l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in Medicine
Transcription Factor HOXA9 is Linked to
the Calcification and Invasion of
Papillary Thyroid Carcinoma
Graduate School of Ajou University
Department of Endocrinology and Metabolism
Yilan Jin
Transcription Factor HOXA9 is Linked to
the Calcification and Invasion of
Papillary Thyroid Carcinoma
Yoon-Sok Chung, M.D., Ph.D., Advisor
I submit this thesis as the
Doctoral thesis in Medicine
February 2020
Graduate School of Ajou University
Department of Endocrinology and Metabolism
Yilan Jin
This certifies that the dissertation
of Yilan Jin is approved.
SUPERVISORY COMMITTEE
Seon-Yong Jeong
Yoon-Sok Chung
Yup Kang
Yong Jun Choi
Insun Song
The Graduate School, Ajou University
December, 9th, 2019
i
-ABSTRACT -
Transcription Factor HOXA9 is Linked to the Calcification and
Invasion of Papillary Thyroid Carcinoma
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, representing 75 to 85 percent of all thyroid cancer cases. Calcification is one of the important characteristic feature for the diagnosis of papillary thyroid carcinoma. Runt-related transcription factor 2 (RUNX2), a master transcription factor associated with osteogenic differentiation, is reportedly related to PTC calcification and invasiveness. However, its regulatory role in this process is somewhat uncharacterized. Here, I attempted to identify gene that regulates RUNX2 and to clarify its function in PTC calcification and carcinogenesis.
The expression of RUNX2-upstream genes was evaluated by semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR in normal thyroid cell line Nthy-Ori 3-1 and the PTC cell lines TPC1 and BHP10-3. After cloning the RUNX2 promoter, luciferase assay and chromatin immunoprecipitation assay were performed with the primary candidate gene - homeobox family A9 (HOXA9). I found that RUNX2 promoter activity and binding ability were enhanced by HOXA9. Over-expression of HOXA9 was found to enhance alkaline phosphatase activity, mineralization, and the ability of in vitro
ii
cell migration and invasion, whereas downregulation of HOXA9 showed the opposite effects. To confirm the results from in vitro promoter, mineralization and carcinogenesis assays, I evaluated haematoxylin and eosin staining in human thyroid cancer tissue specimens from thyroid carcinoma patients and identified immunohistochemistry to verify the expression of RUNX2 and HOXA9 in serial calcified malignant thyroid cancer tissues. Haematoxylin and eosin staining and immunohistochemistry results showed that the expression of RUNX2 and HOXA9 were found in calcified cancer tissues simultaneously. Next, I performed RUNX2 down regulation experiment in the control cell lines and HOXA9 overexpressed system with the normal cell line Nthy-Ori 3-1 and PTC cell lines TPC1 and BHP10-3. Further, mineralization assay, wound healing assay, and transwell assay were performed in these cells. Mineralization were decreased in all RUNX2 downregulated cells, and alkaline phosphatase activity in Nthy-Ori 3-1 and BHP10-3 cells was enhanced in the RUNX2 knockdowned and HOXA9 overexpressed cells compared to the RUNX2 knockdowned in control cells. Furthermore, migration ability was all decreased in RUNX2 knockdowned cells compared to the control cells, and invasion ability was enhanced in the RUNX2 knockdown groups with HOXA9 overexpression compared to that in respective RUNX2 knockdown with control groups. These results indicated that HOXA9, as a positive regulator of RUNX2, can enhance calcification, migration, and invasion in PTC.
iii
These data can improve the understanding of the molecular mechanisms of calcification in papillary thyroid carcinoma as well as tumorigenesis.
iv
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTENTS ··· iv
LIST OF FIGURES ··· vi
LIST OF TABLES ··· vii
LIST OF APPENDIX ··· viii
I. INTRODUCTION ··· 1
II. MATERIALS AND METHODS ··· 4
1. Cell culture ··· 4
2. Candidate regulators of RUNX2 ··· 4
3. Semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR ··· 10
4. Luciferase reporter assay and chromatin immunoprecipitation (ChIP) assay ··· 16
5. Plasmids, lentivirus packaging, and stable cell lines ··· 17
6. Western blotting ··· 20
7. Alkaline phosphatase (ALP) assay and Alizarin Red S (ARS) staining ··· 20
8. Wound healing assay ··· 21
9. Invasion assay ··· 21
10. H&E staining and Immunohistochemisty (IHC) assay ··· 22
11. Statistical analysis ··· 23
v
1. HOXA9 regulates RUNX2 gene expression ··· 24
2. HOXA9 mediates the calcification of thyroid cells ··· 31
3. HOXA9 is associated with thyroid cell migration and invasion ··· 34
4. HOXA9 and RUNX2 can be simultaneously detected in calcified thyroid cancer tissues ··· 38
5. HOXA9 increase PTC calcification and tumor invasion directly or indirectly via RUNX2 ··· 42
IV. DISCUSSION ··· 51
V. CONCLUSION ··· 56
REFERENCES ··· 58
vi
LIST OF FIGURES
Figure 1. pGL3-Basis Vector for RUNX2 promoter cloning ··· 6
Figure 2. pOTB7 Vector for HOXA9 cloning ··· 18
Figure 3. pcDNA3.1 Vector for HOXA9 subcloning ··· 19
Figure 4. Expression of candidate genes in thyroid cells ··· 26
Figure 5. Expression of candidate genes in thyroid cells ··· 27
Figure 6. HOXA9 increased RUNX2 promoter activity and RUNX2 binding ability ···· 29
Figure 7. The expression of HOXA9 and RUNX2 in thyroid cells ··· 30
Figure 8. Effect of HOXA9 on osteoblast differentiation ··· 32
Figure 9. Migration ability of thyroid cells based on HOXA9 expression ··· 35
Figure 10. Invasion ability of thyroid cells based on HOXA9 expression ··· 36
Figure 11. The expression of HOXA9 and RUNX2 in thyroid cancer tissues ··· 40
Figure 12. The expression of RUNX2 and HOXA9 ··· 44
Figure 13. Effect of HOXA9 overexpression and shRUNX2 on osteoblast differentiation ··· 46
Figure 14. Migration ability based on HOXA9 and RUNX2 expression in thyroid cells · ··· 47 Figure 15. Invasion ability based on HOXA9 and RUNX2 expression in thyroid cells · 49
vii
LIST OF TABLES
Table 1. The result of transcription factors (score > 95.00) ··· 7
Table 2. Primers for semi-quantitative reverse-transcriptase PCR ··· 12
Table 3. Primers for quantitative real-time PCR ··· 14
viii
LIST OF APPENDIX
- 1 -
I. INTRODUCTION
Thyroid cancer is a malignant cancer disease which develops from the thyroid gland tissues and has four main types that papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer (https://www.cancer.gov/). Papillary thyroid carcinoma (PTC) is the most common type of all malignant thyroid tumors (DeLellis, 2004; Lloyd, 2010; Sipos and Mazzaferri, 2010). Most of the PTC patients have a good prognosis to show excellent overall survival, but some showed poor effect with conventional chemotherapy and poor prognosis (Sipos and Mazzaferri, 2010). Therefore, early identification of high-risk patients who will probably have a more aggressive disease is vital to find a way to prevent the negative evolution of the disease (Shrestha et al., 2015). Calcifications, characteristic findings of this disease, are important for the diagnosis of PTC (Johannessen and Sobrinho-Simoes, 1980; Carcangiu et al., 1985). However, calcifications are commonly found not only in cancer tissues but also in some benign nodules (Lacout et al., 2016). Psammoma bodies (PBs), concentric lamellated calcified structures, are also most common in histological sections of PTC (Cotran et al., 1999; Underwood and Cross, 2009). PBs are mostly considered as the result of dystrophic calcification, in which deposition occurs locally in dead or dying tissues (Das, 2009). However, rather than being the outcome of dystrophic calcification of nonviable or dying tissues, it has been recommended that PBs might characterize an active biologic process leading to the degeneration/death of tumor cells
- 2 -
and the retardation of growth of the neoplasm (Das, 2009). Nonetheless, the underlying mechanism and the role of calcification in PTC are not fully understood.
Runt-related transcription factor 2 (RUNX2) belongs to the RUNX family and consists of three isoforms, namely types I, II, and III (Harada et al., 1999). The key functions of type I and II RUNX2 are as transcription factors in the process of osteoblast differentiation and bone formation (Zambotti et al., 2002; Cohen, 2009; Soltanoff et al., 2009). Nevertheless, as well as bone metastasis, they are also involved in the carcinogenesis of breast and prostate cancers (Pratap et al., 2011; Wysokinski et al., 2015). Furthermore, some findings have suggested that high expression of RUNX2 can predict breast cancer recurrence (Chang et al., 2014) or induce myeloma progression in the bone (Trotter et al., 2015). RUNX2 also plays a vital role in the process of mesenchymal stem cell differentiation to the osteogenic lineage (Dalle Carbonare et al., 2012).
Recently, it has been reported that thyrocytes show similar characteristics with osteoblasts, and the expression of RUNX2 is increased in PTC patient tissues (Endo et al., 2008). Moreover, PTC patients with microcalcifications were found to express significantly higher levels of RUNX2 mRNA in serum than those who without microcalcifications (Dalle Carbonare et al., 2012). Enhanced RUNX2 signalling has been functionally linked to tumor cell invasion and metastasis in thyroid carcinoma by regulating epithelial-to-mesenchymal transition-related molecules, matrix metalloproteinases, and angiogenic/lymphangiogenic factors (Niu et al., 2012). However,
- 3 -
the regulatory role of RUNX2 in thyroid cell calcification and carcinogenesis has not been fully elucidated.
In this study, my aim was to discover a novel protein that regulates the expression of RUNX2 and to clarify the function of this marker in calcification and carcinogenesis of PTC. For this, I screened several candidate transcription factors, upstream genes of RUNX2, and homeobox family A 9 (HOXA9) was identified as a potential candidate gene. Hox proteins, a group of homeodomain-containing transcription factors, play a key role in oncogenesis and are particularly dysregulated both in solid and haematological malignancies (Samuel and Naora, 2005; Morgan and El-Tanani, 2016; Morgan et al., 2017). The expression of HOXA9, as a member of the HOX gene family, is usually altered in solid cancers (Bhatlekar et al., 2014). Thus, I elucidated the relationship between HOXA9 and RUNX2 and the associated functions in calcification and carcinogenesis of PTC.
- 4 -
II. MATERIALS AND METHODS
1. Cell culture
The human normal thyroid cell line Nthy-Ori 3-1 and PTC cell line BHP10-3 were maintained in complete Roswell Park Memorial Institute 1640 medium (RPMI1640; Welgene, Gyeongsan, Korea) with 10% fetal bovine serum (FBS; Gibco BRL) and 1% antibiotic-antimycotic (Gibco, New York, NY); the other PTC cell line TPC1 was grown in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12, 1:1 Mixture (DMEM/F12; Welgene, Gyeongsan, Korea) with 10% FBS and 1% antibiotic-antimycotic. For osteoblast differentiation, cells were plated at 1.0 x 10⁴cells/well in 48-well plates and used for calcification assays and for PCR, 2.0 x 10⁴cells were seeded in a 60-mm plate and; cells were induced by osteogenic medium after 24 h (for cell adherence; day 0). Medium was changed every 2 days. All cells were incubated in a humidified atmosphere of 5% CO₂ at 37 °C.
2. Candidate regulators of RUNX2
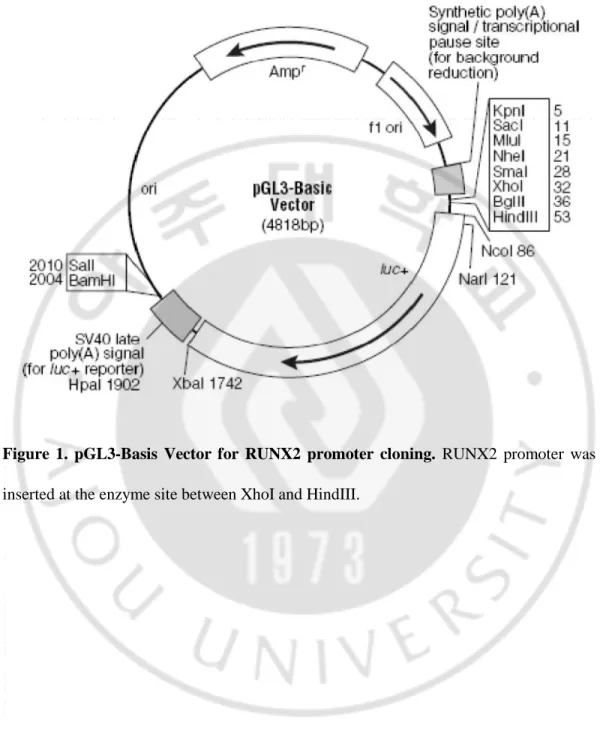
To discover a novel gene that regulates RUNX2, I used the transcription factor search website http://www.cbrc.jp/research/db/TFSEARCH.html with the nearly 3000-bp RUNX2 promoter in pGL3-Basic vector (Figure 1) and identified putative upstream markers. CTNNBIP1, DLX3, HOXA9, NKX2-5, NKX3-2, RUNX1, and SOX9, with
- 5 -
scores greater than 95.00, were selected as candidates [Table 1 (sequence of candidate genes), Appendix 1 (full sequence of RUNX2 promoter)].
- 6 -
Figure 1. pGL3-Basis Vector for RUNX2 promoter cloning. RUNX2 promoter was
- 7 -
Table 1. The result of transcription factors (score > 95.00)
201 TGTATTTCAT TTCCATGGCA TTAAAGTATG TGATTTATAC AGTATACCTA entry score ---> M00101 CdxA 100.0 ---> M00100 CdxA 96.2
351 TGTGGTTTTC AATTTCTGAT TATAACCATG GTATAAATCT ATAATCAAGA entry score ---> M00271 AML-1a 100.0 <--- M00101 CdxA 100.0 <--- M00100 CdxA 96.2
401 GCTTTATTTG CATTGACTTT TCTAAATCAG TATCTCTTCA CCACGTGTAG entry score ---> M00217 USF 98.0
651 GAATTTGATA GGCCTCACAG AGGACAAAAA GTGCATATAT AACAATATCT entry score ---> M00042 Sox-5 95.4
801 AATTCTTATC AGGTCAATTT GAAGAGCCAA AGTTTACATA AAGAAGATTT entry score <--- M00100 CdxA 96.2
951 TCATACTACA GCAGCTACCT ACATATTTTC AATTAAGTGA TTCTGCTATT entry score <--- M00241 Nkx-2. 100.0 ---> M00240 Nkx-2. 97.7
1001 ATAGGTACAC ATGAAACCAT GTTTGGAAAT CCCACAAGCT ATAAGGACAA entry score <--- M00053 c-Rel 96.7
1051 AACCCTCCTT TTGTTTACTT TGGATACTAA TTAGAGATAC AGATAATATG entry score <--- M00148 SRY 100.0 <--- M00160 SRY 95.6
- 8 -
---> M00101 CdxA 100.0 ---> M00100 CdxA 96.2
1551 ACCCCATTTA CTTTATGCCA CTCCTAGTTA CTGTCACACT AGGAAGAAGT entry score ---> M00100 CdxA 96.2
1651 AAAATTGGTC TGTTCGCCTT TATAATTTTG GTTGAAAAAT ACTCCATCGC entry score <--- M00101 CdxA 98.6
1701 TCCCAACTGA TGAAAACAGG AAGCTCTATT CATAAATATA AAATTCACTG entry score <--- M00100 CdxA 100.0 <--- M00101 CdxA 99.3
1901 GCATTATTCC TTACTACACA CAGCATTTTG TAATTTATTT CAAAGCTTCC entry score <--- M00101 CdxA 97.9
1951 ATTAGAAACA AAAAAATACA TAGCTTCTGT TAACCCACTC TATTCTAAGC entry score ---> M00148 SRY 100.0
2051 TTATAGAAGA TCTGCTATCA GAAACTCTAT TAATGTCTAA ACTACTTAAA entry score <--- M00101 CdxA 100.0 ---> M00101 CdxA 99.3
2151 CATAAAGAAA ACTAAGATTC ATCCAATAAA CTATATTACA ATCCCTGTCA entry score <--- M00100 CdxA 96.2
2351 CACTTTCATG ACAGCCAATT ATAGTCAAGC CTAGCAAGCA GTTTGCAACC entry score ---> M00101 CdxA 98.6
2601 TTGTTCATTT TTCCACAGAC ACAATAATGA ACTAAAAAGA GGAGGCAAAA entry score ---> M00101 CdxA 97.9
- 9 -
2801 TCTGGATGCC AGGAAAGGCC TTACCACAAG CCTTTTGTGA GAGAAAGAGA entry score <--- M00271 AML-1a 100.0
2901 TACTTAAGAG TACTGTGAGG TCACAAACCA CATGATTCTG CCTCTCCAGT entry score <--- M00271 AML-1a 100.0
3001 GTGTGAATGC TTCATTCGCC TCACAAACAA CCACAGAACC ACAAGTGCGG entry score <--- M00271 AML-1a 100.0 <--- M00271 AML-1a 100.0 ---> M00148 SRY 96.4
- 10 -
3. Semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR
To determine mRNA expression levels of the bone metabolic markers RUNX2, IBSP, and BGLAP (Osteocalcin) and candidate genes including CTNNBIP1, DLX3, HOXA9, NKX2-5, NKX3-2, RUNX1, and SOX9, semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR were performed. To normalize the efficiency of semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR reactions, human β-actin was used as a standard. Total RNA was extracted from cultured cells in osteogenesis medium at 0, 3, and 8 days cells using TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions and quantified using a spectrophotometer (Beckman Coulter, Brea, USA). Extracted RNA was subsequently reverse transcribed at 42 °C for 1 h using a premix kit with oligo-dT as a primer (iNtRON Biotechnology, Seongnam, Korea). Next, 27–34 cycles of semi-quantitative reverse-transcriptase PCR were performed using a Maxime PCR PreMix kit (i-StarTaq; iNtRON Biotechnology, Seongnam, Korea). All quantitative real-time PCR measurements were performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems; Foster City, USA). All PCR amplifications (25 cycles) were performed in a total volume of 25 μl containing 150 ng cDNA using the SYBR Green I qPCR kit (TaKaRa, Shiga, Japan) according to the manufacturer’s recommendations. The amplification parameters were followed in accordance with the TaKaRa manufacturer’s recommendations. By normalizing to β-actin, relative quantification of gene expression was performed using the comparative threshold (Ct) method as described by the
- 11 -
manufacturer (Applied Biosystems). The values were expressed as fold-change relative to control levels. Relative gene expression was displayed as 2−ΔCt (ΔCt = Ct target gene − Ct β-actin). Fold-change was calculated as 2−ΔΔCt (ΔΔCt = ΔCt control − Ct treatment). Semi-quantitative reverse-transcriptase PCR primers are described in Table 2 and quantitative real-time PCR primers are described in Table 3.
- 12 -
Table 2. Primers for semi-quantitative reverse-transcriptase PCR
Gene Primer (Semi-quantitative reverse-transcriptase PCR) Forward / Reverse β-actin 5`-CCTAAAAGCCACCCCACTTC-3` F 5`-AGGGAGACCAAAAGCCTTCA-3` R RUNX2 5`-TTACTTACACCCCGCCAGTC-3` F 5`-TATGGAGTGCTGCTGGTCTG-3` R IBSP 5`-AACCTACAACCCCACCACAA-3` F 5`-AGGTTCCCCGTTCTCACTTT-3` R BGLAP 5`-GACTGTGACGAGTTGGCTGA-3` F 5`-CTGGAGAGGAGCAGAACTGG-3` R CTNNBIP1 5`-TTGGCTGCAGGAAGAAACTT-3` F 5`-GCAGCCAATCAGACCTCTTC-3` R DLX3 5`-TCTACAAGAACGGGGAGGTG-3` F 5`-GCGTGATACCAGGAGTTGGT-3` R HOXA9 5`-CCACGCTTGACACTCACACT-3` F 5`-CAGTTCCAGGGTCTGGTGTT-3` R NKX2-5 5`-CCTCAACAGCTCCCTGACTC-3` F 5`-CTCATTGCACGCTGCATAAT-3` R NKX3-2 5`-GCTTTAACCACCAGCGCTAC-3` F 5`-ACCTTTACGGCCACCTTCTT-3` R RUNX1 5`-TCTAGCTCAGCACTGCTCCA-3` F 5`-TCATGCAAAACTGGCTTCAG-3` R
- 13 -
Sox9 5`-TTGAGCCTTAAAACGGTGCT-3` F
- 14 - Table 3. Primers for quantitative real-time PCR
Gene Primer (Quantitative real-time PCR) Forward / Reverse β-actin 5`-CAAGATCAACCGGGAAAAGA-3` F 5`-CTGAGGCATAGAGGGACAGC-3` R RUNX2 5`-TTACTTACACCCCGCCAGTC-3` F 5`-CACTCTGGCTTTGGGAAGAG-3` R IBSP 5`-AACCTACAACCCCACCACAA-3` F 5`-CGTACTCCCCCTCGTATTCA-3` R BGLAP 5`-GACTGTGACGAGTTGGCTGA-3` F 5`-CTGGAGAGGAGCAGAACTGG-3` R CTNNBIP1 5`-ACCTTTCCCATCATCGTGAG-3` F 5`-AATCCACTGGTGAACCAAGC-3` R DLX3 5`-AGGCCTAGTTCCTCCTGAGC-3` F 5`-CCTCGTCATGATGTCCACTG-3` R HOXA9 5`-CAATAACCCAGCAGCCAACT-3` F 5`-CAGTTCCAGGGTCTGGTGTT-3` R NKX2-5 5`-GTCAAGCCGCTCTTACCAAG-3` F 5`-TTGTCCGCCTCTGTCTTCTC-3` R NKX3-2 5`-GACGCAGGTGAAAATCTGGT-3` F 5`-ACCTTTACGGCCACCTTCTT-3` R RUNX1 5`-GGCTGGCAATGATGAAAACT-3` F 5`-CCGACAAACCTGAGGTCATT-3` R
- 15 -
SOX9 5`-AGACAGCCCCCTATCGACTT-3` F
- 16 -
4. Luciferase reporter assay and chromatin immunoprecipitation (ChIP) assay
To analyze RUNX2 promoter activity, I cloned RUNX2 promoter and conformed the sequence by DNA sequencing (Table 5). Nthy-Ori 3-1, TPC1, and BHP10-3 cells were plated on 24-well plates at a density of 2 x 10⁴cells/well 1 day before transfection. Plasmid DNA was mixed with TransIT-2020 (Mirus, Madison, WI) and transfected into the cells following the manufacturer’s protocol. After 48 h, cells were washed twice with 1 x PBS and then lysed in reporter lysis buffer (Promega, Madison, WI). Cell extracts were subjected to assays using a luciferase assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Luciferase activity was measured in triplicate, averaged, and then normalized to β-galactosidase activity using o-nitrophenyl-β-D-galactopyranoside (Sigma-Aldrich) as a substrate.
ChIP assays were performed with a ChIP kit (Upstate Biotechnology, Lake Placid. NY), with modified instructions from the manufacturer, using antibodies against HOXA9 (Proteintech, Rosemont, USA) or control IgG (Santa Cruz Biotechnology, Santa Cruz, CA). The precipitated DNA was subjected to PCR amplification with specific primers for the RUNX2 P1 promoter region, which contains HOXA9-binding sites. The following primers were used for PCR: RUNX2 P1 sense, 5′-GCAAAAAGGCAGAGGTTGAG-3′; RUNX2 P1 antisense, 5′- CCCCCTTGCTCTTTCTCTCT-3′.
- 17 -
5. Plasmids, lentivirus packaging, and stable cell lines
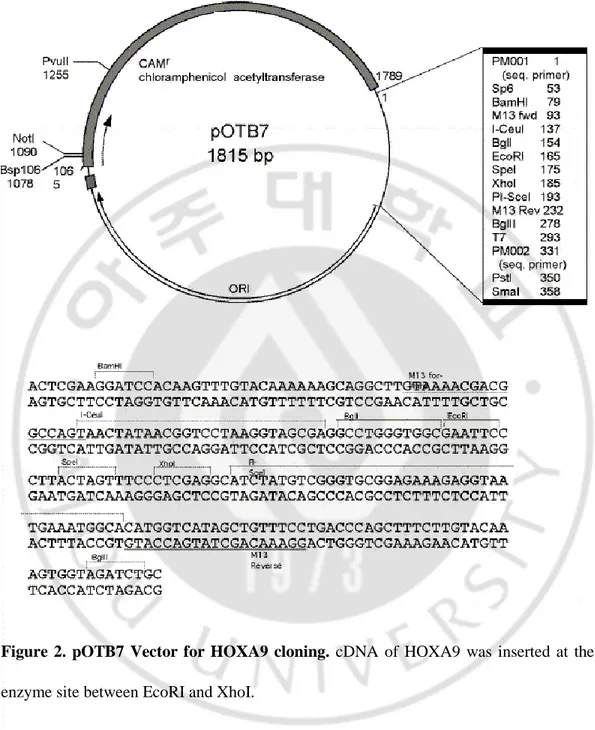
I purchased full-length HOXA9wt, pOTB7-HOXA9 (Figure 2) from the Korea Human Gene Bank, Medical Genomics Research Center, KRIBB, Korea. HOXA9 was subcloned into the pcDNA3.1 (+) vector (Invitrogen) (Figure 3) by ligating EcoRI–XhoI fragments to generate the pcDNA-HOXA9 expression construct. pcDNA-HOXA9 was confirmed by DNA sequencing (Table 8). Stable cell lines were established by transfecting plasmids HOXA9 plasmids into thyroid cells and selecting with G418 in at the a concentration of 400 µg/ml.
Lentivirus harboring HOXA9 or RUNX2 or vector controls were designed and packaged by Merck (Darmstadt, Germany). Lentiviruses were packaged in HEK-293T cells and collected from the medium supernatant. Stable cell lines were established by infecting thyroid cells with HOXA9- or RUNX2-expressing into thyroid cells with lentiviruses and selecting with puromycin in at the a concentration of 10 µg/⁄ml.
- 18 -
Figure 2. pOTB7 Vector for HOXA9 cloning. cDNA of HOXA9 was inserted at the
- 19 -
Figure 3. pcDNA3.1 Vector for HOXA9 subcloning. cDNA of HOXA9 was inserted at
- 20 - 6. Western blotting
Cell lysates were extracted in 0.1 M NaCl, 0.01 M tTris–HCl (pH 7.6), 1 mM EDTA (pH 8.0), 1 mg/ml aprotinin, and 100 mg/ml PMSF; protein concentrations were determined by a Bio-Rad protein assay. Protein (50 μg) was boiled at 95 °C in sodium dodecyl sulphate (SDS) sample buffer for 5 min, electrophoresed on 10% or 12% SDS-PAGE gels, and transferred to polyvinyldifluoridine membranes. Then, blots were incubated overnight at 4 °C with anti-HOXA9 (Abcam, Cambridge, UK, #ab191178; 1:1000), anti-RUNX2 (Abcam, Cambridge, UK, #ab23981; 1:1000), or anti-β-actin (Bethyl, Montgomery, Texas, USA, #A300-491A; 1:8000) antibodies. Membranes incubated with anti-goat or anti-rabbit secondary antibody (Santa Cruz Biotechnology; 1:5000) at room temperature for 60 min. Protein band signals were visualized using an ECF western blotting kit (Amersham Biosciences, Piscataway, NJ, USA) and detected with an LAS3000 luminescent image analyzer (Fuji Photo Film).
7. Alkaline phosphatase (ALP) assay and Alizarin Red S (ARS) staining
For ALP staining, cultured cells were fixed in 10% formalin for 10 min, permeabilized for 30 min with 0.1% triton-100 in 1x PBS, and treated with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate for 10–30 min. To measure calcium deposition in the extracellular matrix, 200 μl of extraction solution was added and samples were incubated overnight at 4 °C. ALP activity was measured in total cell lysates after homogenization in a buffer containing 1 mM tris-HCl (pH 8.8), 0.5% triton
- 21 -
X-100, 10 mM Mg²+, and 5 mM p-nitrophenyl phosphate as substrates. The absorbance
was read at 405 nm (BioTek, USA).
For ARS staining, cultured cells were fixed in 70% ethyl alcohol for 1 h. After washes with 1x PBS, cells were stained with 40 mM ARS solution (pH 4.2) for 10 min to stain calcium deposits. Cells were then washed five times with distilled water followed by 1 x PBS for 15 min and removed non-specific stained cells were removed. To quantify the degree of mineralization, cells were extracted using 10% (w/v) cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0). The concentration was evaluated by measuring the absorbance at 562 nm with a multiplate reader using an Alizarin red S standard curve in the same solution. All values are expressed as fold-change relative to the control.
8. Wound healing assay
A Thyroid cells were seeded in 6-well plates (6 x 10 ⁴cells/well) with 2 ml of complete RPMI1640 or DMEM/F-12. At 24 h, the monolayers were mechanically disrupted with a pipette tip to produce a clean uniform scratch. The assay was performed three times and wells were photographed every 12 h to monitor the closing of the wound. The number of migrating cells into the wound was counted under a microscope. Experiments were repeated three times.
- 22 - 9. Invasion assay
Matrigel was coated on 24-well chambers (Corning, NY, USA, #3415), and thyroid cells were transferred at a density of 3 × 10⁴on top of the Matrigel-coated chambers in serum-free media after drying the Matrigel. After 24 h of incubation, non-invading cells were removed by rinsing, and invaded cells were fixed with ice-cold 100% methanol and stained with 0.5% crystal violet. The number of invading cells was manually counted in four randomly chosen fields under a microscope and images were captured at ×100 magnification. Experiments were repeated three times.
10. H&E staining and Immunohistochemisty (IHC) assay
Paraffin sections from thyroid carcinoma patients were obtained from the Department of Surgery and Pathology of Ajou University School of Medicine (Suwon, Korea). Each tissue was used to generate three serial slides. These tissues contained normal and tumor tissues, as well as benign and malignant nodules with/without calcification. One group of slides was developed using the Dako coverstainer HE autostainer (Santa Clara, United States).
The other two groups of slides were used for Immunohistochemistry (IHC) assays. The primary antibody was HOXA9 (Abcam, Cambridge, UK, #ab191178) or anti-RUNX2 (Abcam, Cambridge, UK, #ab23981), used at a dilution of 1:200. Slides were deparaffinized and rehydrated and were trypsinised before IHC staining. Then, slides
- 23 -
were incubated overnight at 4 °C with the primary antibody and then with biotin-conjugated goat anti-rabbit IgG diluted at 1:200 (Santa Cruz Biotechnology). Slides were dehydrated and stabilized with mounting medium. Staining was viewed under a microscope (SPOT Insight 2Mp scientific CCD digital Camera system, DIAGNOSTIC instruments, Inc, USA; U-CMA3, OLYMPUS, Japan). Image analysis was performed with SPOT Software V4.0 (Diagnostic Instrument, Inc, USA) and Image Pro plus® (Media Cybernetics, Inc, USA).
11. Statistical analysis
The statistical significance of differences was assessed by performing a Student’s t-test. P < 0.05 was considered significant. Results are expressed as the mean ± SEM.
- 24 -
III. RESULTS
1. HOXA9 regulates the expression of RUNX2
To discover a novel protein that regulates the expression of RUNX2, I selected seven candidates [catenin beta interacting protein 1 (CTNNBIP1), distal-less homeobox 3 (DLX3), HOXA9, NK2 homeobox 5 (NKX2-5), NK3 homeobox 2 (NKX3-2), runt related transcription factor 1 (RUNX1), and SRY-Box 9 (SOX9)] from a transcription factor (TF) search website (http://www.cbrc.jp/research/db/TFSEARCH.html). Then, I screened the effect of candidate genes on osteoblastic marker genes including RUNX2, integrin binding sialoprotein (IBSP), and bone gamma-carboxyglutamic acid-containing protein (BGLAP) by measuring mRNA expression levels via semi-quantitative reverse-transcriptase PCR (Figure 4) and quantitative real-time PCR (Figure 5). Total RNA was prepared from the normal cell line Nthy-Ori 3-1 and PTC cell lines TPC1 and BHP10-3, which were cultured in osteogenesis differentiation media for 0, 3, or 8 days; RNA from these cells was used as a template for semi-quantitative reverse-transcriptase PCR and quantitative real-time PCR. Based on these data, HOXA9, for which expression changed over time with RUNX2, was selected as a primary candidate.
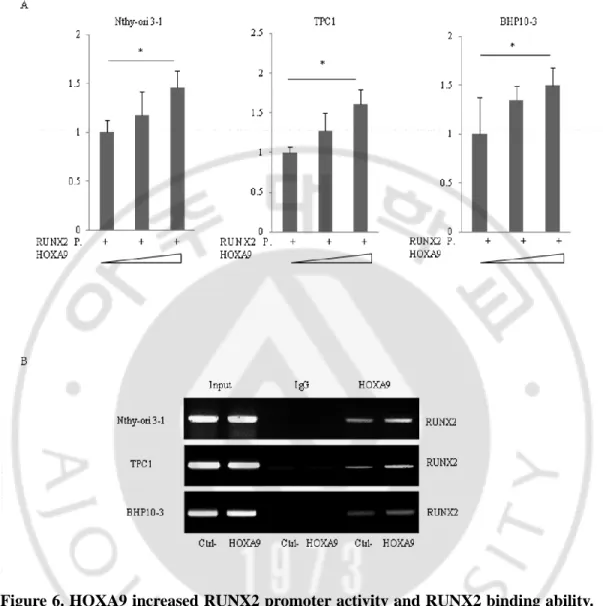
Subsequently, I cloned the RUNX2 P1 promoter and performed luciferase reporter assays to investigate the regulatory interaction between RUNX2 and HOXA9. The promoter activity of RUNX2 P1 was increased with the addition of HOXA9 (Figure 6 A).
- 25 -
Next, I carried out Chromatin Immunoprecipitation (ChIP) assays to evaluate the binding of HOXA9 to the promoter of RUNX2 P1 using both normal cell Nthy-Ori 3-1 and TPC1 and BHP10-3 PTC cells, and with an anti-HOXA9 antibody. Consistent with luciferase reporter assay data, the binding of HOXA9 to the RUNX2 promoter improved in a dose-dependent manner (Figure 6 B).
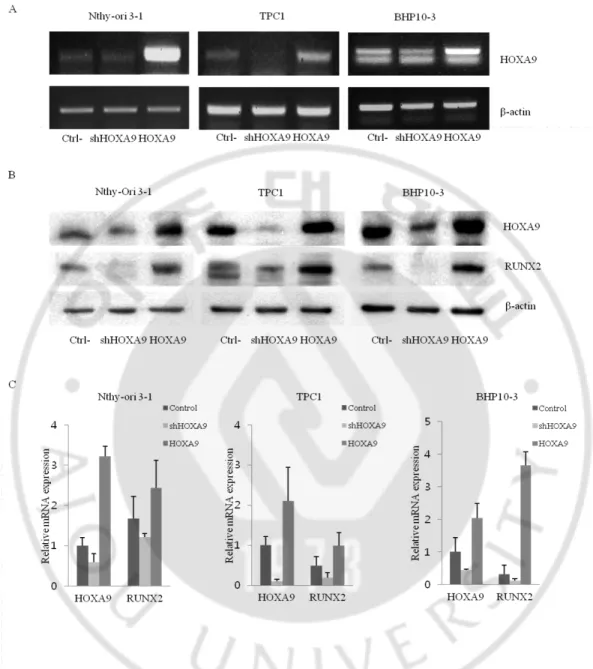
Then I knockdowned or overexpressed HOXA9 in Nthy-Ori 3-1 cell line and TPC1 and BHP10-3 PTC cell lines, and established the expression of RNA and protein levels each by semi-quantitative reverse-transcriptase PCR (Figure 7 A) and western blotting (Figure 7 B). I also confirmed that the expression of RUNX2 was down-regulated and up-regulated depending on the expression of HOXA9. These results suggest that HOXA9 regulates RUNX2 by binding its promoter in two types of thyroid cell lines, and specifically control in PTC (Figure 7 C).
- 26 -
Figure 4. Expression of candidate genes in thyroid cells. (a) The expression of several
selected candidate genes (RUNX2-upstream genes) in thyroid cell lines cultured in osteogenesis medium for 0, 3, and 8 days, as well as HOXA9 expression, was evaluated by semi-quantitative reverse-transcriptase PCR.
- 28 -
Figure 5. Expression of candidate genes in thyroid cells. The expression of several
selected candidate genes (RUNX2-upstream genes) in thyroid cell lines cultured in osteogenesis medium for 0, 3, and 8 days, were evaluated by quantitative real-time PCR. Error bars represent standard deviation (n = 3 biological replicates). *p < 0.05 vs. Control.
- 29 -
Figure 6. HOXA9 increased RUNX2 promoter activity and RUNX2 binding ability.
The RUNX2 promoter (P) was cloned and plasmid DNA encoding HOXA9 was transfected into thyroid cells; HOXA9-binding activity and ability at the RUNX2 promoter region was assessed by luciferase reporter assays (A) and chromatin immunoprecipitation (ChIP) assays (B). Error bars represent standard deviation (n = 3 biological replicates). *p < 0.05 vs. Control.
- 30 -
Figure 7. The expressions of HOXA9 and RUNX2 in thyroid cells. HOXA9 was
knockdowned or overexpressed in two types of thyroid cell lines. (A) The RNA expression of HOXA9 was assessed by qRT-PCR. (B) The protein expressions were evaluated by western blotting. (C) Alterations to the RNA and protein expressions of RUNX2 depended on HOXA9 levels.
- 31 - 2. HOXA9 mediates the calcification of thyroid cells
To assess whether HOXA9 is involved in the process of calcification, alkaline phosphatase (ALP) staining was detected at 3, 5, and 7 days and Alizarin red S (ARS) staining was performed at 10 or 14 days. ALP activity was significantly enhanced in HOXA9-overexpressing Nthy-Ori 3-1 and TPC1 cells lines. In contrast, ALP activities were significantly reduced by the HOXA9-knockdowned groups in TPC1 and BHP10-3 cell lines (Figure 8 A, B). Moreover, mineralization status was increased in overexpressing Nthy-Ori 3-1 and TPC1, but was attenuated in all groups of HOXA9-knockdown cells (Figure 8 A, C). These data suggest that HOXA9 is involved in the process of thyroid calcification .
- 32 -
Figure 8. Effect of HOXA9 on osteoblast differentiation. (A) Alkaline phosphatase
(ALP) staining (upper layer) was performed after 7 days and Alizarin red S staining was carried out on the 10th day using Nthy-Ori 3-1 cells and on the 14th day using papillary thyroid carcinoma (PTC) cells (TPC1 and BHP10-3). (B) ALP activity was measured at 405 nm using alkaline phosphatase yellow (pNPP) liquid substrate system with control,
- 33 -
shHOXA9, and HOXA9-overexpressing cells with for the two types of thyroid cells. (C) Alizarin red S-stained cells were extracted using cetylpyridinium chloride, and the mineralization level was quantified by measuring absorbance at 562 nm. Error bars represent standard deviation (n = 3 biological replicates). * p < 0.05 vs. Control.
- 34 -
3. HOXA9 is associated with thyroid cell migration and invasion
To evaluate the effect of HOXA9 in Nthy-Ori 3-1, TPC1, and BHP10-3 cell migration and invasion, wound healing assay and invasion assay were performed. HOXA9-overexpressing cells migrated more than control cells, whereas in the normal Nthy-Ori 3-1 cell line, HOXA9 knockdown suppressed migration; however, this difference was not significant. In PTC cells, wound healing ability was increased by a small amount marginally in HOXA9-overexpressing cells compared to that in control cells and was significantly decreased in HOXA9-knockdown cells (Figure 9 A, B). Furthermore, cell invasion was enhanced in HOXA9-overexpressing groups and reduced in HOXA9-knockdown groups, and the results expressed the similar appearance compare to outcome of the wound-healing assay (Figure 10 A, B). These data indicate that HOXA9 can mediate migration and invasion in two types of thyroid cells.
- 35 -
Figure 9. Migration ability of thyroid cells based on HOXA9 expression. (A) Thyroid
cells were seeded in 6-well plates (6 x 10⁴ cells/well) with 2 ml of complete growth media. After 24 h, wound-healing assays were performed, and the wells were photographed every 12 h to monitor wound closure. (B) The numbers of migrating cells into the wound were counted under a microscope. Experiments were repeated three times. Error bars represent standard deviation (n = 3 biological replicates). * p < 0.05 vs. Control.
- 36 -
Figure 10. Invasion ability of thyroid cells based on HOXA9 expression. (A) Thyroid
cells were transferred to the top of Matrigel-coated chambers with 3 x 10⁴ cells/well in serum-free media. After 24 h of incubation, the invaded cells were fixed with methanol
- 37 -
and stained with 0.5% crystal violet. (B) The numbers of invading cells was were counted under a microscope. Experiments were repeated three times. Error bars represent standard deviation (n = 3 biological replicates). * p < 0.05 vs. Control.
- 38 -
4. HOXA9 and RUNX2 can be simultaneously detected in calcified PTC tissues
To confirm the results of in vitro promoter, calcification and carcinogenesis assays, next I assessed cancer tissue specimens from thyroid carcinoma patients (Table 4). First, I performed haematoxylin and eosin (H&E) staining using paraffin tissue slides to detect cancer tissues and calcification. In the set of 42 cancer tissue samples, 25 showed calcification (Figure 11 A, B). Next, RUNX2 and HOXA9 were individually evaluated by immunohistochemistry (IHC) in serial tumor tissue slides in 25 samples which with calcification. Among these, RUNX2 was detected in 12 cases and HOXA9 was apparent in 13 cases (Figure 11 B~D). Among these 25 samples, two samples expressed RUNX2 only (Figure 11 C), whereas 3 samples had HOXA9 expression alone (Figure 11 D). Intriguingly, RUNX2 and HOXA9 were simultaneously detected with calcification in 10 cancer tissues (Figure 11 A, B). These data suggest that HOXA9 and RUNX2 are linked to calcification and carcinogenesis in thyroid tumor.
- 39 -
Table 4. The chacterisitics of thyroid carcinoma patients
Metastasis No. Gender Age Opday TumorSize PathType SubclassPTC Multiplicity pT3 LymVascPerm NoLymVascPerm BRAF
Central neck node 1 F 24 2017-03-06 18 1 1 1 4 1 1
2 F 47 2015-07-16 20 1 1 1 4 2 1 3 F 44 2016-09-08 15 1 1 3 4 1 2 4 M 28 2015-04-07 65 1 3 3 4 2 4 2 5 M 48 2015-10-01 70 5 1 4 2 4 6 M 33 2016-08-19 11 1 1 3 4 1 2 7 M 39 2017-07-11 15 1 1 3 2 2 2 8 F 39 2016-06-24 27 1 1 3 4 1 2 9 F 32 2015-04-02 17 1 1 4 2 4 2 10 F 39 2015-07-21 11 1 1 3 4 2 2 11 F 35 2015-12-15 14 1 7 4 2 2 12 F 55 2015-10-08 17 1 1 1 4 1 13 F 30 2016-01-19 13 1 1 1 4 1 2 14 M 35 2016-02-29 65 1 10 1 4 2 1 15 F 26 2016-04-28 41 1 1 3 4 2 16 M 31 2016-08-29 38 1 1 2 4 2 2 17 M 26 2017-02-07 17 1 1 1 4 2 2 18 F 38 2016-07-29 12 1 1 4 4 1 2 19 F 29 2017-03-28 15 1 1 2 2 1 2 20 M 30 2015-10-08 35 1 1 3 4 2
Lateral neck node 1 F 37 2016-05-31 12 1 1 1 4 1
2 M 41 2016-01-12 11 1 1 3 4 1 1 3 M 43 2016-06-23 16 1 1 2 4 2 2 4 F 50 2016-02-04 15 1 1 4 4 2 5 F 31 2017-05-18 14 1 1 1 4 1 2 6 M 34 2016-08-05 35 1 1 1 4 1 7 F 43 2014-02-13 15 1 1 1 4 2 4 2 8 M 42 2016-01-26 10 1 1 2 4 2 2 9 F 40 2015-12-10 14 1 1 1 4 1 2 10 F 37 2016-05-24 17 1 1 2 2 2 1 11 F 43 2016-02-23 12 1 1 1 4 1 1 12 F 42 2016-03-07 13 1 1 1 4 13 M 61 2016-07-14 12 1 1 1 2 1 14 F 21 2016-06-09 15 1 1 1 2 2 2 15 M 28 2016-06-09 12 1 1 3 4 2 2 16 F 52 2016-06-09 22 1 1 1 3 1 2 17 M 33 2016-09-22 22 1 1 1 4 1 18 F 41 2017-01-04 12 1 1 1 4 1 2 19 M 44 2017-02-10 10 1 3 4 2 20 F 58 2016-05-10 12 1 1 4 4 2
Microcalcification/ final pathology
- 40 -
Figure 11. The expression of HOXA9 and RUNX2 in thyroid cancer tissues. (A)
H&E staining was performed using the Dako coverstainer HE autostainer. RUNX2 and HOXA9 were detected by immunohistochemistry; immunostaining is in red. Image
- 41 -
analysis was performed using SPOT Software V4.0 and Image Pro plus® . (B) Numbers of cases based on staining. Among the 42 cancer tissues, 25 cases had concurrent calcification. In these 25 cases, RUNX2 was expressed in 12 cases and HOXA9 in 13 cases. Both RUNX2 and HOXA9 were simultaneously expressed in 10 cancer tissues with calcifications. (1: calcification, 2: cancer tissue.) Scale bar: (A) 200 μm, (C, D) Tissue sample: 1000 μm; calcification: 50 μm; cancer tissue: 200 μm.
- 42 -
5. HOXA9 increase PTC calcification and tumor invasion directly or indirectly via RUNX2
To determine whether the HOXA9-mediated effect on enhanced calcification and carcinogenesis is associated with RUNX2, I utilized a RUNX2-knockdown system and HOXA9-overexpression system with the normal cell line Nthy-Ori 3-1 and PTC cell lines TPC1 and BHP10-3 (Figure 12).
Next, I performed mineralization assays, as previously described, on these cells. ALP activities were significantly decreased in all RUNX2-downregulated cells (Figure 13 A, B). Further, mineralization was also suppressed in all RUNX2-knockdown groups (Figure 13 A, C). ALP activity in Nthy-Ori 3-1 and BHP10-3 cells was marginally enhanced with RUNX2 knockdown and HOXA9 overexpression compared to that RUNX2-knockdown in control cells, and this difference was statistically significant (*p < 0.05, **p < 0.005; Figure 13 B ).
Subsequently, wound healing assay and transwell assay were performed. Similar to the results of mineralization assay, migration was considerably decreased in RUNX2-knockdown cells compared to that in controls (Figure 14 A, B). Likewise, RUNX2 knockdown cells also suppressed invasion capacity in the three thyroid cell lines. Additionally, invasion ability was enhanced in the groups of RUNX2-knockdown cells with HOXA9 overexpression compared to that in respective RUNX2-knockdown control cells (Figure 15 A, B).
- 43 -
These data suggest that HOXA9, as a positive regulator of RUNX2, can enhance calcification and tumor migration and invasion in normal thyroid cell and PTC cells, dependent or independent of RUNX2.
- 45 -
Figure 12. The expression of RUNX2 and HOXA9 in thyroid cell lines. RUNX2 was
knockdowned in three control and HOXA9-overexpressing cell lines. Protein expression was assessed by western blotting (#1: Control, #2: shRUNX2, #3: HOXA9-overexpressing, #4: HOXA9-overexpressing/shRUNX2).
- 46 -
Figure 13. Effect of HOXA9 overexpression and shRUNX2 on osteoblast differentiations. (A) Alkaline phosphatase (ALP) staining (upper layer) on the 7th day
and Alizarin red S staining (lower layer) on the 10th day for Nthy-Ori 3-1 cells and on the
14th day for papillary thyroid carcinoma (PTC) cells (TPC1 and BHP10-3). (B, C)
Quantitative data for ALP and ARS staining in thyroid cells with control, shRUNX2, HOXA9-overexpressing, and HOXA9-overexpressing/shRUNX2 conditions. Error bars represent standard deviation (n = 3 biological replicates). *p < 0.05, **p < 0.005 vs. control.
- 48 -
Figure 14. Migration ability based on HOXA9 and RUNX2 expression in thyroid cells. (A) Migration ability was demonstrated by wound-healing assay. Cells were seeded
in 6-well plates (6 x 104 cells/well), and after 24 h, wound-healing assays were performed
and the wells were photographed every 12 h to monitor wound closure. (B) The number of migrated cells into the wound were counted under a microscope. Experiments were repeated three times. Error bars represent standard deviation (n = 3 biological replicates). * p < 0.05 vs. control, ** p < 0.005 vs. control.
- 49 -
Figure 15. Invasion ability based on HOXA9 and RUNX2 expression in thyroid cells.
(A) Thyroid cells were transferred to the top of Matrigel-coated chambers with 3 x 10⁴ cells/well in serum-free media. After 24 h of incubation, the invaded cells were fixed with methanol and stained with 0.5% crystal violet. (B) The number of cells that invaded
- 50 -
through the filter was counted under a microscope. Experiments were repeated three times. Error bars represent standard deviation (n = 3 biological replicates). * p < 0.05 vs. control, ** p < 0.005 vs. control.
- 51 -
IV. DISCUSSION
In this study, I screened upstream mediator genes of RUNX2, and found one candidate as a primary candidate, HOXA9, which regulates the expression of RUNX2 in two types of thyroid cell lines. Overexpression of HOXA9 was found to enhance ALP activity and mineralization, as well as in vitro tumor cell migration and invasion, while downregulation of this marker inhibited these processes. Moreover, the expression of RUNX2 and HOXA9 were confirmed by IHC in calcified malignant thyroid tumor tissues and the simultaneous expression of both markers was found in the calcified cancer tissues. Furthermore, cells exhibited enhanced migration and invasion in RUNX2-knockdown cells with HOXA9 overexpression compared to those in RUNX2-RUNX2-knockdown control cells. This suggests that HOXA9 could be linked to the calcification and tumor invasion of PTC, which is independent or dependent of RUNX2.
RUNX2 is the master regulator in osteoblast differentiation (Pratap et al., 2003; Soltanoff et al., 2009; Sancisi et al., 2012; Gong et al., 2013). It was also reported to have a role in thyroid carcinogenesis. Specifically, levels of this marker were found to be significantly higher in larger PTC tumors (Gong et al., 2013). Moreover, RUNX2 regulates cellular invasion in thyroid tumor cells (Sancisi et al., 2012). However, there have been few reports regarding regulators of RUNX2 during thyroid calcification and carcinogenesis. Accordingly, I identified one candidate, HOXA9, and confirmed that it regulates the expression of RUNX2 in thyroid cell lines. Homeobox (HOX) family genes encode a class of transcription factors that regulate the expression of numerous genes,
- 52 -
control cell growth, and drive specific tissue differentiation (Grier et al., 2005). Further, the expression of HOX family genes is often dysregulated in tumors (Samuel and Naora, 2005). HOXA9, a transcription factor, is most commonly altered in solid tumors (Bhatlekar et al., 2014). To demonstrate the function of HOXA9 in PTC calcification, I performed ALP and ARS staining assays. ALP activity and calcification were enhanced in overexpressing Nthy-Ori 3-1 and TPC1 cells and reduced in HOXA9-knockdown TPC1 and BHP10-3 cells. These results indicated that calcification could be regulated by HOXA9 in thyroid cell lines.
Many studies have established the function of HOXA9 in cancer cells. There are some reports indicating that knockdowned the expression of HOXA9 can significantly decrease colony formation, invasion, and migration in colorectal cancer cells (Gaspar et al., 2010; Wang et al., 2017; Bhatlekar et al., 2018). However, some others reported that HOXA9 inhibits migration and that the hypermethylation of HOXA9 is especially apparent in the early stages of lung cancer (Hwang et al., 2011; Wrangle et al., 2014; Hwang et al., 2015). Moreover, low expression of this marker was observed in cervical cancer cells and proliferation and migration were suppressed when HOXA9 expression was restored in these cells (Alvarado-Ruiz et al., 2016). This protein was also found to restrict cell growth, survival, and invasion in T4-2 breast cancer cells (Gilbert et al., 2010). In contrast, it was determined that HOXA9 promotes the viability and aggressiveness of glioblastoma cells (Pojo et al., 2015). Furthermore, in ovarian cancer, this factor was shown to induce peritoneal macrophages to obtain an M2 tumor-promoting phenotype (Ko et al., 2014). Likewise, HOXA9 encourages epithelial ovarian cancer growth in mouse xenograft models and supports the generation of a
- 53 -
microenvironment for tumor growth (Ko et al., 2012). However, there have been no reports regarding the role of HOXA9 in thyroid carcinoma. This study showed that the overexpression of HOXA9 significantly enhances in vitro normal cell migration and invasion and that tumor cell migration and invasion can be inhibited by downregulating HOXA9. The migration and invasion ability was increased in HOXA9-overexpressing cells more than control cells in the normal Nthy-Ori 3-1 cell line; whereas this difference was not significant in PTC cell lines. However HOXA9 knockdowned group considerably suppressed the cancer ability compare to the control groups in PTC cell lines, likewise the difference was not significant in normal cell line. About thses results, I infer that TPC1 and BHP10-3 cell lines are cancer cell line, and they already has the high level ability of tumor aggressiveness, therefore, HOXA9 not impressed PTC cell lines as much as normal cell line. My results thus showed that HOXA9 could be related to thyroid cell migration and invasion.
After confirming the relationship between RUNX2 and HOXA9, as well as the function of HOXA9 in thyroid calcification and carcinogenesis in vitro, I further identified the expression of these markers in vivo using thyroid cancer patient tissue samples to confirm these results. IHC results showed that RUNX2 and HOXA9 were concurrently expressed in calcified thyroid cancer tissues indicating that HOXA9, as a positive regulator of RUNX2, could be linked to the calcification and tumor invasion of thyroid cancer via RUNX2.
As stated, HOXA9 is an upstream regulator of RUNX2. Therefore, I investigated whether its effect on enhanced calcification and carcinogenesis occurs through RUNX2.
- 54 -
Thus, I downregulated RUNX2 in two types of thyroid cell lines with or without HOXA9 overexpression. My results indicated that ALP activity, mineralization behaviour, and cell migration and invasion capacity were all significantly decreased upon RUNX2 downregulation. These results were similar to a previous report showing that the downregulation of RUNX2 can impair migration and invasion in thyroid tumor cells (Sancisi et al., 2012). However, all of these activities were increased with HOXA9 overexpression, as compared to those in the RUNX2-knockdown only groups. There have some reports about the molecular mechanisms independent of RUNX2 in the formation of the calcification and tumor progression of PTC. Like the strong correlation between bone sialoprotein (BSP) and osteopontin (OPN) have a role in calcification and tumor progression of PTC (Wu et al., 2015). Moreover, osteocalcin, OPN, and CD44v6 were important in the formation of the calcification in PTC (Gong and Wang, 2012). These results suggested that HOXA9 might enhance the calcification and tumor invasion of PTC not only via RUNX2, but also independently of this marker.
HOXA9, a positive regulator of RUNX2, can enhance calcification and cancer migration and invasion ability in PTC, dependent or independent of RUNX2. My data improved the understanding of the molecular mechanisms of calcification and tumorigenesis in PTC and might lead to the development of novel diagnostic or prognostic biomarkers for this disease.
- 55 -
V. CONCLUSION
I investigated the upstream of RUNX2 at the transcription factor search site to find the regulator of RUNX2. The genes which scores greater than 95.00 were selected as candidate genes. Homeobox family A 9, HOXA 9, was selected as the primary candidate gene via semi-quantitative PCR and real-time PCR, because the expression of HOXA9 was changed over time with RUNX2. And the normal thyroid cell line Nthy-Ori 3-1 and PTC cell line TPC1 and BHP10-3 were used in vitro. I knockdowned and overexpressed HOXA9 in the two types of thyroid cell lines, and found the expression of RUNX2 was changed with the expression of HOXA9. HOXA9 also increased RUNX2 promoter activity and RUNX2 binding ability. Calcification was enhanced in HOXA9 overexpressed thyroid cell lines than control. Overexpression of HOXA9 significantly enhanced in vitro normal cell migration and invasion, while downregulation of HOXA9 inhibited tumor cell migration and invasion. IHC assay was administered in calcified thyroid cancer tissues, and RUNX2 and HOXA9 were simultaneously expressed. Then the expression of RUNX2 was downregulated in two types of thyroid cell lines with or without HOXA9 overexpression. ALP activity, mineralization behaviour, and cancer capacity were all considerably decreased upon RUNX2 downregulation, while all of these activities were increased with HOXA9 overexpression, as compared to those in the RUNX2-knockdown only groups.
- 56 -
This is the first report demonstrating the function of HOXA9 on PTC. Based on the
in vitro and in vivo data, my results suggest that HOXA9 can be linked to calcification
- 57 -
REFERENCES
1. Alvarado-Ruiz L, Martinez-Silva MG, Torres-Reyes LA, Pina-Sanchez P, Ortiz-Lazareno P, Bravo-Cuellar A, Aguilar-Lemarroy A, Jave-Suarez LF: HOXA9 is Underexpressed in Cervical Cancer Cells and its Restoration Decreases Proliferation, Migration and Expression of Epithelial-to-Mesenchymal Transition Genes. Asian Pac J Cancer Prev 17: 1037-1047, 2016
2. Bhatlekar S, Fields JZ, Boman BM: HOX genes and their role in the development of human cancers. J Mol Med (Berl) 92: 811-823, 2014
3. Bhatlekar S, Viswanathan V, Fields JZ, Boman BM: Overexpression of HOXA4 and HOXA9 genes promotes self-renewal and contributes to colon cancer stem cell overpopulation. J Cell Physiol 233: 727-735, 2018
4. Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J: Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer 55: 805-828, 1985
5. Chang CH, Fan TC, Yu JC, Liao GS, Lin YC, Shih AC, Li WH, Yu AL: The prognostic significance of RUNX2 and miR-10a/10b and their inter-relationship in breast cancer. J Transl Med 12: 257, 2014
6. Cohen MM, Jr.: Perspectives on RUNX genes: an update. Am J Med Genet A 149A: 2629-2646, 2009
- 58 -
1999
8. Dalle Carbonare L, Frigo A, Francia G, Davi MV, Donatelli L, Stranieri C, Brazzarola P, Zatelli MC, Menestrina F, Valenti MT: Runx2 mRNA expression in the tissue, serum, and circulating non-hematopoietic cells of patients with thyroid cancer. J Clin Endocrinol Metab 97: E1249-1256, 2012
9. Das DK: Psammoma body: a product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor?
Diagn Cytopathol 37: 534-541, 2009
10. DeLellis RA: Pathology and genetics of tumours of endocrine organs, IARC, 2004
11. Endo T, Ohta K, Kobayashi T: Expression and function of Cbfa-1/Runx2 in thyroid papillary carcinoma cells. J Clin Endocrinol Metab 93: 2409-2412, 2008 12. Gaspar N, Marshall L, Perryman L, Bax DA, Little SE, Viana-Pereira M, Sharp
SY, Vassal G, Pearson AD, Reis RM, Hargrave D, Workman P, Jones C: MGMT-independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3-kinase-mediated HOX/stem cell gene signature. Cancer Res 70: 9243-9252, 2010
13. Gilbert PM, Mouw JK, Unger MA, Lakins JN, Gbegnon MK, Clemmer VB, Benezra M, Licht JD, Boudreau NJ, Tsai KK, Welm AL, Feldman MD, Weber BL, Weaver VM: HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J Clin Invest 120: 1535-1550, 2010
- 59 -
14. Gong T, Wang J: The analysis of the calcification in differentiating malignant thyroid neoplasm and the molecular mechanisms for the formation of the calcification. Lin chuang er bi yan hou tou jing wai ke za zhi= Journal of clinical
otorhinolaryngology, head, and neck surgery 26: 763-766, 2012
15. Gong T, Wang J, Qian M, Zhou Y: [Detection of Runx2 mRNA expression using relatively real-time RT-PCR in papillary thyroid carcinoma]. Lin Chung Er Bi
Yan Hou Tou Jing Wai Ke Za Zhi 27: 193-195, 2013
16. Grier DG, Thompson A, Kwasniewska A, McGonigle GJ, Halliday HL, Lappin TR: The pathophysiology of HOX genes and their role in cancer. J Pathol 205: 154-171, 2005
17. Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M: Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem 274: 6972-6978, 1999
18. Hwang JA, Lee BB, Kim Y, Hong SH, Kim YH, Han J, Shim YM, Yoon CY, Lee YS, Kim DH: HOXA9 inhibits migration of lung cancer cells and its hypermethylation is associated with recurrence in non-small cell lung cancer.
Mol Carcinog 54 Suppl 1: E72-80, 2015
19. Hwang SH, Kim KU, Kim JE, Kim HH, Lee MK, Lee CH, Lee SY, Oh T, An S: Detection of HOXA9 gene methylation in tumor tissues and induced sputum samples from primary lung cancer patients. Clin Chem Lab Med 49: 699-704, 2011
- 60 -
20. Johannessen JV, Sobrinho-Simoes M: The origin and significance of thyroid psammoma bodies. Lab Invest 43: 287-296, 1980
21. Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H: HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J
Clin Invest 122: 3603-3617, 2012
22. Ko SY, Ladanyi A, Lengyel E, Naora H: Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am J Pathol 184: 271-281, 2014
23. Lacout A, Chevenet C, Thariat J, Marcy PY: Thyroid calcifications: a pictorial essay. J Clin Ultrasound 44: 245-251, 2016
24. Lloyd RV: Endocrine Pathology:: Differential Diagnosis and Molecular Advances, Springer Science & Business Media, 2010
25. Morgan R, El-Tanani M: HOX Genes as Potential Markers of Circulating Tumour Cells. Curr Mol Med 16: 322-327, 2016
26. Morgan R, El-Tanani M, Hunter KD, Harrington KJ, Pandha HS: Targeting HOX/PBX dimers in cancer. Oncotarget 8: 32322-32331, 2017
27. Niu DF, Kondo T, Nakazawa T, Oishi N, Kawasaki T, Mochizuki K, Yamane T, Katoh R: Transcription factor Runx2 is a regulator of epithelial-mesenchymal transition and invasion in thyroid carcinomas. Lab Invest 92: 1181-1190, 2012 28. Pojo M, Goncalves CS, Xavier-Magalhaes A, Oliveira AI, Goncalves T, Correia
- 61 -
Sousa N, Costa BM: A transcriptomic signature mediated by HOXA9 promotes human glioblastoma initiation, aggressiveness and resistance to temozolomide.
Oncotarget 6: 7657-7674, 2015
29. Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ: Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63: 5357-5362, 2003
30. Pratap J, Lian JB, Stein GS: Metastatic bone disease: role of transcription factors and future targets. Bone 48: 30-36, 2011
31. Samuel S, Naora H: Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer 41: 2428-2437, 2005 32. Sancisi V, Borettini G, Maramotti S, Ragazzi M, Tamagnini I, Nicoli D, Piana S,
Ciarrocchi A: Runx2 isoform I controls a panel of proinvasive genes driving aggressiveness of papillary thyroid carcinomas. J Clin Endocrinol Metab 97: E2006-2015, 2012
33. Shrestha RT, Karunamurthy A, Amin K, Nikiforov YE, Caramori ML: Multiple mutations detected preoperatively may predict aggressive behavior of papillary thyroid cancer and guide management—a case report. Thyroid 25: 1375-1378, 2015
34. Sipos JA, Mazzaferri EL: Thyroid cancer epidemiology and prognostic variables.
- 62 -
35. Soltanoff CS, Yang S, Chen W, Li YP: Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene
Expr 19: 1-46, 2009
36. Trotter TN, Li M, Pan Q, Peker D, Rowan PD, Li J, Zhan F, Suva LJ, Javed A, Yang Y: Myeloma cell-derived Runx2 promotes myeloma progression in bone.
Blood 125: 3598-3608, 2015
37. Underwood JC, Cross SS: General and Systematic Pathology, International Edition E-Book: with STUDENT CONSULT Access, Elsevier Health Sciences, 2009
38. Wang X, Bu J, Liu X, Wang W, Mai W, Lv B, Zou J, Mo X, Li X, Wang J, Niu B, Fan Y, Hou B: miR-133b suppresses metastasis by targeting HOXA9 in human colorectal cancer. Oncotarget 8: 63935-63948, 2017
39. Wrangle J, Machida EO, Danilova L, Hulbert A, Franco N, Zhang W, Glockner SC, Tessema M, Van Neste L, Easwaran H, Schuebel KE, Licchesi J, Hooker CM, Ahuja N, Amano J, Belinsky SA, Baylin SB, Herman JG, Brock MV: Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin Cancer Res 20: 1856-1864, 2014
40. Wu G, Guo J-J, Ma Z-Y, Wang J, Zhou Z-W, Wang Y: Correlation between calcification and bone sialoprotein and osteopontin in papillary thyroid carcinoma. International journal of clinical and experimental pathology 8: 2010-2017, 2015
- 63 -
41. Wysokinski D, Blasiak J, Pawlowska E: Role of RUNX2 in Breast Carcinogenesis. Int J Mol Sci 16: 20969-20993, 2015
42. Zambotti A, Makhluf H, Shen J, Ducy P: Characterization of an osteoblast-specific enhancer element in the CBFA1 gene. J Biol Chem 277: 41497-41506, 2002
- 64 - Appendix 1. The result of transcription factors
** TFSEARCH ver.1.3 ** (c)1995 Yutaka Akiyama (Kyoto Univ.) <Warning> Scoring scheme is so straightforward in this version. score = 100.0 * ('weighted sum' - min) / (max - min) The score does not properly reflect statistical significance!
Database: TRANSFAC MATRIX TABLE, Rel.3.3 06-01-1998 Query: untitled (3100 bases)
Taxonomy: Vertebrate Threshold: 85.0 point
TFMATRIX entries with High-scoring:
1 GTATTAAAAT ATGATCATTA GTTAAGAAAA TAAAAAATTA CTCAAATGGT entry score <--- M00101 CdxA 92.9 <--- M00130 HFH-2 89.3 <--- M00131 HNF-3b 86.7 <--- M00101 CdxA 86.4 <--- M00129 HFH-1 86.2 ---> M00116 C/EBPa 85.7 ---> M00101 CdxA 85.0
51 TACAATGAAG ACACACATAG CATTTAAACA CAAATACCTT GGTATTTTCA entry score ---> M00148 SRY 92.7 <--- M00100 CdxA 91.0 ---> M00100 CdxA 88.5 <--- M00241 Nkx-2. 85.3
- 65 -
101 AAAGTTCAAC CAATTCTAAC ACATTTTATT CACTACAAAG CAATGGAGAA entry score ---> M00101 CdxA 92.9
151 GCAAGATACC AACAACTTTT TTTCTTTGAA ATTGATTTCA AGATCAAAAC entry score <--- M00148 SRY 90.0 ---> M00106 CDP CR 87.7 ---> M00076 GATA-2 87.4
201 TGTATTTCAT TTCCATGGCA TTAAAGTATG TGATTTATAC AGTATACCTA entry score ---> M00101 CdxA 100.0 ---> M00100 CdxA 96.2 <--- M00101 CdxA 92.1 <--- M00162 Oct-1 87.8 <--- M00101 CdxA 85.0
251 AACTTGTTGC TTACTTCAAA ACAGCAAATA CTAACATGTC CCCAGAGAGG entry score ---> M00101 CdxA 92.9 <--- M00083 MZF1 87.8 <--- M00131 HNF-3b 86.7 ---> M00148 SRY 86.4 --- M00261 Olf-1 85.4
301 ATAAAATTTT ATGGGTCTTT AAAAAGCAAA AATAAAAATA AAAATAAATC entry score ---> M00101 CdxA 92.1 ---> M00216 TATA 91.8 ---> M00100 CdxA 91.0 <--- M00101 CdxA 90.0 <--- M00100 CdxA 89.7 <--- M00216 TATA 89.6 <--- M00131 HNF-3b 87.3 <--- M00100 CdxA 87.2
- 66 - ---> M00148 SRY 86.4 ---> M00101 CdxA 85.7 ---> M00101 CdxA 85.7 ---> M00101 CdxA 85.7 ---> M00261 Olf-1 85.4
351 TGTGGTTTTC AATTTCTGAT TATAACCATG GTATAAATCT ATAATCAAGA entry score ---> M00271 AML-1a 100.0 <--- M00101 CdxA 100.0 <--- M00100 CdxA 96.2 ---> M00101 CdxA 93.6 <--- M00137 Oct-1 91.6 ---> M00100 CdxA 89.7 ---> M00137 Oct-1 87.3 ---> M00101 CdxA 86.4 <--- M00101 CdxA 86.4
401 GCTTTATTTG CATTGACTTT TCTAAATCAG TATCTCTTCA CCACGTGTAG entry score ---> M00217 USF 98.0 ---> M00055 N-Myc 94.7 <--- M00217 USF 91.7 ---> M00131 HNF-3b 89.0 ---> M00123 c-Myc/ 88.6 <--- M00077 GATA-3 88.1 --- M00122 USF 87.5 <--- M00122 USF 87.5 ---> M00101 CdxA 87.1 <--- M00055 N-Myc 86.9 <--- M00109 C/EBPb 86.8 <--- M00076 GATA-2 86.6 <--- M00001 MyoD 86.0 ---> M00100 CdxA 85.9
- 67 - <--- M00100 CdxA 85.9 --- M00121 USF 85.5 <--- M00121 USF 85.5 <--- M00126 GATA-1 85.2 <--- M00267 XFD-1 85.0 <--- M00101 CdxA 85.0
451 TAAGCTATTT CTAATGAAAC ACACTGTTAT CAAATGTGTA CCAATTATCA entry score ---> M00101 CdxA 94.3 ---> M00148 SRY 92.7 --- M00099 S8 91.7 <--- M00241 Nkx-2. 88.2 > M00122 USF 87.5 - M00122 USF 87.5 ---> M00101 CdxA 86.4 ---> M00100 CdxA 85.9 > M00121 USF 85.5 - M00121 USF 85.5 ---> M00137 Oct-1 85.5
501 AATGTGCCCC AGTTGTCATT GTTTTTTAAA TTTTTACAAC AAAGTAATAT entry score --- M00131 HNF-3b 94.2 <--- M00042 Sox-5 92.2 > M00099 S8 91.7 <--- M00148 SRY 90.0 ---> M00101 CdxA 88.6 <--- M00100 CdxA 88.5 ---> M00130 HFH-2 85.5
551 TTGCTCCAAA GAGGATATAT TTTCGTTTTA GGAAAAAAAT TCATGTGGAT entry score ---> M00131 HNF-3b 94.2 --- M00137 Oct-1 90.5