1-1 / H. Takezoe
• IMID 2009 DIGEST
Abstract
After reviewing the principle of a new display utilizing bent-core liquid crystals, we summarize the advantages and problems of this display. Then we will introduce our effort to overcome these problems mostly by synthesizing new materials. We obtained a variety of newly synthesized compounds showing the SmAPR phase. Mixing was effective
to decrease and widening the temperature range of the SmAPR phase.
1. Introduction
(1 line spacing)
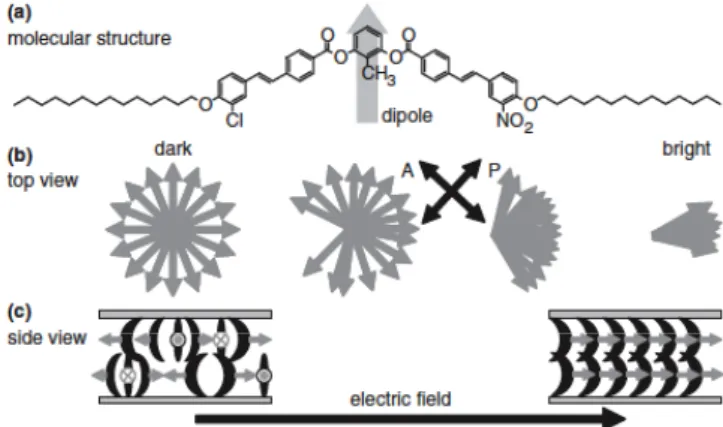
We proposed and demonstrated a novel display device utilizing bent-core liquid crystals in the SmAPR phase [1], where the subscript “R” stands for “random” polarization (or tip) orientation. Because of the random orientation, this phase looks uniaxial SmA phase, so that very dark view is achieved under crossed polarizers if it is homeotropically aligned. By applying an in-plane field, polarization parallel to the smectic layer starts to align, resulting in a biaxial order (Figure 1). Hence this state can be used as a bright state, if the crossed polarizer and analyzer make 45 degrees with respect to the field direction. The switching is not dielectric but ferroelectric, so that the response time is very fast [1]. By analyzing the second-harmonic generation (SHG) as a function of an applied electric field, we found that the bent-core molecules form polar domains consisting of a few hundreds of molecules [2]. In this way, the field application is IPS-like, the orientation is VA-like, and the switching is FLC-like. Hence, the display devices seem to be ideal; wide viewing angle, high contrast ratio, and fast response time.
Unfortunately, however, there are many problems to be solved: We need to have (1) wider temperature
range including room temperature, (2) stabler layer structure, (3) larger biaxiality, (4) lower operating voltages, and (5) negligible temperature dependence of physical parameters. In this work, we concentrate on synthesizing new materials which shows the SmAPR phase.
Fig. 1. Principle of our display using the SmAPR
phase: (a) molecular structure and dipole (arrow); (b) top view and (c) side view of the dipole reorientation process with increasing an electric field.
2. Results
Three series of asymmetric bent-core compounds were synthesized and their mesomorphic properties were studied [3]. All the compounds possess 2-methylresorcinol as a central unit and stilbene bridge as a main element in the structure. Molecules of series I and series II possess only saturated terminal chains
Liquid crystal display utilizing bent-core liquid crystals: advantages and problems
Hideo Takezoe1, Kinga Gomola1, Lingfeng Guo1, Surajit Dhara1, Yoshio Shimbo1, Ewa Gorecka2, Damian Pociecha2, and Jozef Mieczkowski2
1Dept. of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo, Japan
Tel.:81-3-5734-2436, E-mail: takezoe.h.aa@m.titech.ac.jp
2Chemistry Department, Warsaw University, Al. Zwirki i Wigury 101, 02-089 Warsaw, Poland
1-1 / H. Takezoe
IMID 2009 DIGEST • while those of series III one or both unsaturated
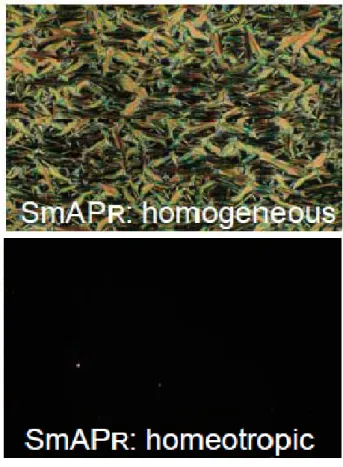
chain/s. The mesophases were characterized by using optical polarizing microscopy, differential scanning calorimetry, X-ray diffraction, electrooptic and dielectric measurements, and miscibility studies. All the compounds exhibit the isotropic to SmAPR phase transition and the B1RevTilted phase at lower temperatures. As an example textures in homogeneous (left) and homeotropic (right) cells of one homologue
11d-NCl-14 at 123°C (SmAPR phase) are shown in Figure 2. A very dark view was observed in the homeotropic cell.
Fig. 2. Textures in homogeneous (upper) and homeotropic (lower) cells of one homologue 11d-NCl-14 at 123°C (SmAPR phase).
Mixing experiments were made to expand and lower the temperature range of SmAPR. One of the examples is shown in Figure 2. The SmAPR phase range was expanded and lowered.
0 20 40 60 80 100 90 100 110 120 130 140 B1RevTilted SmAPR Iso Te mp era tur e( oC) Concentration of 11d-NCl-14 (wt%)
Fig. 3. Phase diagram of a binary mixture of
14-NCl-14 and 11d-NCl-14.
3. Summary
We synthesized new materials showing the SmAPR phase. Lowering and widening of the SmAPR phase have been achieved to some extent by mixing a original classical bent core compound and one of newly synthesized compounds.
4. References
1. Y. Shimbo, Y. Takanishi, K. Ishikawa, E. Gorecka, D. Pociecha, J. Mieczkowski, K. Gomola and H. Takezoe, Jpn. J. Appl. Phys., 45, p.L282 (2006). 2. Y. Shimbo, E. Gorecka, D. Pociecha, F. Araoka,
M. Goto, Y. Takanishi, K. Ishikawa, J.
Mieczkowski, K. Gomola and H. Takezoe, Phys.
Rev. Lett.,97, p.113901 (2006).
3. K. Gomola, Guo Lingfeng, Surajit Dhara, Yoshio Shimbo, Ewa Gorecka, Damian Pociecha, Jozef Mieczkowski, and Hideo Takezoe, J. Mater. Chem.