저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Effect of TIS21 on DNA Damage Repair induced
by Etoposide
by
Kyu-Sung Choi
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Effect of TIS21 on DNA Damage Repair induced
by Etoposide
by
Kyu-Sung Choi
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the
Degree of Master of Biomedical Sciences
Supervised by
Tae Jun Park, M.D., Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Kyu-Sung Choi is approved.
SUPERVISORY COMMITTEE
Tae Jun Park
In Kyoung Lim
Gyesoon Yoon
The Graduate School, Ajou University
June, 25th, 2010
- ABSTRACT -
Effect of TIS21 on DNA Damage Repair Induced by
Etoposide
TIS21
/BTG2/PC3(
12-O-tetradecanoyl phorbol-13-acetate-inducible sequence 21)
has been known as a p53 target gene and functions as a tumor suppressor in
carcinogenesis of thymus, prostate, kidney, and liver. Although it has been
known that the expression of TIS21
/BTG2/PC3was induced during
chemotherapy-mediated DNA damage in cancer cells, a role of TIS21
/BTG2/PC3in DNA repair
process remains to be elucidated.
In this study, the mechanism and role of TIS21 involved in eotposide
induced DNA damage signaling were examined.
gH2AX foci formation, representative marker for DNA damage, was increased in TIS21-/- mouse embryo fibroblasts (MEFs) compared to wild type MEFs. Foci formation of gH2AX after etoposide treatment in LacZ infected Huh7 cells were more retained than TIS21 infected Huh7 cells. Comet assay also showed that DNA damage induced by etoposide were smaller in TIS21 expressed cells. In order to examine TIS21 involved in DNA damage repair and apoptosis, immunoblot analysis was performed. The phosphorylation level of Chk2T68 and p53S20 in the present of etoposide in TIS21 infected Huh7 cells (harboring mutant p53) was significantly decreased as compared to LacZ infected cells.In addition to wild type p53 harboring cells such as MCF7, HeLa, and H9C2 cell lines were also confirmed, and it showed same phenomenon with Huh7 cells. Bcl-2-associated X protein (BAX) and p53 upregulated modulator of apoptosis(PUMA), which are known to be pro-apoptotic proteins in p53-dependent apoptosis, were not induced in TIS21 infected Huh7 cells. Caspase 3/7 activity, chromatin condentation and annexin V staining data suggest that TIS21 inhibited Chk2-p53 DNA damage pathway. Furthermore, TIS21 regulated PRMT1 activity and induced hypermethylation of Mre11 protein. In conclusion, TIS21 involved DNA damage signaling and inhibited Chk2-p53 pathway via Mre11 methylation.
Keywords : ATM, Chk2, BTG2, TIS21, DNA damage, Apoptosis, Mre11
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅱ LIST OF FIGURES ··· ⅲ LIST OF TABLES ··· iv . Ⅰ INTRODUCTION ··· 1 . Ⅱ PURPOSE ··· 8Ⅲ. MATERIALS AND METHODS ··· 9
A. Cell preparation··· 9 B. Immunoblot analysis··· 9 C. Immunocytochemistry ··· 10 D. Comet assay ··· 10 E. FACS analysis ··· 11 F. Immunoprecipitation ··· 12
G. Caspase3/7 activity assay··· 12
H. In vitro and in vivo methylation assay ··· 13
I. Cloning of Chk2 and Mre11··· 13
Ⅳ. RESULTS ··· 15
A. The foci formation of gH2AX was depending upon TIS21 expression. ··· 15
B. TIS21 blocked the Chk2-p53 signal pathway via inhibition of Chk2 phosphorylation.··· 20
C. TIS21 blocked apoptosis via inhibition of p53-dependent pro-apoptotic proteins. ···26
D. TIS21 inhibited Chk2 phosphorylation through regulation of Mre11. ···33
Ⅴ. DISCUSSION ··· 37
Ⅵ. CONCLUSION ··· 41
REFERENCES ··· 42
LIST OF FIGURES
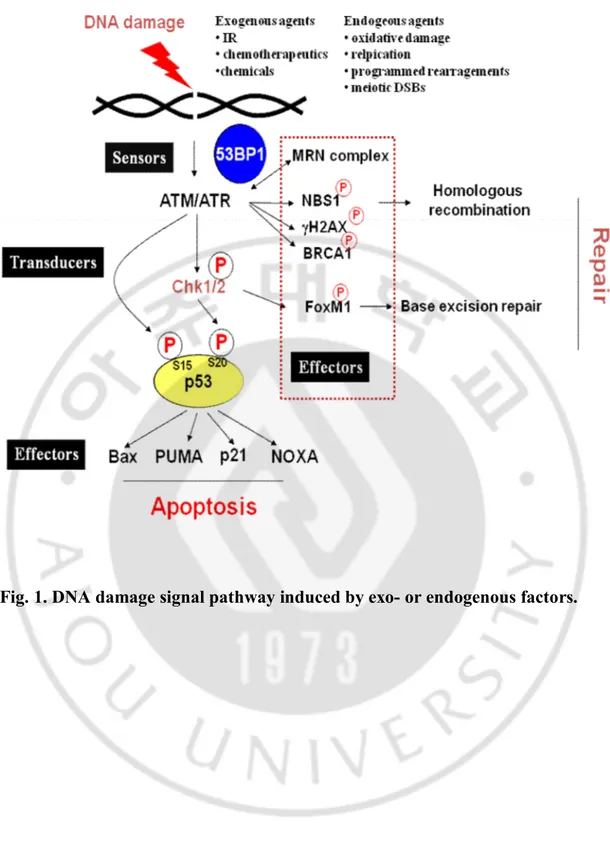
Fig. 1. DNA damage signal pathway induced by exo- or endogenous factors.··· 5
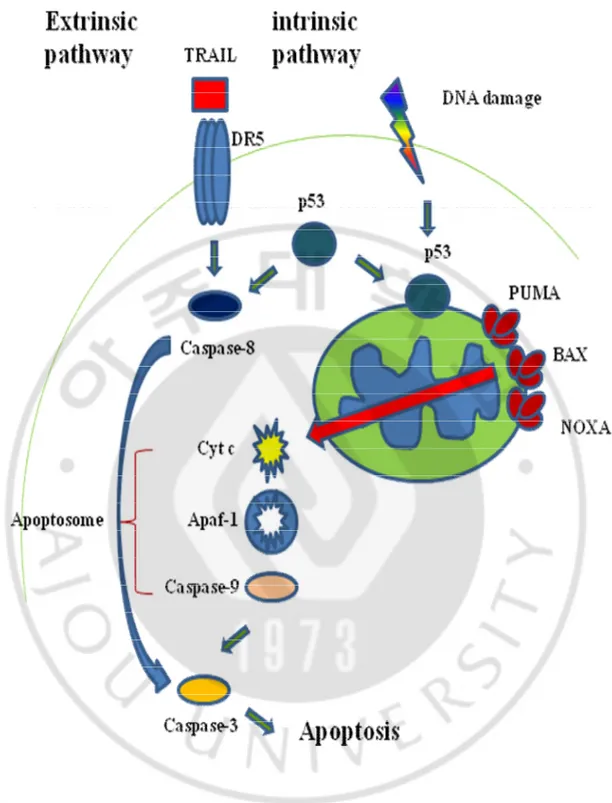
Fig. 2. Two distinct apoptotic pathways; extrinsic-, or intrinsic-apoptosis.··· 6
Fig. 3. The gH2AX foci were increased in TIS21-/-MEFs. ··· 17
Fig. 4. The DNA damage repair induced by etoposide was increased by TIS21. ··· 18
Fig. 5. Etoposide induced DNA damage was less in TIS21 infected Huh7 cells. ··· 19
Fig. 6.
TIS21 inhibited the phosphorylation levels of Chk2 at Thr68 residue and
p53 at Ser20 residue.
··· 22Fig. 7. TIS21 inhibited the phosphorylation levels of Chk2 at Thr68 residue. ··· 23
Fig. 8. The inhibition of Chk2 phosphorylation by TIS21 was confirmed in the various cell lines. ··· 24
Fig. 9.
The phosphorylation level of Chk2 was increased in TIS21
-/-MEFs.
··· 25Fig. 10. The expression levels of
BAX, p21
Waf1/Cip1, and NOXA, were decreased
by TIS21.
··· 27Fig. 11. The cleavage of PARP and caspase 3 was decreased by TIS21. ··· 28
Fig. 12. The activities of caspase 3/7 were decreased by TIS21. ··· 29
Fig. 13. Etoposide-induced apoptosis was attenuated by TIS21 overexpression in Huh7 cells.··· 30
Fig. 14. Etoposide-induced apoptosis was attenuated by TIS21 overexpression in H9C2 cells.··· 31
Fig. 15. PRMT1 bound to MRE11 and TIS21. ··· 34
Fig. 17.
The phosphorylation level of Chk2 was inhibited by TIS21 and Mre11
overexpression.
··· 36LIST OF TABLES
I. INTRODUCTION
A. TIS21
/BTG2/PC3TIS21/BTG2/PC3, orthologs of mouse, human, and rat respectively, was initially identified as one of early growth response genes and induced by various stimulation (Sukhatme et al., 1987). TIS21 (12-O-tetradecanoyl phorbol-13-acetate-inducible sequence 21), was isolated in mice 3T3 fibroblast after treatment with 12-O-tetradecanoyl phorbol-13-acetate (TPA) (Fletcher et al., 1991). The human homolog of TIS21, named BTG2 (B cell translocation gene 2), was cloned from cDNA library of human lymphoblastoid cells as a gene associated with DNA damage response (Rouault et al., 1998). The rat homolog of TIS21, named PC3 (pheochromocytoma cell 3) was isolated as an immediate early response gene when activated by nerve growth factor (NGF) at the onset of neuronal differentiation in rat PC12 cells (Bradbury et al., 1991). TIS21/BTG2/PC3 was highly expressed in thymus, lung alveolar epithelium, proximal tubule of kidney and basal cell layer of prostate acini (Lim, 2006). Potential roles of TIS21 have been reported as transcriptional co-regulator, differentiation and anti-apoptotic factor in neurogenesis, key mediator of the stage-specific expansion of thymocyte and negative regulator of hematopoietic progenitor expansion, and tumor suppressor gene in both mouse and human. TIS21 as pan-cell cycle regulator induced G1/S arrest by both pRB dependently and pRB independently, and also could induce G2/M arrest by inhibition of cyclin B1/cdk activity (summarized in Table. 1.). TIS21 also known to be a binding partner of protein arginine methyltransferase (PRMT1), which methylates lots of proteins, involved in many cellular processes such as RNA processing and DNA repair (Berthet et al., 2002).
B. DNA double-strand breakage and DNA repair mechanisms
1. Sensor of DNA damage signal
The double strand breakages (DSBs) induced by the exogenous or endogenous agents are recognized and repaired by the DNA repair system machinery. If unrepaired DNA remains, it would be threatened not only the integrity of genome but also the survival of organism.
As depicted in Fig. 1, cell responds to DSBs through the action systems that detect the DNA damage lesion and then trigger various downstream events. Classical signal-transduction cascades can explain that DNA damage signal is detected by sensor proteins (DNA-damage binding proteins) and then trigger the activation of transducer proteins (protein kinase cascades) which amplify the signal by targeting a series of downstream effector proteins of the DNA-damage response.
The DSB signaling system should be rapidly responded and needed to be sensitive. If DSBs left unrepaired, they can result in permanent cell cycle arrest, apoptosis, or mitotic cell death (Olive, 1998).
There are two major mechanisms for DSBs repair: non-homologous end-joining (NHEJ) and homologous recombination repair (HR). HR and NHEJ are mechanistically distinct DSBs repair pathways which function as stabilizer for the integrity of DNA (Lieber et al., 2003; O'Driscoll and Jeggo, 2006). NHEJ is the predominant mechanism in mammalian cells and repairs broken ends with no requirement for sequence homology. Activated repair pathway is depending upon the cell cycle, with NHEJ and HR being available in G0/G1 and S/G2 phase, respectively. During HR, the damaged chromosome enters into synapsis with, and retrieves genetic information from an undamaged DNA with
which it shares extensive sequence homology. There are a number of proteins required for triggering DNA damage stress responses that act as sensors for damage that are also important in cell cycle regulation. These sensors include ATM (ataxia talengiectaxia mutated) and ATR (Ataxia telangiectasia and Rad3 related) (Abraham, 2001; Durocher and Jackson, 2001). MRN complex (Mre11, Rad50, and Nbs1) also detected at damaged lesion, then RAD51 protein was recruited. This pre-synapsis complex involves a group of proteins associated with RAD51 paralogs and XRCC2 (X-ray repair complementing defective repair in Chinese hamster cells 2) or XRCC3. BRCA2 (Breast Cancer Type 2 susceptibility protein) is also essential for HR, and join in RAD51 complex (Pellegrini et al., 2002). After loading of RAD51 and its accessory protein, the DNA strand invades the sister chromatid with a homologous template. The final step in the four DNA strands of the two DNA duplexes function as the Holiday junction ultimately results in repaired DNA (Liu et al., 2004; Wyman et al., 2004).
2. Transducers of DNA damage signal
Defects of DNA damage repair and cell cycle checkpoints cause genetic instability, resulting in the accumulation of genetic mutations and eventually cause cancer development. The ATM–Chk2–p53 pathway is involved in the DNA damage repair network that is activated in response to DNA damage or errors in cell cycle events (Bartek and Lukas, 2003; Kastan and Bartek, 2004; Deng, 2006). Upon the occurrence of double-stranded DNA breaks (DSBs), ATM directly phosphorylates p53 on Ser 15 residue and Chk2 on Thr 68 residue, which, in turn, phosphorylates p53 on Ser 20 residue, thereby helping to regulate the cell cycle and apoptosis (Matsuoka et al., 1998; Hirao et al., 2000). Thus, Chk2 works as both
transducer acting in the ATM–Chk2–p53 cascade and candidate tumor suppressor (Fig. 1.). Chk1/2 phosphorylates cdc25 phosphatase and induced G1/S or G2/M cell cycle arrest. Indeed, Chk2 mutations are found in some hereditary malignancies, such as Li-Fraumeni Syndrome (Bell et al., 1999).
3. Effector of DNA damage signal
Apoptosis, or programmed cell death, is one of cell death mechanism which is essential for maintenance the genome stability. There are at least two apoptotic pathways. One is extrinsic pathway, the other is intrinsic pathway. p53 which mainly function as transcription factor, cell cycle regulator, and tumor suppressor is major regulator in response to apoptosis as well as autophagy. p53 is implicated in the induction of two distinct apoptotic signaling pathway that leads to the activation of the aspartate-specific cysteine proteases (caspases) that mediate apoptosis.
The extrinsic pathway involves engagement of particular death receptors that belong to the tumor necrosis factor receptor (TNF-R) family and, through the formation of the death-inducing-signaling-complex (DISC) (Ashkenazi and Dixit, 1998), leads to a cascade of activation of caspases, including caspase-8 and caspase-3 (Fig. 2.).
The intrinsic pathway is triggered by various DNA damage and is associated with mitochondrial depolarization by BAX, then released cytochrome c from the mitochondrial intermembrane space into the cytoplasm (Fig. 2.).
Table. 1. Potential roles of TIS21/BTG2/PC3
Potential function Reference
Transcriptional co-regulator
Modulate the activities of its interacting proteins
(Rouault et al., 1998; Prevot et al., 2001), (Morel et al., 2003; Duriez et al., 2004)
Neuronal differentiation factor Inhibit neuronal cell proliferation
(Canzoniere et al., 2004; Corrente et al., 2002; el-Ghissassi et al., 2002; Iacopetti et al., 1999) Survivor factor in PC12 cells
Differentiate neuroepithelial cells
(Canzoniere et al., 2004; el-Ghissassi et al., 2002; Wang et al., 2005; Calegari et al., 2002; Kosodo et al., 2004; Haubensak et al., 2004)
Stage-specific expansion of thymocyte and hematopoietic progenitors, Thymocyte differentiation, Expansion of hematopoietic progenitors
(Konrad and Zuniga-Pflucker, 2005; Oswald et al., 2006)
Tumor suppressor gene
Thymic carcinoma in SV40T mice, Renal cell carcinoma and prostate tumors, Regulate senescent phenotype in breast cancer
(Lim et al., 1995)
(Ficazzola et al., 2001; Struckmann et al., 2004; Ryu et al., 2004; Elmore et al., 2005; Kawakubo et al., 2004)
Pan-cell cycle regulator
G1/S arrest by pRB dependent manner, G1/S arrest by pRB independent manner, G2/M arrest and cell death in the p53 null U937 cells
(Guardavaccaro et al., 2000) (Lim et al., 1998)
(Ryu et al., 2004; Hong et al., 2005)
Developmental regulator
Vertebrate patterning in TIS21 knock out mice, Paraxial mesoderm development in zebrafish, Notochord development in Xenopus
(Park et al., 2004) (Sakaguchi et al., 2001) (Sugimoto et al., 2005)
II. PURPOSE
TIS21 was identified as tumor suppressor gene, and induced cell cycle arrest in G1/S or G2/M. TIS21 protein was markedly induced after DNA damage and there were few studies about TIS21 function during DNA damage response. This study was determined to investigate how TIS21 works to repair DNA damage induced by etoposide treatment.
III. MATERIALS & METHODS
A. Cell preparation
Huh7, Chang, HeLa, MCF7, and H9C2, which were human hepatoma, human normal liver, human cervical cancer, human breast cancer and rat myoblast cell line, respectively, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) from GIBCO-BRL (Grand island, NY). Wild type mouse embryo fibroblasts (MEFs) and TIS21-/- MEFs were prepared from 13.5 day old embryos of the wild type and TIS21-/- mice and then cultured in DMEM with 10% FBS.
B. Immunoblot analysis
Cell pellets were solubilized in 1 x protein sample buffer (0.35M Tris-HCl, pH 6.8, 10% SDS, 30% glycerol, 6% b-mercaptoethanol, 0.012% bromophenol blue). Samples were boiled for 5 to 10 minutes. The cell lysates were resolved on 8 to 13% SDS-PAGE in 25mM Tris-glycine buffer. The gel-separated proteins were then transferred to nitrocellulose membrane (Whatman, Germany). The membranes were blocked with 5% nonfat skim milk in phosphate-buffered saline (PBS) containing 0.05% Tween20 (PBST) for 1 hour. and then incubated with antibodies against p-ATMS1981 (Epitomics, CA), p-Chk2T68 (Cell signaling, MA), Chk2 (Millipore, MA), p-p53S15 (Cell signaling, MA), p-p53S20 (Cell signaling, MA), p53 (Santa Cruz Biotechonology, CA), gH2AX (Millipore, MA), p-NBS1S343 (Cell signaling, MA), BAX (Cell signaling, MA), PUMA (Santa Cruz Biotechonology, CA), NOXA (Santa Cruz Biotechonology, CA), p21Waf1/Cip1 (Santa Cruz Biotechonology, CA),
temperature for 1 hour, or at 4℃ for overnight.
C. Immunocytochemistry analysis
Huh7 cells were cultured on a cover glass and fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) at 4℃ for 20 min, and then washed 3 times with PBST. Cells were incubated with 1.5% horse serum in PBST for 1 hour at room temperature, and a primary antibody was then applied overnight at 4℃. The secondary antibody (1:500 in PBST) was applied for 1 hour. After incubating secondary antibody, DAPI were applied for 10 min and the cells were observed under fluorescence microscope (Carl Zeiss, Axioimager M1, x100 EC Plan-NEOFLUAR and AxioVision 4.5 software).
D. Comet assay
Huh7 cells were harvested by using a rubber policeman. The cells were scraped and centrifuged at 3000 rpm for 3min, and then counted to 1 x 105 cells/ml. Low melting point agarose (LMA) were prepared at 37℃, mixing with counted cells. The cells (1 x 103 cells) were put on the Cometslide (R&D systems, MN). The slides were placed flat to gel at 4℃ in the dark for 10 min, then immersed in prechilled lysis solution (R&D systems, MN) for 30 min. After discarding the lysis solution, the slides were immersed in fresh alkaline solution (NaOH pellets 0.6 g, 200 mM EDTA, pH 10 250 ml in DW 49.75 ml) for 40 min. For electrophoresis, the slides were washed with 1 x Tris borate acid electrophoresis buffer (TBE) twice, placing slides onto a gel tray and align equidistant from the electrodes. The slides were immersed with 1 x TBE buffer, and applied 30 voltage for 10 min. After electrophoresis, the slides were washed twice with DW and immersed slides in 70% ethanol
for 5 min, leaving it until it dry. Fifty ml of diluted SYBR green I were put on the gels. Every slide was confirmed in fluorescence microscopy (SYBR Green I’s maximum excitation and emission are 494 nm and 521 nm, respectively).
E. FACS analysis
Huh7 cells were prepared for two-dimensional flow cytometry by using propidium iodide (PI), a marker of DNA content. Cells were collected by centrifugation, resuspended in PBS, fixed by the addition of 70% ethanol at 4°C for 30 min, and then stored at -20°C pending preparation for analysis. Cells were then pelleted and resuspended in PBS and centrifuged at 2000 x g for 5 min. Cells were resuspended in solution A containing trypsin in citrate buffer and incubated for 10 min at room temperature. Cells in solution A were added to solution B containing trypsin inhibitor and ribonuclease A in citrate stabilizing buffer and then incubated for 10 min at room temperature. Finally, the cells in solutions A and B were added to solution C containing propidium iodide and spermine tetrahydrochloride in citrate stabilizing buffer, and were incubated for 10 mim at 4°C (Solution A, B and C : Becton Dickinson, Cycle TESTTM PLUS DNA REAGENT KIT, USA). Data were collected using a FACScan flow cytometer (Becton Dickinson). For each sample, 20,000 events were collected.
For analysis of cellular apoptosis, we used FITC Annexin V Apoptosis Detection Kit I (BD Pharmingentm, USA). Briefly, cells were washed twice with cold PBS and then resuspended cells in 1 x binding buffer at a concentration of 1x105 cells/100 ml. The cells were added to 5 ml FITC annexin V and 5 ml PI solution and then incubated for 15 min at RT
in the dark. The cells were added 300 ml of 1 x binding buffer to each tube and analyzed by flow cytometry within 1 hour.
F. Immunoprecipitation
Immunoprecipitation was performed using anti-PRMT1 and anti-HA antibodies with Ad-LacZ and Ad-TIS21-HA infected Huh7 cell lysates (500 mg) in immunoprecipitation buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% deoxycholic acid, 1% NP-40, 1 mM PMSF, and 1 mg/ml leupeptin. Whole cell lysates were pre-cleared with protein G-agarose beads (Invitrogen, Carlsbad, CA) for 1 hour at 4℃ before precipitation with the antibodies. The immunoprecipitaties were washed 3 times with immunoprecipitation buffer, and then performed immuneblot analysis with anti-MRE11, anti-PRMT1, and anti-HA antibodies respectively.
G. Caspase 3/7 activity assay
To determine caspase 3/7 activity on apoptosis, Caspase-Glo 3/7 assay (Promega, CA) was performed to Ad-LacZ and Ad-TIS21-HA infected Huh7 cells after treatment of 50 mM etoposide for 24 hours. Five thousand cells which were infected with LacZ or TIS21 adenovirus for 2 days were seeded in 96 wells plate and treated with 50 mM etoposide for 24 hours. Hundred ml of Caspase-Glo 3/7 reagent was added directly to the cells in 96-well plates and incubated for 1 hour before recording luminescence using TD-20/20 luminometer (Turner Designs, CA)
H. In vitro and In vivo methylation assay
In order to perform protein arginine methylation assay, we generated recombinant GST tagged PRMT1, MRE11, and TIS21. The recombinant proteins were purified in E. coli (BL21). GST tagged Mre11 recombinant protein were incubated with GST tagged PRMT1 with or without TIS21 at 25mM Tris-HCl, pH 7.4, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, and 160 μM S-[methyl-3H] Adenosyl-L-methionine, (Perkin Elmer, CA) at 37 °C for 1 hour, and then terminated by adding 6 x SDS sample buffer. Methylated Mre11 protein was examined autoradiography after SDS-PAGE.
In order to examine protein methylation in vivo, GST-tagged Mre11 plasmid transfected Huh7 cells were infected by Ad-LacZ and Ad-TIS21-HA for 2 days, and then incubated with cycloheximide (100 mg/ml) and chloroamphenicol (40 mg/ml) in DMEM for 30 min to inhibit neo-biosynthesis of proteins. The medium was then replaced with DMEM without methionine, supplemented with penicillin, streptomycin and containing 10 mCi of L-[methyl-3H] methionine (Perkin Elmer, CA) per ml as a methyl donor and cells were further incubated for an additional 3 hours in the presence of the same protein synthesis inhibitors. The cells were performed immunoprecipitation with anti-Mre11 antibody. After SDS-PAGE, the protein gel was incubated with amplify solution (GE Healthcare Biosciences, PA) for 1 hour, and it was dried and exposed to Kodak film at -70°C for a month.
I. Cloning of Chk2 and Mre11
To generate flag-tagged Chk2 and flag-tagged Mre11, human Chk2 and Mre11 were cloned from Huh7 hepatoma cells. cDNAs were amplified with sense 5’-AAAGAATTCAATGTCTCGGGAGTCGGA-3’ and anti-sense
5’-AAAGTCGACTCACAACACGCAGCACA-3’ for Chk2 and sense 5’-AAAGTCGACATGAGTACTGCAGAT-3’ and anti-sense 5’-AAGCGGCCGCTTATCTTCTATTTCT-3’ for Mre11 at 94OC 30 sec, 58OC 45 sec, 72OC 45 sec. Amplified PCR products were digested by EcoR1 and Sal I for Chk2 and Sal I and Not I for Mre11, respectively and then inserted to p3xFlag-CMV7.1 vector.
J. Statistical analysis
Numerical data were presented as mean + standard deviation (SD) of the independent determinations. The statistical analysis was performedwith Student's t test. Differenceswith
IV. RESULTS
A. The foci formation of gH2AX was depending upon TIS21 expression.
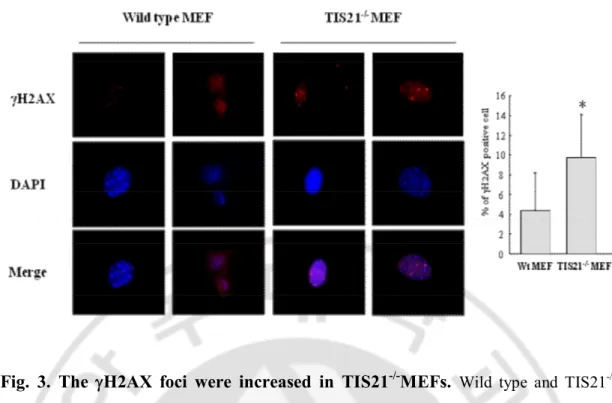
Many studies have reported that gH2AXS139 is one of the DNA damage markers. In order to check whether TIS21 is involved in DNA damage response, we examined spontaneous DNA damage signal in wild type and TIS21-/- MEFs by using immunocytochemistry assay with anti-gH2AX antibodies (Fig. 3.). Wild type MEFs expressed gH2AX in 4% of the cells (Fig. 3.), while TIS21-/- MEFs showed 10% of gH2AX expression (Fig. 3.). These data suggested that TIS21-/-MEFs were more spontaneously DNA damaged compared to wild type MEFs.
The foci formation of gH2AX was examined to determine whether TIS21 involved in DNA repair or not. In the presence of etoposide, foci formation of gH2AX was markedly increased in both of Ad-LacZ and Ad-TIS21-HA infected Huh7 cells. However, foci formation of gH2AX in Ad-TIS21-HA infected Huh7 cells were markedly decreased compared to Ad-LacZ infected Huh7 cells after etoposide treatment for 6 hours. In addition to that, the protein expression of gH2AX was performed by immunoblot analysis. gH2AX was rapidly phosphorylated in 1 hour. After 48 hour, gH2AX expression in Ad-TIS21-HA infected Huh7 cells was not detected, while gH2AX expression in Ad-LacZ infected Huh7 cells was still remained (Fig. 4.). When we removed etoposide after 1 hour by media change, gH2AX expression was markedly decreased in TIS21 infected cells at 24 hour after media change. These data indicated that TIS21 involved in DNA repair responses.
To determine DNA damage, comet assay, named single cell electrophoresis, were performed in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells. In the present of DNA
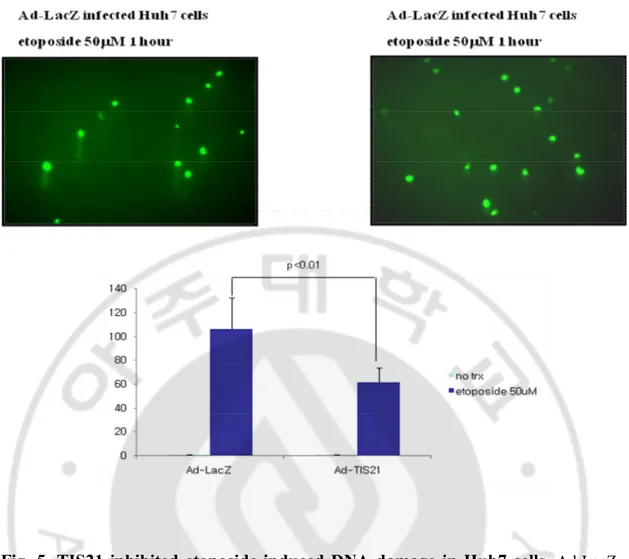
damage, each cells showed comet tails, and its length indicates how the cell be damaged. When we treated 50 mM of etoposide to Ad-LacZ and Ad-TIS21-HA infected Huh7 cells, the comet tails in Ad-LacZ infected Huh7 cells was longer than Ad-TIS21-HA infected Huh7 cells (Fig. 5.). These data indicated that Ad-TIS21-HA infected Huh7 cells were not sensitive to DNA damage drugs compared to Ad-LacZ infected Huh7 cells.
Fig. 3. The
gH2AX foci were increased in TIS21
-/-MEFs.
Wild type and TIS21 -/-MEFs were immunostained with anti-gH2AX antibody and DAPI staining. gH2AX foci and nucleus were strained by red color and blue color, respectively. Positive cells of gH2AX foci were counted and performed statistically. Total 300 cells were counted. The p value between wild type MEFs and TIS21-/-MEFs was less than 0.01.Fig. 4. The DNA damage repair induced by etoposide was increased by TIS21.
(A) Ad-LacZ and Ad-TIS21-HA infected Huh7 cells were treated by 20 mM etoposide for 1 hour and 6 hours, then immunocytochemistry analysis was performed with anti-gH2AX antibody. Merged images were shown. (B) gH2AX expression was examined in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells after treatment of 50 mM etoposide for 48 hours
. (C)
gH2AX expression was examined in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells after remove of etoposide after 1 hour.
Fig. 5. TIS21 inhibited etoposide induced DNA damage in Huh7 cells.
Ad-LacZ and Ad-TIS21-HA infected Huh7 cells were treated by 50 mM of etoposide for 1 hour. Electrophoresis were performed to 1,000 cells mixed with LMA on a comet slide in 1 x TBE buffer, then the slide was immersed with SYBR green I. After that, comet tails were measured under fluorescence microscopy.B. TIS21 blocked the Chk2-p53 signal pathway via inhibition of Chk2 phosporylation.
Next, we examined how TIS21 involved in DNA damage pathway during eotposide induced DSBs. In the presence of etoposide, Atacxia telangiectaxia (ATM), the highest upstream of double strand DNA breakages (DSBs), is rapidly activated in its Ser 1891 residue. When ATM is activated, downstream proteins such as gH2AX, p53, and Chk2 were also rapidly phosphorylated by ATM. We checked p-ATMS1891, p-p53S20, p-p53S15, and p-Chk2T68 in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells after treatment of 50 mM etoposide in time dependent manner (Fig. 6.). The phosphorylation level of ATMS1891 showed the same expression between both cells, suggesting that TIS21 does not effect to ATM activation. Surprisingly, Chk2 phophorylation in Ad-TIS21 infected Huh7 cells was dramatically decreased than Ad-LacZ infected Huh7 cells (Fig. 6.). ATM phosphorylates p53 at Ser 15 residue directly, however p53 at Ser 20 residue was phosphorylated by Chk2 kinase. In order to check whether TIS21 is involved in this pathway, the phosphorylation level of p53S15 and p53S20 were detected in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells after treatment 50 mM etoposide in time dependent manner (Fig. 6.). The phosphorylation level of p53S15 showed almost same expression between those cells, while the phosphorylation level of p53S20 in Ad-TIS21-HA infected Huh7 cells was not induced as compared to Ad-LacZ infected Huh7 cells, indicating that TIS21 down-regulates Chk2-p53 pathway. There are several Chk2 protein phosphorylation residues which can be phosphorylated by several kinases. The phosphorylation level of Chk2T68, Chk2T387, Chk2S33/35, andChk2S19 in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells were screened to examine effects of TIS21. The phosphorylation level of Chk2T68 was only affected by TIS21 overexpression (Fig. 7.). Next, we examined Chk1 phosphorylation in TIS21 infected cells,
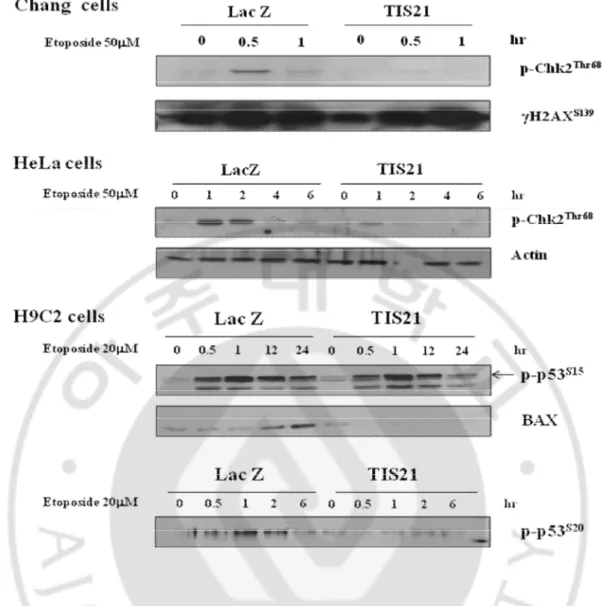
However, the phosphorylation level of Chk1S345 was not different between LacZ and TIS21 infected cells. In addition to that, the phosphorylation level of Chk2T68, p53S15, and p53S20 was confirmed in different cell lines such as Chang, HeLa, and H9C2 cells. The phosphorylation level of Chk2T68 and p53S20 but not p53S15 was downregulated in TIS21 infected cells (Fig. 8.).
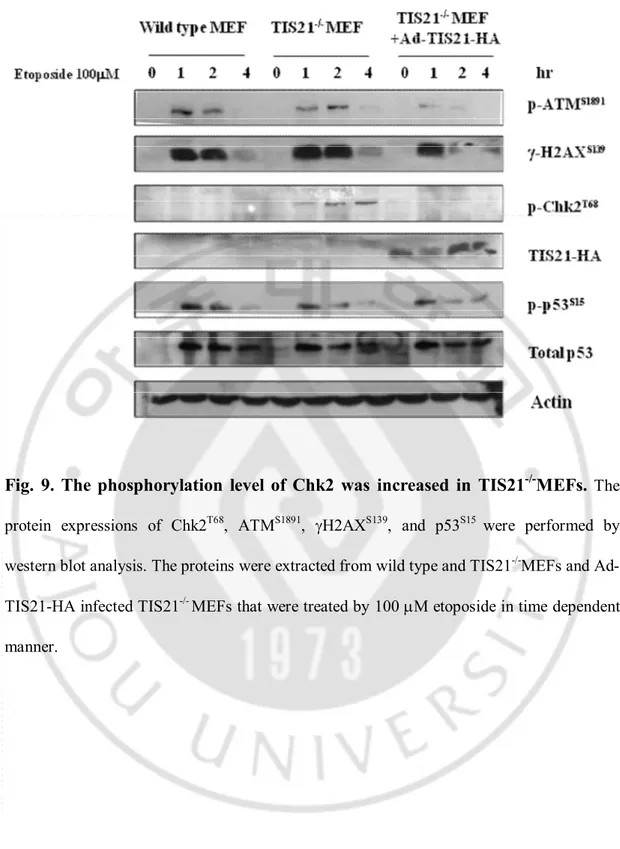
The phosphorylation level of ATM, gH2AX, p53 and Chk2 was examined in wild type MEFs, TIS21-/-MEFs, and Ad-TIS21-HA infected TIS21-/-MEFs after treatment of etoposide. As shown in Fig. 6, 7, only Chk2 phosphorlyation was not induced in Ad-TIS21-HA infected Huh7 cells, indicating that TIS21 downregulated phosphorylation of Chk2 in MEFs (Fig. 9.).
Fig. 6. TIS21 inhibited the phosphorylation levels of Chk2 at Thr68 residue and
p53 at Ser20 residue.
The protein expression of p-Chk2T68, p-p53S20, p-ATMS1891 and p-p53S15 were performed by western blot analysis. The proteins were extracted from Ad-LacZ and Ad-TIS21-HA infected Huh7 cells that were treated by 50 mM etoposide in time dependent manner.Fig. 7. TIS21 inhibited the phosphorylation level of Chk2 at Thr68 residue.
The phosphorylation levels of Chk2T68, Chk2T387, Chk2S33/35, Chk2S19, and Chk2S345 were examined after exposure to etoposide in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells.Fig. 8. The inhibition of Chk2 phosphorylation by TIS21 was confirmed in the
various cell lines.
The phosphorylation levels of Chk2T68 and p53S20 were confirmed by immunoblot analysis in Chang liver cells, HeLa cervical cancer cells, and H9C2 rat cardiomyoblast cells.Fig. 9. The phosphorylation level of Chk2 was increased in TIS21
-/-MEFs.
The protein expressions of Chk2T68, ATMS1891, gH2AXS139, and p53S15 were performed by western blot analysis. The proteins were extracted from wild type and TIS21-/-MEFs and Ad-TIS21-HA infected TIS21-/- MEFs that were treated by 100 mM etoposide in time dependent manner.C. TIS21 blocked apoptosis via inhibition of p53-dependent-proapoptotic proteins.
As we mentioned above, the phosphorylation level of p53 at Ser 20 residue was down-regulated by TIS21 expression. To determine whether TIS21 is involved in p53-dependent apoptosis, we examined the expression of p53-dependent pro-apoptotic protein such as PUMA, p21, and BAX. Pro-apoptotic proteins were markedly increased in Ad-LacZ infected cells after etoposide treatment, however, TIS21 expressed cells did not show pro-apoptotic protein induction. The protein level of p53 and BAX in Ad-TIS21-HA infected MCF7 cells was not induced compared to Ad-LacZ infected MCF7 (Fig. 10.). These data indicated that TIS21 inhibited induction of BAX, p21, and NOXA via inhibition of Chk2-p53 mediated apoptotic pathway.
To determine etoposide induced apoptosis, caspase 3 activation and PARP cleavage were analyzed by immunoblot. Cleaved form of caspase 3 and PARP in Ad-LacZ infected Huh7 was significantly induced in time dependent manner than Ad-TIS21-HA infected Huh7 after treatment of etoposide (Fig. 11.). Caspase 3/7 activity was also measured in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells. Caspase 3/7 activity was induced by etoposide treatment for 24 hours, however caspase 3/7 activity in Ad-TIS21-HA infected Huh7 cells was not induced compared to Ad-LacZ infected Huh7 cells (Fig. 12.). FACS analysis showed that annexin V stained cells were more increased in Ad-LacZ infected cells (Fig. 13.). To confirm apoptosis in p53 wild type cell line, H9C2 cells were infected by Ad-LacZ and Ad-TIS21-HA, then treated with 20 mM etoposide for 8, 12, 24 hours. Apoptotic cells were strained by Hoechst 33258 to detect chromatin condensation. Apoptosis was markedly inhibited in TIS21 infected H9C2 cells (Fig. 14.).
Fig. 10. The expression levels of BAX, p21
Waf1/Cip1, and NOXA, were decreased
by TIS21.
The protein expression of BAX, p21Waf1/Cip1, and NOXAwas determined by western blot analysis. The proteins were extracted from Ad-LacZ and Ad-TIS21-HA infected Huh7 and MCF7 cells that were treated by 50 mM etoposide in time dependent manner.Fig. 11. The cleavage of PARP and caspase 3 was decreased by TIS21.
The protein expression of cleaved PARP and cleaved caspase 3 was detected in Ad-LacZ infected and Ad-TIS21-HA infected Huh7 cells after 50 mM etoposide in time dependent manner.Fig. 12. The activities of caspase 3/7 were decreased by TIS21.
LacZ and Ad-TIS21-HA infected Huh7 cells were treated by 50 mM etoposide for 24 hours. Caspase 3/7 activity in both cells was measured in luminescence after incubating 1 hour with Caspase-Glo 3/7 reagent.Fig. 13. Etoposide-induced apoptosis was attenuated by TIS21 overexpression in
Huh7 cells.
Apoptosis was evaluated after treatment with 50 mM etoposide for 24 hours, and staining with annexin V. Flow cytometry profile represents annexin-V-FITC staining inFig. 14. Etoposide-induced apoptosis was attenuated by TIS21 overexpression in
H9C2 cells.
(A) The pictures were taken to examine the apoptotic cells in Ad-LacZ and Ad-TIS21-HA infected H9C2 rat cardiomyoblast cells after treatment of etoposide for 8 and 12 hours. (B) Chromatin condensation were stained with hoechst 33258 and counted in Ad-LacZ and Ad-TIS21-HA infected H9C2 cells. White arrows showed chromatin condensation cells. Total 800 cells were counted.D. TIS21 inhibited Chk2 phosphorylation through regulation of Mre11.
TIS21 was well known as a PRMT1 regulator. TIS21 can bind to PRMT1 and regulated its activity. PRMT1 methylates DNA damage response proteins Mre11 and 53BP1. Mre11 form a complex with RAD50 and NBS1 and is referred to as the MRN complex. Recently, PRMT1 has been shown to regulate Mre11 and 53BP1. To determine the interaction of TIS21 with PRMT1 and Mre11, immunoprecipitation assay was performed. PRMT1 was pulled by anti-PRMT1 antibody in Ad-LacZ and Ad-TIS21-HA infected Huh7 cells, then performed immunoblot analysis to detect Mre11 and TIS21. Figure 15 showed that PRMT1 interacts with Mre11 and TIS21. To determine the function of their interaction, in vitro methylation assay was performed. In the presence of recombinant TIS21, the methylation of Mre11 protein was markedly increased (Fig. 16.). Furthermore, the methylation of Mre11 protein was increased in TIS21 infected Huh7 cells (Fig. 16.).
When we transfected Mre11 transiently and treated with etoposide in Huh7 cells, the phosphorylation of Chk2T68 was markedly decreased, these data indicated that Mre11 overexpression decreased DNA damaging signal (Fig. 17). These results suggested that TIS21 induced Mre11 methylation via regulation of PRMT1 activity and increased Mre11 activity.
Fig. 15. PRMT1 bound to Mre11 and TIS21.
PRMT1 was pulled down with anti-PRMT1 antibody from Ad-LacZ and Ad-TIS21-HA infected Huh7 cells in the absence or presence of etoposide, and then immunoblot was performed with anti PRMT1, Mre11 and HA antibodies.Fig. 16. TIS21 increased the methylation of Mre11 through binding PRMT1.
(A, B) We generated recombinant proteins of GST-MRE11, GST-PRMT1, and GST-TIS21 fromE. coli to examine methylation assay. The recombinant proteins, MRE11 and
GST-PRMT1, were incubated with or without GST-TIS21 recombinant protein. (C) Flag-Mre11 was transfected to Ad-LacZ and Ad-TIS21-HA infected Huh7 cells, and then performed immunoprecipitation assay. Figure B, C and upper band of A were autoradiography and the lower band of A was coomassie brilliant blue staining.
Fig. 17. The phosphorylation level of Chk2 was inhibited by TIS21 and Mre11
overexpression. (A) Flag vector and Flag-tagged Mre11 were transfected to
Ad-LacZ infected Huh7 cells. (B) Flag vector and Flag-tagged Mre11 were transfected
to TIS21 adenovirus infected Huh7 cells.
V. DISCUSSTION
DSBs induced by any exogenous agent such as IR, UV, or topoisomerase II inhibitor such as etoposide are recognized by the DNA repair machinery. Etoposide is anti-cancer drug. It inhibits the DNA topoisomerase II which unwinds DNA, and by doing so causes DNA strands to break.
In this study, we wanted to clarify whether TIS21 was involved in etoposide induced DNA damage. First, we observed spontaneous gH2AX foci positive cells in wild type MEFs and TIS21-/-MEFs (Fig. 3.). Wild type MEFs and TIS21-/-MEFs were stained by gH2AX and DAPI, counting gH2AX foci positive cells. And we also examined the DNA repair capacity using by retention of gH2AX foci formation in time dependent manner. After 6 hours, gH2AX foci in Ad-TIS21-HA infected Huh7 cells were significantly decreased, meaning DNA damage recovered, while Ad-LacZ infected Huh7 cells still had foci of gH2AX (Fig. 4.). In addition to that, we used comet assay as DNA damage marker in LacZ and Ad-TIS21-HA infected Huh7 cells. Ad-LacZ and Ad-Ad-TIS21-HA cells were treated with etoposide for 1 hour, then comet tails were measured. Figure 5 data indicated that TIS21 have DNA repair function as regulator in DNA damage signal.
To clarify how TIS21 involved in DNA damage signal pathway, we examined the phosphorylation level such as ATM, Chk2, and p53 which were involved in DNA damage signal pathway (Fig. 1.). ATM and ATR directly phosphorylates p53 on Ser 15 (Banin et al., 1998; Canman et al., 1998) and mediate the phosphorylation of p53 on additional residues through the activation of other kinases, such as Chk1, Chk2 (Chehab et al., 2000; Hirao et al., 2000; Shieh et al., 2000). Our data showed that TIS21 suppressed Chk2-p53 phosphorylation
but ATM, gH2AX, and the other residue of p53 in p53 mutant harboring Huh7 cells (Fig. 6.). The amino-terminal domain of Chk2 contains a series of seven serine or threonine residues (Ser19, Thr26, Ser28, Ser33, Ser35, Ser50 and Thr68) each followed by glutamine (SQ or TQ motif). These are known to be preferred sites for phosphorylation by ATM/ATR kinases (Kastan and Lim, 2000; Matsuoka et al., 2000). After DNA damage by ionizing radiation (IR), UV irradiation or hydroxyurea treatment, Thr68 and other sites in this region become phosphorylated by ATM/ATR (Ahn et al., 2000; Matsuoka et al., 2000; Melchionna et al., 2000). The SQ/TQ cluster domain, therefore, seems to have a regulatory function. Phosphorylation at Thr68 of Chk2 is a prerequisite for the subsequent activation step, which is attributable to autophosphorylation of Chk2 on residues Thr383 and Thr387 in the activation loop of the kinase domain (Lee and Chung, 2001).We examined a series of five serine of threonine residue (Ser19, Ser33, Ser35, Thr68, and Thr387) in LacZ and Ad-TIS21-HA infected Huh7 cells. Six residues except of Thr68 were shown same phosphorylation level in both cells. And we examined whether TIS21 inhibited Chk2-p53 phosphorylation in wild type p53 harboring cells, such as MEF, Chang, HeLa, and H9C2 cell lines. Figure 8 data showed that inhibition of Chk2 phosphorylation by TIS21 expression was independent of p53. It was expected that TIS21 might regulate p53-dependent-apoptosis, because previous study has reported that mutation Ser 20 to Ala of p53 do not induce apoptosis compared to wild type (Unger et al., 1999). To confirm these phenomena in wild type MEFs, TIS21-/- MEFs, and Ad-TIS21-HA infected TIS21-/- MEFs, the phosphorylation level of ATM, gH2AX, Chk2, and p53 was detected using by immunoblot analysis (Fig. 9.). Only Chk2T68 phosphorylation in MEFs was inhibited depending upon TIS21 expression. These data indicated that TIS21 blocked Chk2-p53
pathway in MEFs, as well as p53-mediated apoptosis. To prove that, we examined the protein expression levels of p53’s downstream such BAX, p21Waf1/Cip1, and NOXA (Fig. 10.). NOXA and PUMA are two BH3-only proapoptotic proteins that act upstream of BAX/BAK to promote mitochondrial depolarization. NOXA is essentially thought to sensitize cells to the action of activator BH3-only pro-apoptotic proteins by disrupting their interaction with anti-apoptotic proteins (Kim et al., 2006). BAX, p21Waf1/Cip1, and NOXA were dramatically induced in Ad-LacZ infected Huh7 cells during etoposide induced cell death, however the level of the proteins in TIS21-HA infected Huh7 cells were not induced compared to Ad-LacZ infected Huh7 cells. These data indicated that TIS21 partially blocked p53-mediated transcription (Fig. 10.). To determine cellular apoptosis, we applied several apoptotic markers such as PARP cleavage, caspase activation, and annexin V & PI staining method as mentioned above. The enzyme poly (ADP-ribose) polymerase was one of the first proteins identified as a substrate for caspases. PARP is involved in repair of DNA damage and functions by catalyzing the synthesis of poly (ADP-ribose) and by binding to DNA strand breaks and modifying nuclear proteins. The ability of PARP to repair DNA damage is prevented following cleavage of PARP by caspase 3. PARP cleavage and caspase 3 activation in Ad-TIS21-HA infected Huh7 cells were not induced compared to Ad-LacZ infected Huh7 cells. Annexin V is used as a probe in the annexin V affinity assay to detect cells that have expressed phosphatidylserine on the cell surface, a feature found in apoptosis as well as other forms of cell death. Propidium iodide (PI) is membrane impairment and generally excluded from viable cells. PI is commonly used for identifying dead cells in a population and as a counterstaining in multicolor fluorescent techniques. Annexin V positive and PI negative indicates that status of the cells is early apoptosis, and both annexin V & PI
positive indicates that the cells are apoptotic status. We measured annexin V & PI positive cells using flow cytometry in Ad-LacZ and HA infected Huh7 cells. Ad-TIS21-HA infected Huh7 cells showed less shifted than Ad-LacZ infected Huh7 cells (Fig. 13.). These data indicated that TIS21 certainly blocked etoposide-induced apoptosis.
We examined how TIS21 inhibited Chk2 phosphorylation. At first we examined whether TIS21 interacted with Chk2. However, TIS21 could not bind TIS21 directly. Next, we examined Chk2 upstream protein, such as ATM, 53BP1 and MRN complex. TIS21 was well known PRMT1 regulator. PRMT1 could methylate DNA damage response proteins Mre11 and 53BP1. Mre11 form a complex with RAD50 and NBS1 and is referred to as the MRN complex. Recently, PRMT1 has been shown to regulate Mre11 and 53BP1. Mre11 was markedly methylated by PRMT1 and methylated Mre11 has fully activity. TIS21 can bind to PRMT1 and regulated its activity. TIS21 interacted with PRMT1 and Mre11 furthermore, TIS21 induced Mre11 protein methylation in vitro and in vivo also. These data suggested that TIS21 involved in DNA damage signaling. When we transfected Mre11 cDNA and treated etoposide, Chk2 phosphorylation was markedly decreased. Mre11 has exonuclease activity and involve homologous recombination DNA repair. These data suggested that Mre11 overexpression induced homologous recombination DNA repair activity and decreased DNA damage signal such as p-Chk2 and p-p53. In the present study, we examined that TIS21 increased Mre11 protein methylation in vitro and in vivo. These data suggested that TIS21 upregulated Mre11 homologous recombination DNA repair activity and resulted in decreasing of DNA damage signaling.
VI. CONCLUSION
In the present study, downregulation of p-Chk2T68 and p-p53S20 in Ad-TIS21-HA infected Huh7 cells after treatment of etoposide were observed during DNA damage signal. It was also confirmed in several different cell lines. Inhibition of p-Chk2T68 caused downregulation of p53-dependent-apoptotic protein such as BAX, p21Waf1/Cip1, and NOXA in the same condition, leading to anti-apoptotic responses. We confirmed the apoptosis by using apoptotic marker such as cleaved form of caspase3, PARP, caspase3/7 activity, FACS, and chromatin condensation. Furthermore, TIS21 induced Mre11 protein methylation and we thought TIS21 increased Mre11 DNA repair activity.
Therefore, TIS21 involved DNA damage pathway. However, it is still more identified of which TIS21 inhibits Chk2 phosphorylation.
REFERENCES
1.
Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR
kinases. Genes Dev 15, 2177-2196, 2001
2. Ahn, J.Y., Schwarz, J.K., Piwnica-Worms, H., and Canman, C.E. Threonine 68
phosphorylation by ataxia telangiectasia mutated is required for efficient
activation of Chk2 in response to ionizing radiation. Cancer Res 60, 5934-5936,
2000
3. Ashkenazi, A., and Dixit, V.M. Death receptors: signaling and modulation.
Science 281, 1305-1308, 1998
4. Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C.W., Chessa, L.,
Smorodinsky, N.I., Prives, C., Reiss, Y., Shiloh, Y., et al. Enhanced
phosphorylation of p53 by ATM in response to DNA damage. Science 281,
1674-1677, 1998
5. Bartek, J., and Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer.
Cancer Cell 3, 421-429, 2003
6. Bell, D.W., Varley, J.M., Szydlo, T.E., Kang, D.H., Wahrer, D.C., Shannon, K.E.,
Lubratovich, M., Verselis, S.J., Isselbacher, K.J., Fraumeni, J.F., et al.
Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science
286, 2528-2531, 1999
7. Berthet, C., Guehenneux, F., Revol, V., Samarut, C., Lukaszewicz, A., Dehay, C.,
Dumontet, C., Magaud, J.P., and Rouault, J.P. Interaction of PRMT1 with
BTG/TOB proteins in cell signalling: molecular analysis and functional aspects.
Genes Cells 7, 29-39, 2002
8. Bradbury, A., Possenti, R., Shooter, E.M., and Tirone, F. Molecular cloning of
PC3, a putatively secreted protein whose mRNA is induced by nerve growth
factor and depolarization. Proc Natl Acad Sci U S A 88, 3353-3357, 1991
9. Calegari, F., Haubensak, W., Yang, D., Huttner, W.B., and Buchholz, F.
Tissue-specific RNA interference in postimplantation mouse embryos with
endoribonuclease-prepared short interfering RNA. Proc Natl Acad Sci U S A 99,
14236-14240, 2002
10. Canman, C.E., Lim, D.S., Cimprich, K.A., Taya, Y., Tamai, K., Sakaguchi, K.,
Appella, E., Kastan, M.B., and Siliciano, J.D. Activation of the ATM kinase by
ionizing radiation and phosphorylation of p53. Science 281, 1677-1679, 1998
11. Canzoniere, D., Farioli-Vecchioli, S., Conti, F., Ciotti, M.T., Tata, A.M.,
Augusti-Tocco, G., Mattei, E., Lakshmana, M.K., Krizhanovsky, V., Reeves,
S.A., et al. Dual control of neurogenesis by PC3 through cell cycle inhibition
and induction of Math1. J Neurosci 24, 3355-3369, 2004
12. Chehab, N.H., Malikzay, A., Appel, M., and Halazonetis, T.D. Chk2/hCds1
functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev
14, 278-288, 2000
13. Corrente, G., Guardavaccaro, D., and Tirone, F. PC3 potentiates NGF-induced
differentiation and protects neurons from apoptosis. Neuroreport 13, 417-422,
2002
14. Deng, C.X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage
response and cancer evolution. Nucleic Acids Res 34, 1416-1426, 2006
15. Durocher, D., and Jackson, S.P. DNA-PK, ATM and ATR as sensors of DNA
damage: variations on a theme? Curr Opin Cell Biol 13, 225-231, 2001
16. el-Ghissassi, F., Valsesia-Wittmann, S., Falette, N., Duriez, C., Walden, P.D.,
and Puisieux, A. BTG2(TIS21/PC3) induces neuronal differentiation and
prevents apoptosis of terminally differentiated PC12 cells. Oncogene 21,
6772-6778, 2002
17. Elmore, L.W., Di, X., Dumur, C., Holt, S.E., and Gewirtz, D.A. Evasion of a
single-step, chemotherapy-induced senescence in breast cancer cells:
implications for treatment response. Clin Cancer Res 11, 2637-2643, 2005
18. Ficazzola, M.A., Fraiman, M., Gitlin, J., Woo, K., Melamed, J., Rubin, M.A.,
and Walden, P.D. Antiproliferative B cell translocation gene 2 protein is
down-regulated post-transcriptionally as an early event in prostate carcinogenesis.
Carcinogenesis 22, 1271-1279, 2001
19. Fletcher, B.S., Lim, R.W., Varnum, B.C., Kujubu, D.A., Koski, R.A., and
Herschman, H.R. Structure and expression of TIS21, a primary response gene
induced by growth factors and tumor promoters. J Biol Chem 266, 14511-14518,
1991
20. Guardavaccaro, D., Corrente, G., Covone, F., Micheli, L., D'Agnano, I., Starace,
G., Caruso, M., and Tirone, F. Arrest of G(1)-S progression by the
p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1
transcription. Mol Cell Biol 20, 1797-1815, 2000
21. Haubensak, W., Attardo, A., Denk, W., and Huttner, W.B. Neurons arise in the
basal neuroepithelium of the early mammalian telencephalon: a major site of
neurogenesis. Proc Natl Acad Sci U S A 101, 3196-3201, 2004
22. Hirao, A., Kong, Y.Y., Matsuoka, S., Wakeham, A., Ruland, J., Yoshida, H.,
Liu, D., Elledge, S.J., and Mak, T.W. DNA damage-induced activation of p53
by the checkpoint kinase Chk2. Science 287, 1824-1827, 2000
23. Hong, J.W., Ryu, M.S., and Lim, I.K. Phosphorylation of serine 147 of
tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death.
J Biol Chem 280, 21256-21263, 2005
24. Iacopetti, P., Michelini, M., Stuckmann, I., Oback, B., Aaku-Saraste, E., and
Huttner, W.B. Expression of the antiproliferative gene TIS21 at the onset of
neurogenesis identifies single neuroepithelial cells that switch from proliferative
to neuron-generating division. Proc Natl Acad Sci U S A 96, 4639-4644, 1999
25. Kastan, M.B., and Bartek, J. Cell-cycle checkpoints and cancer. Nature 432,
26. Kastan, M.B., and Lim, D.S. The many substrates and functions of ATM. Nat
Rev Mol Cell Biol 1, 179-186, 2000
27. Kawakubo, H., Carey, J.L., Brachtel, E., Gupta, V., Green, J.E., Walden, P.D.,
and Maheswaran, S. Expression of the NF-kappaB-responsive gene BTG2 is
aberrantly regulated in breast cancer. Oncogene 23, 8310-8319, 2004
28. Kim, H., Rafiuddin-Shah, M., Tu, H.C., Jeffers, J.R., Zambetti, G.P., Hsieh, J.J.,
and Cheng, E.H. Hierarchical regulation of mitochondrion-dependent apoptosis
by BCL-2 subfamilies. Nat Cell Biol 8, 1348-1358, 2006
29. Konrad, M.A., and Zuniga-Pflucker, J.C. The BTG/TOB family protein TIS21
regulates stage-specific proliferation of developing thymocytes. Eur J Immunol
35, 3030-3042, 2005
30. Kosodo, Y., Roper, K., Haubensak, W., Marzesco, A.M., Corbeil, D., and
Huttner, W.B. Asymmetric distribution of the apical plasma membrane during
neurogenic divisions of mammalian neuroepithelial cells. EMBO J 23,
2314-2324, 2004
31. Lee, C.H., and Chung, J.H. The hCds1 (Chk2)-FHA domain is essential for a
chain of phosphorylation events on hCds1 that is induced by ionizing radiation.
J Biol Chem 276, 30537-30541, 2001
32. Lieber, M.R., Ma, Y., Pannicke, U., and Schwarz, K. Mechanism and regulation
of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 4, 712-720,
2003
33. Lim, I.K. TIS21 (/BTG2/PC3) as a link between ageing and cancer: cell cycle
regulator and endogenous cell death molecule. J Cancer Res Clin Oncol 132,
417-426, 2006
34. Lim, I.K., Lee, M.S., Lee, S.H., Kim, N.K., Jou, I., Seo, J.S., and Park, S.C.
Differential expression of TIS21 and TIS1 genes in the various organs of Balb/c
mice, thymic carcinoma tissues and human cancer cell lines. J Cancer Res Clin
35. Lim, I.K., Lee, M.S., Ryu, M.S., Park, T.J., Fujiki, H., Eguchi, H., and Paik,
W.K. Induction of growth inhibition of 293 cells by downregulation of the
cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21.
Mol Carcinog 23, 25-35, 1998
36. Liu, Y., Masson, J.Y., Shah, R., O'Regan, P., and West, S.C. RAD51C is
required for Holliday junction processing in mammalian cells. Science 303,
243-246, 2004
37. Matsuoka, S., Huang, M., and Elledge, S.J. Linkage of ATM to cell cycle
regulation by the Chk2 protein kinase. Science 282, 1893-1897, 1998
38. Matsuoka, S., Rotman, G., Ogawa, A., Shiloh, Y., Tamai, K., and Elledge, S.J.
Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc
Natl Acad Sci U S A 97, 10389-10394, 2000
39. Melchionna, R., Chen, X.B., Blasina, A., and McGowan, C.H. Threonine 68 is
required for radiation-induced phosphorylation and activation of Cds1. Nat Cell
Biol 2, 762-765, 2000
40. Morel, A.P., Sentis, S., Bianchin, C., Le Romancer, M., Jonard, L., Rostan,
M.C., Rimokh, R., and Corbo, L. BTG2 antiproliferative protein interacts with
the human CCR4 complex existing in vivo in three cell-cycle-regulated forms. J
Cell Sci 116, 2929-2936, 2003
41. O'Driscoll, M., and Jeggo, P.A. The role of double-strand break repair - insights
from human genetics. Nat Rev Genet 7, 45-54, 2006
42. Olive, P.L. The role of DNA single- and double-strand breaks in cell killing by
ionizing radiation. Radiat Res 150, S42-51, 1998
43. Oswald, J., Steudel, C., Salchert, K., Joergensen, B., Thiede, C., Ehninger, G.,
Werner, C., and Bornhauser, M. Gene-expression profiling of CD34+
hematopoietic cells expanded in a collagen I matrix. Stem Cells 24, 494-500,
2006
44. Park, S., Lee, Y.J., Lee, H.J., Seki, T., Hong, K.H., Park, J., Beppu, H., Lim,
I.K., Yoon, J.W., Li, E., et al. B-cell translocation gene 2 (Btg2) regulates
vertebral patterning by modulating bone morphogenetic protein/smad signaling.
Mol Cell Biol 24, 10256-10262, 2004
45. Pellegrini, L., Yu, D.S., Lo, T., Anand, S., Lee, M., Blundell, T.L., and
Venkitaraman, A.R. Insights into DNA recombination from the structure of a
RAD51-BRCA2 complex. Nature 420, 287-293, 2002
46. Prevot, D., Morel, A.P., Voeltzel, T., Rostan, M.C., Rimokh, R., Magaud, J.P.,
and Corbo, L. Relationships of the antiproliferative proteins BTG1 and BTG2
with CAF1, the human homolog of a component of the yeast CCR4
transcriptional complex: involvement in estrogen receptor alpha signaling
pathway. J Biol Chem 276, 9640-9648, 2001
47. Rouault, J.P., Prevot, D., Berthet, C., Birot, A.M., Billaud, M., Magaud, J.P.,
and Corbo, L. Interaction of BTG1 and p53-regulated BTG2 gene products with
mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional
regulatory complex. J Biol Chem 273, 22563-22569, 1998
48. Ryu, M.S., Lee, M.S., Hong, J.W., Hahn, T.R., Moon, E., and Lim, I.K.
TIS21/BTG2/PC3 is expressed through PKC-delta pathway and inhibits binding
of cyclin B1-Cdc2 and its activity, independent of p53 expression. Exp Cell Res
299, 159-170, 2004
49. Sakaguchi, T., Kuroiwa, A., and Takeda, H. Expression of zebrafish btg-b, an
anti-proliferative cofactor, during early embryogenesis. Mech Dev 104, 113-115,
2001
50. Shieh, S.Y., Ahn, J., Tamai, K., Taya, Y., and Prives, C. The human homologs
of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple
DNA damage-inducible sites. Genes Dev 14, 289-300, 2000
51. Struckmann, K., Schraml, P., Simon, R., Elmenhorst, K., Mirlacher, M.,
Kononen, J., and Moch, H. Impaired expression of the cell cycle regulator
BTG2 is common in clear cell renal cell carcinoma. Cancer Res 64, 1632-1638,
2004
52. Sugimoto, K., Hayata, T., and Asashima, M. XBtg2 is required for notochord
differentiation during early Xenopus development. Dev Growth Differ 47,
435-443, 2005
53. Sukhatme, V.P., Kartha, S., Toback, F.G., Taub, R., Hoover, R.G., and
Tsai-Morris, C.H. A novel early growth response gene rapidly induced by fibroblast,
epithelial cell and lymphocyte mitogens. Oncogene Res 1, 343-355, 1987
54. Unger, T., Juven-Gershon, T., Moallem, E., Berger, M., Vogt Sionov, R.,
Lozano, G., Oren, M., and Haupt, Y. Critical role for Ser20 of human p53 in the
negative regulation of p53 by Mdm2. EMBO J 18, 1805-1814, 1999
55. Wang, Y., Shao, C., Shi, C.H., Zhang, L., Yue, H.H., Wang, P.F., Yang, B.,
Zhang, Y.T., Liu, F., Qin, W.J., et al. Change of the cell cycle after flutamide
treatment in prostate cancer cells and its molecular mechanism. Asian J Androl 7,
375-380, 2005
56. Wyman, C., Ristic, D., and Kanaar, R. Homologous recombination-mediated
double-strand break repair. DNA Repair (Amst) 3, 827-833, 2004
- 국문요약 -
TIS21 이 Etoposide 에 의해 유도되는 DNA 손상 후
수복기작에 미치는 영향 연구
아주대학교 대학원 의생명과학과
최규성
(지도교수: 박태준)
TIS21/BTG2/PC3 (12-O-tetradecanoyl phorbol-13-acetate-inducible
sequence 21) 은 p53 의 타깃 유전자로써 갑상선, 전립선, 신장 및 간에서의 암 발생과정 중에서 암을 억제하는 기능을 하는 것으로 알려져 있다. 또한 암을 치료하기 위한 화학요법에 의해 발생하는 세포사멸 중 TIS21 의 발현됨을 관찰함으로써, 세포사멸 과정 중의 TIS21 이 중요시되었다. 이 연구는 암 억제유전자인 TIS21 이 etoposide 에 의해 일어나는 세포 사멸조절 기작의 관여에 대해 연구하기 위하여 실시되었다. DNA 손상의 마커인 gH2AX 의 foci 형성을 wild type MEF 와 TIS21
-/-mouse embryo fibroblasts(MEFs)에서 관찰 해 본 결과 TIS21-/- MEFs 에서 gH2AX foci
형성되는 세포들이 wild type MEFs 보다 더 많이 관찰 되었으며, 이는 TIS21 이 DNA 수복 기작에 관여를 한다는 것을 의미하였다. 다음으로 DNA 손상 마커인 single cell electrophoresis 라고 불리는 comet assay 를 시행 하였다. Etoposide 를 사용하여 adenovirus LacZ 와 TIS21 이 과발현 된 간암세포주인 Huh7 세포에 DNA 손상을 주었으며, 이때 TIS21 이 과발현 된 세포에서의 comet tail 이 더 짧은 것으로 보아, TIS21 이 DNA 수선 역할을 할 수 있다는 증거를 관찰하였다. 다음으로 DNA 손상 시그널에 TIS21 이 관여하는지를 연구하기 위해, ATM-mediated DNA 수복과 p53-mediated apoptosis 에 관여되는 단백질들의 발현과 인산화 정도를 관찰하였다. Chk2 의 Thr68 잔기의 인산화는 DNA 손상 후 시그널을 하위로 내려 보내는 중요한 단백질로 Ad-TIS21-HA 가 과발현된 세포에서 Thr68 잔기의 인산화와 Chk2 의 직접적인 하위 시그널인 p53 의 Ser20 잔기의 인산화가 Ad-LacZ 가 과 발현 된 세포보다 적은 것을 관찰 하였다. p53 의 인산화는 단백질의 안정화 및 전사조절에 중요한 역할을 하며, 나아가 세포주기 억제와 세포사멸에 영향을 미치게 된다. Wild type p53 을 발현하는 MCF7, HeLa, H9C2 와 같은 다양한 세포주에서도 동일한 현상을 관찰하여 mutant p53 을 가지고 있는 Huh7 에서 관찰되는 Chk2, p53 인산화의 저해가 p53 비 의존적으로 관찰됨을 알게 되었다.
p53 의 Ser20 잔기 인산화의 감소가 세포사멸 기작에 미치는 영향을 연구하기 위해 BAX, p21Waf1/Cip1, NOXA 와 같은 세포사멸에 관계된 단백질 발현을
관찰해본 결과, TIS21 이 과발현 세포에서 세포사멸에 관계된 단백질의 발현이 감소 되어있는 것을 관찰하였다. 또한 caspase 3/7 활성도 및 annexin V 염색이 TIS21 과 발현 세포에서 감소함을 관찰하였다. TIS21 은 protein arginine methyltransferase 1(PRMT1) 과 결합을 통하여 PRMT1 의 활성도를 조절한다는 보고를 바탕으로 TIS21 이 과 발현되어있을 때 PRMT1 의 기질 중 DNA 수선에 관여하는 Mre11 의 메칠화가 증가하는 것을 관찰 하였다. Mre11 의 과 발현이 또한 Chk2 의 인산화를 억제하는 것으로 관찰되어 TIS21 이 Mre11 의 메칠화를 통해 Chk2 의 인산화에 영향을 미친다는 것을 관찰 하였다. TIS21 은 Mre11 과의 결합 및 메칠화를 통해 Chk2 의 인산화를 억제하는 것으로 보아 Chk2-p53 를 통한 세포 사멸을 억제하며 DNA 수복에 관여 한다는 결론을 낼 수 있다. 핵심어: ATM, Chk2, BTG2, TIS21, Mre11, DNA damage, apoptosis