD8+CD122+-expressing cells were analyzed by Flow cytometry. The data shows the frequencies of CD122+ (A), CD8+122+ cells in PBMC and lymph node cells (B) and the dot plot of CD8+CD122+ T cells in lymph node cells from BDN, PBS or Poly I:C injected BD mice group (C).
G. Poly I:C down-regulates IL-23 receptor and IL-17A in BD mice
Recent studies have showed that the IL-23R gene is strongly associated with Behcet’s disease (Jiang, et al., 2010; Mizuki, et al., 2010; Remmers, et al., 2010) as well as several autoimmune diseases, such as Crohn’s disease (Duerr, et al., 2006), Rheumatoid arthritis (Hollis-Moffatt, et al., 2009), and Ankylosing spondylitis (Rueda, et al., 2008; Sung, et al., 2009). The IL-23R is essential for the terminal differentiation of IL-17-producing effector T helper cells in vivo (McGeachy, et al., 2009). Th17 cells are also present in human patients with various autoimmune diseases, including rheumatoid arthritis, multiple sclerosis (Matusevicius, et al., 1999), systemic lupus erythematous (Wong, et al., 2000), and asthma (Hashimoto, et al., 2005). Therefore, we investigated the mRNA level of IL-23R and IL-17A cytokine serum level from BD, BDN and Poly I:C injected BD mice. IL-23R mRNA levels in lymph nodes of BD mice were up-regulated when compared to BDN by RT-PCR. At 2 and 17 days after the last injection, IL-23R mRNA levels in lymph nodes down-regulated in Poly I:C injected BD mice compared to BDN mice (Fig. 7A). Th17 cells mainly produce IL-17A, therefore the serum level of IL-17A was measured by ELISA. Serum levels of IL-17A were 9.87±4.46 pg/ml in BDN (n=9) and 21.30±11.79 pg/ml in BD (n=12) by ELISA. As a result, the serum IL-17A levels were higher in BD mice than BDN mice (p=0.006). Serum IL-17A levels were down-regulated by injection of Poly I:C in BD mice at 2 days after the last injection though statistically not significant (Fig. 7B) (Poly I:C (n=5) vs. PBS (n=6))(11.70 ±1.60 vs. 18.32 ±9.95 pg/ml, p=0.18). At 17 days after the last injection, serum IL-17A levels were returned to PBS injected control levels.
Fig. 7. mRNA expression of IL
I:C injected BD mice. Lymph nodes cells and serum were collected from Poly I:C injected BD mice isolated at 2
expression was analyzed by RT ELISA .
IL-23 receptor and serum protein levels of IL-17A
Lymph nodes cells and serum were collected from BDN, BD and ected BD mice isolated at 2 or 17 days after the last injection. (A)
expression was analyzed by RT-PCR. (B) The serum levels of IL-17A were measured by 17A in Poly BDN, BD and (A) IL-23R 17A were measured by
Ⅳ. DISCUSSION
In BD patients, the frequencies of CD45RO+ cells were lower than disease control group has been reported (Takase, et al., 2006) and also, in our laboratory we observed that the frequencies of CD8+CD44+ and CD8+CD62L- memory T cells in BD mice were significantly lower than BDN mice. Therefore, we investigated the expression of IL-15Rα and IL-7Rα which as known as association with generation of memory T cells (Kimberly, et al., 2003) in the HSV-induced BD mouse model. The expression of IL-15Rα was significantly lower in BD mice than BDN mice. Although serum level of IL-15 was not analyzed because mouse IL-15 ELISA kit was not available, IL-7 was not different between BD mice and BDN mice. Therefore, we focused in induction of IL-15Rα to up-regulate memory T cells in BD mice. There has been reported, the IL-15Rα mRNA induced during Poly I:C bystander responses in vivo (Lodolce, et al., 2001). It was also reported that administrated Poly I:C enhanced in type I IFN and TGFβ-mediated inflammation in systemic sclerosis (Rothstein, et al., 2010), airway inflammation in a rat model of asthma (Takayama, et al., 2011), and EAE (Experimental Autoimmune Uveitis) retinal autoimmunity model (Ren, et al., 2011). In contrast, many observations supports a protective role of Poly I:C supplementation in the diabetes prone BB rat (Sobel, et al., 1998), a murine Experimental autoimmune encephalomyelitis model (Touil, et al., 2006), and inflammatory arthritis model by IFN-α involvement (Yarilina, et al., 2007). In this experiment, we found low dose of Poly I:C effectively increased the level of IL-15Ra by dose dependent manner in vivo. Then, 0.2
µg/g of Poly I:C was injected intra-peritoneally into BD mice to induce IL-15Rα level. At 2 days after the last injection, we observed that cutaneous symptoms were particularly improved and the severity score of BD mice was decreased compared to PBS injected control group. In the same time, we observed the increased frequencies of IL-15Rα in PBMC and splenocytes significantly but there was no difference in the frequencies of IL-7Rα between Poly I:C and PBS injected BD mice group. Furthermore, the frequencies of CD8+CD44+ memory T cells were significantly increased in PBMC and splenocytes but the frequencies of CD4+CD44+ memory T cells were not different between Poly I:C and PBS injected BD mice group at 17 days after the last injection. In this result, CD8+CD44+ memory T cells were selectively up-regulated in BD mice by Poly I:C injection. Therefore, the increased CD8+CD44+ memory T cells could be correlated to IL-15Rα that was up-regulated by Poly I:C. Interestingly, although the frequency of IL-7Rα were not affected, the serum level of IL-7 significantly up-regulated by Poly I:C injection in BD mice. It is possible that the increased IL-7 cytokine also could be involved in up-regulation of CD8+CD44+ memory T cells. There are many papers associated with potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15 (Zhang, et al., 1998; Becker, et al., 2002; Becker, et al., 2003;Oh, et al., 2004; Mortier, et al., 2009) but are not required for memory-phenotype CD4+ T cells (Tan, et al., 2002). In addition, the expression of IL-15Rα in PBMC was steadily elevated by 17 days in contrast with return to PBS control level in splenocytes and lymph node cells. As has been reported that IL-15 and IL-15Rα, that form stable complexes on the cell surface of activated monocytes, recycled after endosomal
Sato, et al., 2007). It has been thought that highly IL-15Rα-expressing cells such as monocytes and macrophages were abundant in blood compared to spleen and lymph nodes. The expression of IL-15Rα on cell surfaces enable sustained IL-15 activity by recycling. In our result, also has shown steady expression of IL-15Rα in PBMC. As a result, IL-15Rα induced CD8+ memory T cells were up-regulated at 17 days and the improvement of cutaneous symptoms of BD mice was also observed after Poly I:C injection. Then, what kind of factors affect improvement in HSV-induced BD mice model in early phase after poly I:C injection? Recent data has been revealed that Poly I:C stimulation of dendritic cells and fibroblasts limits herpes simplex virus type 1 infection in an IFN-β-dependent way (Gaajetaan, et al., 2011). And another data showed, Poly I:C suppress the effector phase of inflammatory arthritis and it is mediated by IFN-α involved in type I IFNs (Yarilina, et al., 2007). Also, Interferon-α2a (IFN-α2a) has been reported to be clinically effective in several patient groups with BD (Zouboulis, et al., 1998). When we evaluated the serum level of IFN-α in the PBS or Poly I:C injected BD mice using VeriKineTM Mouse Interferon Alpha ELISA Kit from R&D Systems according to the manufacturer’s recommendations, the IFN-α levels in the mouse sera tested were below the detection limit of the kit (data not shown). We observed the mRNA level of IL-23R and serum level of IL-17A, as known as pro-inflammatory factor, were down-regulated in Poly I:C injected mice compared to control group at 2 days after the last injection. Also we found the frequencies of CD8+CD122+ T cells as known as newly identified regulatory T cells were significantly elevated in Poly I:C injected group compared with control group in lymph node cells at 17 days after the last Poly I:C injection. In addition, in our laboratory study, we observed that the frequencies of
IL-15Rα were down-regulated in peripheral blood mononuclear cells of BD patients compared with healthy control as a similar pattern with HSV-induced BD mouse model (data not shown). In conclusion, these results suggest that IL-15Rα is an important factor for the regulation of symptoms in HSV-induced BD mice.
Ⅴ. CONCLUSION
In BD patients, the frequencies of CD45RO+ cells were lower than disease control group has been reported (Takase, et al., 2006) and also, in our laboratory we observed that the frequencies of CD8+CD44+ and CD8+CD62L- memory T cells in BD mice were significantly lower than BDN mice. In this study, we investigated the expression of IL-15Rα and IL-7Rα which as known as association with generation of memory T cells in the HSV-induced BD mouse model that developed in our laboratory. Interestingly, the expression of IL-15Rα was significantly lower in BD mice than BDN mice. The serum level of IL-7 was not different between BD and BDN mice, although serum level of IL-15 was not analyzed because mouse IL-15 ELISA kit was not available. In vivo, we found that Poly I:C effectively increased the level of IL-15Rα by dose dependent manner. Therefore, we administrated Poly I:C into BD mice to up-regulate IL-15Rα and observed that cutaneous symptoms were improved and the severity score of BD mice was decreased compared to PBS injected control group. In the ameliorated BD mice, the frequencies of IL-15Rα and CD8+CD44+ memory T cells significantly increased in Poly I:C group compared to control group. Although the frequencies of IL-7Rα were not different, but serum level of IL-7 significantly increased in Poly I:C group compared to control group. The frequencies of CD8+CD122+ T cells as known as newly identified regulatory T cells were significantly elevated in Poly I:C group compared with control group in lymph node cells. In addition, the mRNA level of IL-23R and serum level of IL-17A were down-regulated in Poly I:C injected
group compared to PBS infected control group. In conclusion, these results suggest that IL-15Rα is an important factor for the regulation of symptoms in HSV-induced BD mice.
REFERENCES
1. Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541-1548, 2002
2. Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A 100: 4724-4729, 2003
3. Direskeneli H, Ergun T, Yavuz S, Hamuryudan V, Eksioglu-Demiralp E. Thalidomide has both anti-inflammatory and regulatory effects in Behcet's disease. Clin Rheumatol 27: 373-375, 2008
4. Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 17: 537-547, 2002
5. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314: 1461-1463, 2006
6. Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, Rifkin IR, Marshak-Rothstein A, Radstake TR, Lafyatis R. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol 130: 2583-2593, 2010
7. Gaajetaan GR, Geelen TH, Grauls GE, Bruggeman CA, Stassen FR. CpG and poly(I:C) stimulation of dendritic cells and fibroblasts limits herpes simplex virus type 1 infection in an IFNβ-dependent and -independent way. Antiviral Res, 2011
8. Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol 137: 51-54, 2005
9. Hollis-Moffatt JE, Merriman ME, Rodger RA, Rowley KA, Chapman PT, Dalbeth N, Gow PJ, Harrison AA, Highton J, Jones PB, O'Donnell JL, Stamp LK, Merriman TR. Evidence for association of an interleukin 23 receptor variant independent of the R381Q variant with rheumatoid arthritis. Ann Rheum Dis 68: 1340-1344, 2009
10. Jiang Z, Yang P, Hou S, Du L, Xie L, Zhou H, Kijlstra A. IL-23R gene confers susceptibility to Behcet's disease in a Chinese Han population. Ann Rheum Dis 69: 1325-1328, 2010
11. Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med 7: 935-946, 2002
12. Kamiya S, Nakamura C, Fukawa T, Ono K, Ohwaki T, Yoshimoto T, Wada S. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast ifferentiation. J Bone Miner Metab 25: 277–285, 2007
13. Kimberly S. Schluns and Leo Lefrancois. Cytokine control of memory T-cell development and survival. Nature Reviews Immunology 3: 269–279, 2003
14. Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol 180: 825-832, 2008
15. Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15R alpha signals are required for bystander proliferation. J Exp Med 194: 1187-1194, 2001
16. Lorenzen I, Dingley AJ, Jacques Y, Groetzinger J. The structure of the interleukin-15 alpha receptor and its implications for ligand binding. J Biol Chem 281: 6642-6647, 2006
17. Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 5: 101-104, 1999
18. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10: 314-324, 2009
19. Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, Ito N, Kera J, Okada E, Yatsu K, Song YW, Lee EB, Kitaichi N, Namba K, Horie Y, Takeno M, Sugita S, Mochizuki M, Bahram S, Ishigatsubo Y, Inoko H. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet's disease susceptibility loci. Nat Genet 42: 703-706, 2010
20. Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity 31: 811-822, 2009
21. Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A
22. Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, Le JM, Yang B, Korman BD, Cakiris A, Aglar O, Emrence Z, Azakli H, Ustek D, Tugal-Tutkun I, Akman-Demir G, Chen W, Amos CI, Dizon MB, Kose AA, Azizlerli G, Erer B, Brand OJ, Kaklamani VG, Kaklamanis P, Ben-Chetrit E, Stanford M, Fortune F, Ghabra M, Ollier WE, Cho YH, Bang D, O'Shea J, Wallace GR, Gadina M, Kastner DL, Gül A. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet's disease. Nat Genet 42: 698-702, 2010
23. Ren X, Zhou H, Li B, Su SB. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid enhances autoimmune disease in a retinal autoimmunity model. Int Immunopharmacol 11: 769-773, 2011
24. Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol 20: 937-947, 2008
25. Rueda B, Orozco G, Raya E, Fernandez-Sueiro JL, Mulero J, Blanco FJ, Vilches C, González-Gay MA, Martin J. The IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitis. Ann Rheum Dis 67: 1451-1454, 2008
26. Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves' hyperthyroidism in a murine model. Endocrinology 148: 6040-6046, 2007
27. Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A 104: 588-593, 2007
28. Schluns KS, Klonowski KD, Lefrançois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood 103: 988-994, 2004
29. Shen CH, Ge Q, Talay O, Eisen HN, García-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol 1: 171-178, 2008
30. Sobel DO, Goyal D, Ahvazi B, Yoon JW, Chung YH, Bagg A, Harlan DM. Low dose poly I:C prevents diabetes in the diabetes prone BB rat. J Autoimmun 11: 343-352, 1998
32. Sung IH, Kim TH, Bang SY, Kim TJ, Lee B, Peddle L, Rahman P, Greenwood CM, Hu P, Inman RD. IL-23R polymorphisms in patients with ankylosing spondylitis in Korea. J Rheumatol 36: 1003-1005, 2009
33. Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4: 329-336, 1996
34. Takase H, Sugita S, Taguchi C, Imai Y, Mochizuki M. Capacity of ocular infiltrating T helper type 1 cells of patients with non-infectious uveitis to produce chemokines. Br J Ophthalmol 90: 765–768, 2006
35. Takayama S, Tamaoka M, Takayama K, Okayasu K, Tsuchiya K, Miyazaki Y, Sumi Y, Martin JG, Inase N. Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma. Immunology 134: 140-150, 2011
36. Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 195: 1523-1532, 2002
37. Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol 177: 7505-7509, 2006
38. Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol 16: 205-226, 1998
39. Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol 184: 2751-2755, 2010
40. West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, Haining WN, Ahmed R. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity 35: 285-298, 2011
41. Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9: 589-593, 2000
Bao-mei Gao, Hui Guan, Wanda Niedbala, Gavin K. Paterson, Iain B. McInnes, and Foo Y. Liew. The Sushi Domain of Soluble IL-15 Receptor a Is Essential for Binding IL-15 and Inhibiting Inflammatory and Allogenic Responses In Vitro and In Vivo. The Journal of Immunology 167: 277–282, 2001
43. Yamashita N, Kaneoka H, Kaneko S, Takeno M, Oneda K, Koizumi H, Kogure M, Inaba G, Sakane T. Role of gammadelta T lymph node cells in the development of Behçet's disease. Clin Exp Immunol 107: 241-247, 1997
44. Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol 178: 2204-2211, 2007
45. Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8: 591-599, 1998
46. Zouboulis CC, Orfanos CE. Treatment of Adamantiades-Behçet disease with systemic interferon alfa. Arch Dermatol 134: 1010-1016, 1998
- 국문요약 -
단순포진 바이러스로 유도한 베체트병 마우스모델에서 Poly I:C
에 의해 유도된 IL-15Rα의 항 염증 효과에 관한 연구
아주대학교 대학원 의생명과학과
최 주 영
(지도교수: 손 성 향)
1937년 터키의 피부과 의사인 Hulusi Behcet 에 의해 처음 명명된 베체트병은 구강궤양, 외음부궤양 및 안 증상의 3가지 주 증상을 반복적으로 보이는 만성 염증성 질환으로 호전과 악화가 반복되는 재발성 질병 양상을 보이며, 실명의 가장 흔한 원인 질환으로 알려져 있다. 이 질환은 전 세계적으로 발생하고 있으나 아직까지도 발병 원인이 정확하게 규명되지 않았으며 치료법 또한 연구 중에 있다.활성화된 단핵세포의 표면에서 발현되는 IL-15 수용체 alpha 는 IL-15와 높은 친화성으로 안정적인 복합체를 형성하며, 단핵세포의 표면에 전시된 IL-15-IL-15Rα 복합체는 IL-15/2 수용체 beta와 γ-chain을 발현하는 기억 CD8+ T 세포와의 세포간 상호작용으로 인해 기억 CD8+ T 세포의 증식을 조절한다고 알려져 있다. 최근 연구에 의하면 Polyinosinic:polycytidylic acid (Poly I:C)는
면역자극제로서 보고되어있다. 또한 베체트 병 환자에서 기억 T 세포의 빈도가 질병 대조군 집단에서 보다 낮다는 보고가 있다.
이번 연구에서 단순포진바이러스로 유도한 베체트병 마우스모델에서 IL-15 수용체 alpha의 발현이 대조군 마우스와 비교했을 때 감소된 것을 관찰하였다. 따라서 IL-15와 IL-15 수용체 alpha를 유도하여 기억 세포를 조절한다고 알려진 Poly I:C 의 투여로 인해 베체트병 마우스 모델의 염증 증상을 감소시킬 수 있는지에 대해 알아보기 위해 수행되었다. 우선, in vivo에서 Poly I:C 에 의해 IL-15 수용체 alpha의 발현이 증가되는지를 관찰하기 위해 정상 마우스에 0.05 μg/g, 0.2 μg/g, 1 μg/g 농도의 Poly I:C 또는 대조군으로 PBS를 복강주사 하였다. 최종적으로 IL-15 수용체 alpha가 가장 높게 증가한 0.2 μg/g 농도의 Poly I:C 를 베체트병 마우스 모델에 3일 간격으로 4회 복강 주사 한 뒤, 마지막으로 Poly I:C 가 투여된 지 17일 째 되는 날까지 증상 변화를 관찰하였다. 베체트병 마우스 모델에 마지막으로 Poly I:C 가 투여된 후 2일 째 되는 날에 IL-15 수용체 alpha의 발현이 유의하게 증가되었고 17일 째 되는 날에는 기억 CD8+ T 세포의 빈도가 유의하게 증가하였다. 또한 대조군 집단에 비해서 Poly I:C를 투여한 집단에서 증상의 호전, 중증 척도 값의 감소, 염증성 사이토카인의 감소, CD8+CD122+ 조절 T 세포의 증가가 관찰되었다. 따라서 Poly I:C 의 투여로 인해 증가된 IL-15 수용체 alpha를 단순포진 바이러스에 의해 유도된 베체트 병 마우스 모델의 증상을 조절하는 중요한 인자로 제시하였다.
핵심어 : IL-15Rα, Poly I:C, 기억 CD8+ T 세포, 단순포진 바이러스, 베체트병 마우스모델