저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
specific Antigen Nadir and Time to
Prostate-specific Antigen Nadir Following Maximal Androgen
Blockade Independently Predict the Prognosis in Patients
with Metastatic Prostate Cancer
By
Seok Young Hong
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
specific Antigen Nadir and Time to
Prostate-specific Antigen Nadir Following Maximal Androgen
Blockade Independently Predict the Prognosis in Patients
with Metastatic Prostate Cancer
By
Seok Young Hong
A Dissertation Submitted to the Graduate School of
Ajou University in Partial Fulfillment of the Requirements for the
Degree of Master of Medicine
Supervised by
Se Joong Kim, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
ofSeok Young Hong is approved.
SUPERVISORY COMMITTEE
SeJoong Kim
Young Soo Kim
Hyun SooAhn
The Graduate School, Ajou University
December, 14th, 2012
감사의 글
바쁜병원생활 틈틈이 논문을 쓰겠다고수많은 차트를 뒤적여가며 데이터를 수집해 나가는 동안 과연 이 많은 차트를 언제 다 확인할 수 있을까 하는 의구심이 드는 순간도 있었던 것 같은데, 어느덧 논문을 완성하고 이렇게 감사의 글을 쓰게 되는 순간이 오니 감회가 새롭습니다. 많은 시행착오를 겪으면서 한 편의 논문을 쓴다는 것이 이처럼 어려운 일이라는 것을 깨닫게 되었고, 그만큼 이번 경험은 저에게 추후 다른 연구를 수행하거나 이해하는 데 있어서 좋은 자양분이 되었을 거라 생각합니다. 우선 연구의 시작부터 끝까지, 너무나 부족한 점이 많았던 저에게 세세한 부분 하나 하나 살펴봐 주시면서 조언과 도움을 아끼지 않으셨던 김세중 교수님께 무한한 감사를 드립니다. 아울러 데이터 수집과 분석 과정에서, 난관에 봉착할 때마다 문제 해결에 아이디어와 도움을 주셨던 김선일 교수님, 조대성 선생님께도 감사 드립니다. 바쁘신 와중에 논문을 세심히 검토해 주시고 심사해 주신 김영수 교수님, 안현수 교수님, 역시 많은 조언과 격려를 아끼지 않으셨던 최종보 교수님께 감사를 드립니다. 그리고 4 년간 함께 하면서 힘든 시간 서로 의지할 수 있었던 동기 지용이, 같이 고생한 비뇨기과 선후배 모두에게 감사를 드립니다. 마지막으로 저를 키워주시고, 그저 믿음으로 묵묵히 저의 뒤에서 후원을 해주신 아버지, 어머니, 멀리 떨어져 있지만 늘 격려의 말을 아끼지 않았던 형까지, 우리 가족 모두에게 감사 드립니다.i
- Abstract -
Prostate-specific Antigen Nadir and Time to Prostate-specific
Antigen Nadir Following Maximal Androgen Blockade
Independently Predict the Prognosis in Patients with Metastatic
Prostate Cancer
Purpose: To evaluate the influence of prostate-specific antigen (PSA) kinetics following
maximal androgen blockade (MAB) on the disease progression and cancer-specific survival in patients with metastatic, hormone sensitive prostate cancer.
Materials and Methods: One hundred thirty-one patients with metastatic, hormone
sensitive prostate cancer treated with MAB at our institution were included in this study. Patients’ characteristics, PSA level at MAB initiation, PSA nadir, time to PSA nadir (TTN), and PSA decline were analyzed by using univariate and multivariate analysis.
Results:At a median follow-up of 30 months, 97 patients (74.0%) showed disease
progression and 65 patients (49.6%) died. Fifty-nine patients (45.0%) died from prostate cancer. On univariate analysis, PSA at MAB initiation, PSA nadir, TTN, and PSA decline were significant predictors of progression-free survival. And PSA nadir, TTN, and PSA decline were significant predictors of cancer-specific survival. On multivariate analysis, higher PSA nadir (≥0.2 ng/ml) and shorter TTN (<8 months) were independent predictors of shorter progression-free and cancer-specific survivals. In the combined analysis of PSA nadir and TTN, patients with higher PSA nadir and shorter TTN had the worst progression-free survival (hazard ratio 14.098, p<0.001) and cancer-specific survival (hazard ratio 14.050, p<0.001) compared to those with lower PSA nadir and longer TTN.
Conclusions: Our results suggest that higher PSA nadir level and shorter TTN following
MAB are associated with higher risk of disease progression and poorer survival in patients with metastatic, hormone sensitive prostate cancer. Furthermore, these two variables have synergistic effect on the patients’outcome.
ii
Table of Contents
ABSTRACT ··· i
TABLE OF CONTENTS ··· ii
LIST OF FIGURES ··· iii
LIST OF TABLES ···iv
ABBREVIATION ··· v
I. INTRODUCTION ···1
II. MATERIALS AND METHODS ···3
III. RES ULTS ··· ··· ··· ··· ··· ··· ··· ··· ···5
IV. DISCUSSION ··· 11
V. CONCLUSIONS ···15
REFERENCES ···16
iii
List of Figures
Fig. 1.Kaplan-Meier progression-free survival curves according to prostate-specific antigen
(PSA) at maximal androgen blockade (MAB) initiation (A), PSA nadir (B), time to PSA nadir (TTN) (C), and PSA decline (D)···7
Fig. 2.Kaplan-Meier cancer-specific survival curves according to prostate-specific antigen
(PSA) at maximal androgen blockade (MAB) initiation (A), PSA nadir (B), time to PSA nadir (TTN) (C), and PSA decline (D)···8
Fig. 3.Kaplan-Meier progression-free (A) and cancer-specific (B) survival curves according
iv
List of Tables
Table 1.Characteristics of the 131 patients who underwent MAB···6
Table 2.Univariate analysis of potential prognostic factors for progression-free and
cancer-specific survival···9
Table 3.Multivariate analysis of potential adverse prognostic factors for progression-free and
- 1 -
I. INTRODUCTION
Prostate cancer is the most common cancer of men in the United States and the fifth most common cancer of men in Korea(Kim and Kim, 2010). Recently, with the increase in use of prostate-specific antigen (PSA) as a screening tool, the number of men presenting with metastatic disease has been decreasing(Hori et al, 2011).
Androgen deprivation therapy (ADT) remains the mainstay of treatment for metastatic, hormone sensitiveprostate cancer. Although the majority of patients with metastatic disease initially respond well to ADT, almost all patients will eventually progress to castration-resistant prostate cancer (CRPC). Treatment options for CRPC remain limited, and the prognosis of patients with CRPC is dismal(Hori et al, 2011). Therefore, an accurate prediction of the response to ADT and individual survival would allow better prognostic evaluation and decision making for the best treatment strategy.
The serum PSA level correlates with tumor volume and increases 6 to 12 months before definitive radiological or clinical proof of disease progression(Arai et al, 1990; Cooper et al, 1990; Mulders et al, 1992). PSA kinetics has been used as a useful prognostic indicator of disease progression or survival in different clinical settingsincluding radical prostatectomy and radiation therapy (D’Amico et al, 1998; Zhou et al, 2005; May et al, 2005; King et al, 2008). However, its prognostic ability for patients with metastatic prostate cancer treated with ADT is still not well understood. Nadir PSA level has been suggested to be the most significant predictor of progression to CRPC in many studies (Ercole et al, 1987; Miller et al, 1992; Benaim et al, 2002; Kwak et al, 2002; Morote et al, 2004; Morote et al, 2005; Park et al, 2009; Huang et al, 2011). However, there is controversy about the time to PSA nadir (TTN). Whereas earlier reports suggested that shorter TTN correlated with longer remission periods (Stamey et al, 1989; Arai et al, 1990; Cooper et al, 1990; Petros and Andriole, 1993; Furuya et al, 1998; Park et al, 2003) or TTN did not correlate with progression to CRPC(Park et al, 2007), several recent reports suggested that longer TTN correlated with longer remission and survival(Benaim et al, 2002; Morote et al, 2004; Morote et al, 2005; Choueiri et al, 2009; Park et al, 2009; Hori et al, 2011; Huang et al, 2011; Sasaki et al, 2011; Huang et al, 2012).
- 2 -
ofpatients with metastatic, hormone sensitive prostate cancer to evaluate the influence of PSA kinetics following maximal androgen blockade (MAB) on the disease progression and cancer-specific survival.
- 3 -
II. MATERIALS AND METHODS
A total of 131 patients with newly diagnosed metastatic, hormone sensitive prostate cancer who started primary ADT with MAB (combination of luteinizing hormone-releasing hormone (LHRH) agonists or bilateral orchiectomy and antiandrogen) at our institution between July1996 and July 2010 were included in this study. Prostate cancer was diagnosed pathologically in all patients. In 4 patients with the pathologic diagnosis obtained through the biopsy of metastatic site, Gleason score could not be identified. LHRH agonist was used in 114patients (87.0%)and bilateral orchiectomy was done in 17 patients (13.0%)as part of MAB. All patients had not received any treatment such as prostatectomy or radiation therapy for the primary lesion.
Patients’ characteristics, PSA at MAB initiation, PSA nadir, TTN, and PSA decline were analyzed. The definitions used in this study followed those described by Huang et al(Huang et al, 2011; Huang et al, 2012). The PSA nadir was defined as the lowest PSA value achieved during ADT. TTN was defined as the duration of time from the initiation of ADT to the date of PSA nadir. PSA decline was calculated from the slope of the linear regression of the PSA values over time from the beginning of ADT to the nadir PSA. Progression was defined as a serial rise in PSA, at least two consecutive rises in PSA (>1 week apart) greater than the PSA nadir. The date of progression was defined as the date of first PSA rise.
Patients were dichotomized according to the median value of continuous variables except for the PSA nadir. Dichotomization according to PSA nadir of 0.2 ng/ml was used because it has been previously reported to correlate with prostate cancer-specific survival and disease progression in other studies (Morote et al, 2004; Stewart et al, 2005; Hussain et al, 2006; Choueiri et al, 2009; Huang et al, 2011; Sasaki et al, 2011; Huang et al, 2012). To evaluate the interactive effect of PSA nadir and TTN on the disease progression and survival, patients were stratified into four groups: (1) PSA nadir <0.2 ng/ml and TTN ≥8 months, (2) PSA nadir <0.2 ng/ml and TTN <8 months, (3) PSA nadir ≥0.2 ng/ml and TTN ≥8 months, and (4) PSA nadir ≥0.2ng/ml and TTN <8 months. The number of each group was 39, 14, 29 and 49, respectively. The progression-free and cancer-specific survival curves were obtained by Kaplan-Meier method, and univariate and multivariate analyses were performed by using the
- 4 -
log-rank test and the Cox’s proportional hazards regression model, respectively. All statistical analyses were performed by using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Values of p<0.05 were considered to be statistically significant in all of the analyses.
- 5 -
III. RESULTS
The clinicopathological characteristics of the 131 patients included in the study are summarized in Table 1. At MAB initiation, the median age of the patients was 72 years and median PSA level was151 ng/ml. During MAB, the median PSA nadir was 0.5ng/ml and 53 patients (40.5%) achieved PSA nadir <0.2 ng/ml. The median TTN was 8 months and median PSA decline was 242 ng/ml/year. The median follow-up period was 30 months (range, 7 to 133 months). Of the total 131 patients, 97 patients (74.0%) showed disease progression and 65 patients (49.6%) died during the follow-up. Among them, 59 patients (45.0%) died from prostate cancer.
- 6 -
Table 1. Characteristics of the 131 patients who underwent MAB
Characteristic Value At MAB initiation Age (yr) 72 (42-92) Clinical stage M1 105 (80.2) ≥N1 88 (67.2)
Biopsy Gleason score
<7 5 (3.8) 7 32 (24.4) ≥8 90 (68.7) PSA (ng/ml) 151 (10.38-5,000) Types of MAB LHRH agonists + Antiandrogen 114 (87.0)
Bilateral orchiectomy + Antiandrogen 17 (13.0)
During MAB
Nadir PSA (ng/ml) 0.5 (0.003-595.7)
PSA nadir <0.2 ng/ml 53 (40.5)
PSA nadir ≥0.2 ng/ml 78 (59.5)
Time to PSA nadir (months) 8 (1-48)
PSA decline after MAB (ng/ml/yr) 242 (2.9-29878)
Progression/death
Progression 97 (74.0)
Total death 65 (49.6)
Cancer-specific death 59 (45.0)
Values are presented as median (range) or number (%). LHRH, luteinizing hormone-releasing hormone; MAB, maximal androgen blockade; PSA, prostate-specific antigen.
- 7 -
The mean progression-free survival in patients with PSA <151 ng/ml at MAB initiation was significantly longer than those in patients with PSA ≥151 ng/ml at MAB initiation (34.4 months vs. 17.9 months, p=0.005) (Fig. 1A), but there was no significant difference in cancer-specific survival between the two groups(p=0.236) (Fig. 2A). The mean progression-free and cancer-specific survival in patients with PSA nadir <0.2 ng/ml were significantly longer than those in patients with PSA nadir ≥0.2 ng/ml (46.5 months vs. 13.0 months, p<0.001; 91.7 months vs. 49.8 months, p<0.001) (Figs. 1B, 2B). The mean progression-free and cancer-specific survival in patients with TTN ≥8 months were significantly longer than those in patients with TTN <8 months (35.1 months vs. 13.9 months, p<0.001; 88.4 months vs. 42.7 months, p<0.001) (Figs. 1C, 2C). The mean progression-free and cancer-specific survival in patients with PSA decline <242 ng/ml/year were significantly longer than those in patients with PSA decline ≥242 ng/ml/year (31.8 months vs. 16.3 months, p<0.001; 79.2 months vs. 50.0 months, p<0.001) (Figs. 1D, 2D).
- 8 -
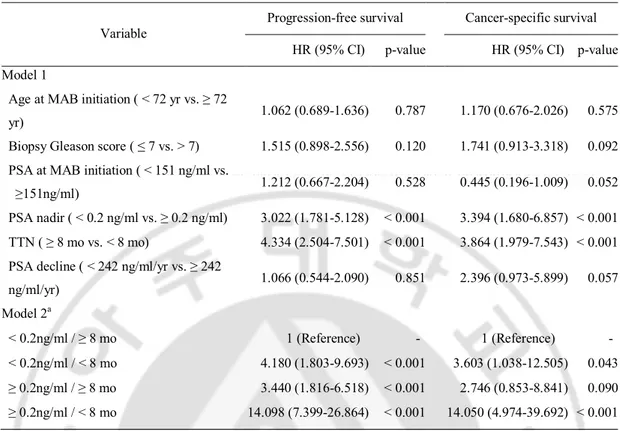
On univariate analysis, PSA at MAB initiation, PSA nadir, TTN, and PSA decline were significant predictors of progression-free survival. And PSA nadir, TTN, and PSA decline were significant predictors of cancer-specific survival (Table 2). On multivariate analysis, higher PSA nadir (≥0.2 ng/ml) and shorter TTN (<8 months) were independent predictors of shorter progression-free and cancer-specific survivals (Table 3). In the combined analysis of PSA nadir and TTN, patients with higher PSA nadir and shorter TTN had the worst progression-free survival (hazard ratio 14.098, p<0.001) and cancer-specific survival (hazard ratio 14.050, p<0.001) compared to those with lower PSA nadir and longer TTN (Table 3, Fig. 3).
- 9 -
Table 2.Univariate analysis of potential prognostic factors for progression-free and cancer-specific survival Variable p-value Progression-free survival Cancer-specific survival
Age at MAB initiation (<72yr vs. ≥72yr) 0.054 0.347
Biopsy Gleason score (≤7 vs. >7) 0.060 0.064
PSA at MAB initiation (<151ng/ml vs.≥151
ng/ml) 0.005 0.236
PSA nadir (<0.2 ng/ml vs. ≥0.2 ng/ml) <0.001 <0.001
Time to PSA nadir (≥8mo vs. <8 mo) <0.001 <0.001
PSA decline (<242ng/ml/yr vs. ≥242ng/ml/yr) <0.001 <0.001 MAB, maximal androgen blockade; PSA, prostate-specific antigen.
- 10 -
Table 3. Multivariate analysis of potential adverse prognostic factors for progression-free and cancer-specific survival
Variable Progression-free survival Cancer-specific survival HR (95% CI) p-value HR (95% CI) p-value Model 1
Age at MAB initiation ( < 72 yr vs. ≥ 72
yr) 1.062 (0.689-1.636) 0.787 1.170 (0.676-2.026) 0.575 Biopsy Gleason score ( ≤ 7 vs. > 7) 1.515 (0.898-2.556) 0.120 1.741 (0.913-3.318) 0.092 PSA at MAB initiation ( < 151 ng/ml vs.
≥151ng/ml) 1.212 (0.667-2.204) 0.528 0.445 (0.196-1.009) 0.052 PSA nadir ( < 0.2 ng/ml vs. ≥ 0.2 ng/ml) 3.022 (1.781-5.128) < 0.001 3.394 (1.680-6.857) < 0.001 TTN ( ≥ 8 mo vs. < 8 mo) 4.334 (2.504-7.501) < 0.001 3.864 (1.979-7.543) < 0.001 PSA decline ( < 242 ng/ml/yr vs. ≥ 242
ng/ml/yr) 1.066 (0.544-2.090) 0.851 2.396 (0.973-5.899) 0.057 Model 2a < 0.2ng/ml / ≥ 8 mo 1 (Reference) - 1 (Reference) - < 0.2ng/ml / < 8 mo 4.180 (1.803-9.693) < 0.001 3.603 (1.038-12.505) 0.043 ≥ 0.2ng/ml / ≥ 8 mo 3.440 (1.816-6.518) < 0.001 2.746 (0.853-8.841) 0.090 ≥ 0.2ng/ml / < 8 mo 14.098 (7.399-26.864) < 0.001 14.050 (4.974-39.692) < 0.001 HR, hazard ratio; CI, confidence interval; MAB, maximal androgen blockade; PSA, prostate-specific antigen; TTN, time to PSA nadir.a:Combined analysis of PSA nadir/TTN.
- 11 -
IV. DISCUSSION
It has generallybeen accepted that serum PSA parameters, such as pre-treatment PSA level, PSA nadir level after treatment, time to PSA nadir and the pattern of PSA decline after treatment, are useful indicators for evaluating the response to ADT in patients with metastatic prostate cancer. However, the prognostic significance of these PSA parameters is still controversial.
While the pre-treatment PSA level has been suggested to predict the response to ADT in some reports(Cooper et al, 1990; Kwak et al, 2002; Morote et al, 2004; Ross et al, 2008), it did not predict the interval to progression to CRPC in other studies(Miller et al, 1992; Lee et al, 2000; Benaim et al, 2002). In our study, the pre-treatment PSA level was a significant predictor of progression-free survival on univariate analysis, but it lost the statistical significance on multivariate analysis.
Nadir PSA level after ADT has been suggested to be the most significant predictor of progression to CRPC (Ercole et al, 1987; Miller et al, 1992; Kwak et al, 2002; Benaim et al, 2002; Morote et al, 2004; Morote et al, 2005; Park et al, 2009; Huang et al, 2011)and survival (Choueiri et al, 2009; Sasaki et al, 2011; Huang et al, 2012) in many studies.Our study also demonstrated that lowerPSA nadir after MAB is an independent predictor of longer progression-free and cancer-specific survivals. However, there is no consensus on the optimal threshold of PSA nadir after ADT for predicting the disease progression and survival.Kwak et al. (Kwak et al, 2002) reported that a lower limit for the nadir PSA level of 1.1 ng/ml gives optimal sensitivity and specificity for predicting the progression to CRPC. Park et al. (Park et al, 2007),Morote et al. (Morote et al, 2005)and Miller et al.(Miller et al, 1992)suggested that the nadir PSA level of 0.5, 2, and 4ng/mlwas the optimal threshold for predicting the progression to CRPC, respectively.Hussain et al. (Hussain et al, 2006) reported that a PSA of <4 ng/ml after 7 months of ADT was a strong predictor of survival. However, in many other studies, the nadir PSA level of 0.2 ng/ml was suggested to be the optimal threshold for predicting the progression to CRPC (Morote et al, 2004; Huang et al, 2011) and survival(Stewart et al, 2005; Choueiri et al, 2009; Sasaki et al, 2011; Huang et al, 2012).Morote et al. (Morote et al, 2004) reported that the nadir PSA level of 0.2 ng/ml was
- 12 -
the optimal threshold for predicting the progression to CRPC and the failure to achieve a nadir PSA level of 0.2 ng/ml was associated with a 20 times likelihood of progression to CRPC within 24 months. Stewart et al. (Stewart et al, 2005) suggested that a PSA nadir of >0.2 ng/ml after 8 months of ADT was significantly associated with prostate cancer-specific mortality in patients with biochemical recurrence after radical prostatectomy or radiation therapy. Therefore, the nadir PSA level of 0.2 ng/ml was chosen as the threshold in our study and it showed that higher PSA nadir (≥0.2 ng/ml) after MAB wasan independent predictor of shorter progression-free and cancer-specific survivals.
The prognostic significance of TTN after ADT on the disease progression and survival is also controversial.Earlier reports suggested that shorter TTN correlated with longer remission periods (Stamey et al, 1989; Arai et al, 1990; Cooper et al, 1990; Petros and Andriole, 1993; Furuya et al, 1998; Park et al, 2003). Stamey et al. (Stamey et al, 1989) reported that patients whose PSA decreased rapidly to an undetectable levelafter ADT had prolonged survivaland showed that PSA level at 6 months after ADT was capable of distinguishing patients with a favorable responsefrom those with a limited response to ADT. Cooper et al. (Cooper et al, 1990) also reported that PSA nadir reached within 6 months after ADT was a predictor of survival in a univariate analysis. Arai et al. (Arai et al, 1990) showed that patients whose PSA decreased rapidly to an undetectable level within 1 month after ADT had the best prognosis and patients whose PSA level remained elevated for more than 3 months had a risk of disease progression within 2 years. Petros et al. (Petros and Andriole, 1993) andFuruya et al. (Furuya et al, 1998) also demonstrated that normalization of PSA at 3 months after ADT was associated with a more favorable prognosis. However, Park et al. (Park et al, 2007) reported that TTN after MAB did not correlate with progression to CRPC.
On the contrary, several recent reports suggested that longer TTN correlated with longer remission and survival (Benaim et al, 2002; Morote et al, 2004; Morote et al, 2005; Choueiri et al, 2009; Park et al, 2009; Hori et al, 2011; Huang et al, 2011; Sasaki et al, 2011; Huang et al, 2012).Morote et al. (Morote et al, 2004)suggested that patients with longer TTN (>12 months)after ADT had 18 times higher likelihood to be free of progression to CRPC within 24 months than those with shorter TTN (≤12 months). Huang et al. (Huang et al, 2011) also reported that patients with shorter TTN (<10 months) after ADT had significantly shorter time to disease progression. Choueiri et al. (Choueiri et al, 2009), Huang et al. (Huang et al,
- 13 -
2012) andSasaki et al. (Sasaki et al, 2011) suggested that longer TTN (≥6 months, ≥10 months, >9 months, respectively) after ADT correlated with longer survival duration. Moreover, Huang et al. (Huang et al, 2011; Huang et al, 2012) reported that the patients with higher PSA nadir (≥0.2 ng/ml) and shorter TTN (<10 months) had significantly higher risk of disease progression, cancer-specific mortality and all-cause mortality compared to those with lower PSA nadir (<0.2 ng/ml) and longer TTN (≥10 months) (hazard ratio 3.11, 6.30, 4.79, respectively, all p<0.001). Sasaki et al. (Sasaki et al, 2011)also suggested that the combination of lower PSA nadir (<0.2 ng/ml) and longer TTN (>9 months) after ADT was the most important early predictor of longer survival.The results of our study also support these recent findings. Shorter TTN (<8 months) after ADT was independent predictor of shorter progression-free and cancer-specific survivals on multivariate analysis.Moreover, in the combined analysis of PSA nadir and TTN, patients with higher PSA nadir (≥0.2 ng/ml) and shorter TTN (<8 months) had the worst progression-free and cancer-specific survival compared to those with lower PSA nadir (<0.2 ng/ml) and longer TTN(≥8 months) (hazard ratio 14.098, 14.050, respectively, all p<0.001).The mechanismsresponsible for the association of shorter TTN with worse prognosis are not clear. The rapid decrease in PSA level may be related toa transcriptional effect of ADTon PSA production rather than prostate cancer cell death (Choueiri et al, 2009; Sasaki et al, 2011). The rapid decrease in PSA level after ADT may be due to ablation of androgen receptor function and the quick suppression of androgen / androgen receptorduring ADT may have a negative effect on disease progression, because androgen receptor can act as a tumor suppressor for the prostate cancer (Huang et al, 2011). Another possibility is that a rapid removal of hormone-sensitive prostate cancer cells may induce an adequate environment for the growth of hormone-resistant prostate cancer cells (Sasaki et al, 2011). But, further studies will be needed to verify these hypotheses.
Whereas biopsy Gleason score was not a predictor of progression to CRPC (Kwak et al, 2002) or overall survival (Sasaki et al, 2011) in patients treated with ADT in some reports, it was an independent predictor of progression to CRPC (Benaim et al, 2002; Morote et al, 2005; Park et al, 2007; Ross et al, 2008; Park et al, 2009; Huang et al, 2011)or survival (Hussain et al, 2006; Choueiri et al, 2009; Huang et al, 2012) in many other studies. In our study, biopsy Gleason score showed only marginal significance as a predictor of progression-free and cancer-specific survivalsin a univariate analysis (p=0.060, p=0.064,
- 14 -
respectively).While other studies included patients without metastasis (Benaim et al, 2002; Park et al, 2007; Ross et al, 2008; Huang et al, 2011; Huang et al, 2012) or applied ADT as primary treatment inonly 56 to 56.9% of patients(Benaim et al, 2002; Huang et al, 2011; Huang et al, 2012), all of our patients had metastasis at diagnosis and received ADT as the primary treatment. Moreover, among the studies with the same homogeneity of patients’ characteristics as ours, the proportion of patients with Gleason score >7(68.7%) in our study was much higher compared with that (46.9 to 58.9%) in other studies (Morote et al, 2005; Hussain et al, 2006; Choueiri et al, 2009). This might explain the lack of significance of Gleason score as a prognostic factor in our study.
This study had several limitations. First, this study was conducted in a single center. Second, it was retrospective in nature and the size of the study population was small. Third,some important factors, such as performance status andlactate dehydrogenase were not included in this study, because these data were not available in all patients. Nevertheless, we believe that the results of our study support the already reported role of PSA nadir and TTN following ADT as independent predictors of disease progression and survival in patients with metastatic prostate cancer.
- 15 -
V. CONCLUSIONS
Our results demonstrated that higher PSA nadir level and shorter TTN following MAB are associated with higher risk of disease progression and poorer survival in patients with metastatic, hormone sensitive prostate cancer. Furthermore, these two variables have synergistic effect on the outcome. These results may be helpful in predicting the prognosis after MAB and guiding decision making of the best treatment strategyin patients with metastatic, hormone sensitive prostate cancer.
- 16 -
REFERENCES
1. Arai Y, Yoshiki T, Yoshida O. Prognostic significance of prostate specific antigen in endocrine treatment for prostatic cancer. J Urol 144:1415-9, 1990
2. Benaim EA, Pace CM, Lam PM, Roehrborn CG. Nadir prostate-specific antigen as a predictor of progression to androgen-independent prostate cancer. Urology59:73-8, 2002 3. Choueiri TK, Xie W, D’Amico AV, Ross RW, Hu JC, Pomerantz M, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer 115:981-7, 2009
4. Cooper EH, Armitage TG, Robinson MR, Newling DW, Richards BR, Smith PH, et al. Prostatic specific antigen and the prediction of prognosis in metastatic prostatic cancer. Cancer 66(5 Suppl):1025-8, 1990
5. D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA280:969-74, 1998
6. Ercole CJ, Lange PH, Mathisen M, Chiou RK, Reddy PK, Vessella RL. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J Urol138:1181-4, 1987
7. Furuya Y, Akimoto S, Akakura K, Igarashi T, Murakami S, Shimazaki J, et al. Response of prostate-specific antigen after androgen withdrawal and prognosis in men with metastatic prostate cancer. UrolInt 60:28-32, 1998
8. Hori S, Jabbar T, Kachroo N, Vasconcelos JC, Robson CN, Gnanapragasam VJ. Outcomes and predictive factors for biochemical relapse following primary androgen deprivation therapy in men with bone scan negative prostate cancer. J Cancer Res ClinOncol137:235-41, 2011
9. Huang SP, Bao BY, Wu MT, Choueiri TK, Goggins WB, Huang CY, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in
- 17 -
prostate cancer treated with androgen-deprivation therapy. Prostate71:1189-97, 2011 10. Huang SP, Bao BY, Wu MT, Choueiri TK, GogginsWB,Liu CC, et al. Significant
associations of prostate-specific antigen nadir and time to prostate-specific antigen nadir with survival in prostate cancer patients treated with androgen-deprivation therapy. Aging Male 15:34-41, 2012
11. Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J ClinOncol24:3984-90, 2006 12. Kim SJ, Kim SI. Incidence, epidemiology and patterns of progression of prostate cancer.
J Korean Med Assoc53:92-7, 2010
13. King CR, Presti JC, Brooks JD, Gill H, Spiotto MT. Postoperative prostate-specific antigen velocity independently predicts for failure of salvage radiotherapy after prostatectomy. Int J RadiatOncolBiolPhys70:1472-7, 2008
14. Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol168:995-1000, 2002
15. Lee SY, Kim YS, Hong SJ. Clinical response of combined androgen blockade in metastatic prostate cancers. Korean J Urol41:361-6, 2000
16. May M, Gunia S, Helke C, Braun KP, Pickenhain S, Hoschke B. Is it possible to provide a prognosis after radical prostatectomy for prostate cancer by means of a PSA regression model? Int J Biol Markers20:112-8, 2005
17. Miller JI, Ahmann FR, Drach GW, Emerson SS, Bottaccini MR. The clinical usefulness of serum prostate specific antigen after hormonal therapy of metastatic prostate cancer. J Urol147:956-61, 1992
18. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventos CX, et al. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers 20:209-16, 2005
- 18 -
best predicts the progression to androgen-independent prostate cancer. Int J Cancer108:877-81, 2004
20. Mulders PF, Fernandez del Moral P, Theeuwes AG, Oosterhof GO, van Berkel HT, Debruyne FM. Value of biochemical markers in the management of disseminated prostatic cancer. EurUrol21:2-5, 1992
21. Park BJ, Lee YG, Ahn HK. Prognostic significance of prostate-specific antigen level two months after maximal androgen blockade in metastatic prostate cancer. Korean J Urol44:855-60, 2003
22. Park SC, Choi HY, Kim CS, Hong SJ, Kim WJ, Lee SE, et al. Predictive variables of the progression to androgen independent prostate cancer after combined androgen blockade. Korean J Urol48:408-15, 2007
23. Park YH, Hwang IS,Jeong CW, Kim HH, Lee SE, Kwak C. Prostate specific antigen half-time and prostate specific antigen doubling time as predictors of response to androgen deprivation therapy for metastatic prostate cancer. J Urol181:2520-4, 2009 24. Petros JA, Andriole GL. Serum PSA after antiandrogen therapy. UrolClin North Am
20:749-56, 1993
25. Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer112:1247-53, 2008
26. Sasaki T, Onishi T, Hoshina A. Nadir PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis 14:248-52, 2011 27. Stamey TA, Kabalin JN, Ferrari M, Yang N. Prostate specific antigen in the diagnosis
and treatment ofadenocarcinoma of the prostate. IV. Anti-androgen treated patients. J Urol 141:1088-90, 1989
28. Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW, et al. Prostate-specificantigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J ClinOncol 23:6556-60, 2005
- 19 -
29. Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D’Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J ClinOncol23:6992-8, 2005
- 20 - - 국문 요약 –
전이성 전립선암으로 최대남성호르몬차단요법을 시행 받은
환자에서 전립선특이항원 최저점과 전립선특이항원
최저점까지의 시간은 독립적 예후 인자이다
아주대학교 대학원 의학과 홍석영 (지도교수: 김세중) 목적: 전이성 전립선암으로 최대남성호르몬차단요법 (maximal androgen blockade; MAB)을 시행 받은 환자들을 대상으로 전립선특이항원 (prostate- specific antigen; PSA) 역동학이 질병 진행 및 암특이 생존율에 미치는 영향을 알아보고자 하였다.대상 및 방법: 본원에서 전이성 전립선암으로 MAB를 시행 받은 131명의 환자 들을 대상으로 연구를 진행하였다. 환자들의 기본적인 특성, MAB 시작 시의 PSA, PSA 최저점, PSA 최저점까지의 시간 (time to PSA nadir; TTN), PSA decline을 분석하였다.
결과: 30개월의 정중 추적 관찰기간 동안 97명의 환자가 질병 진행을 보였고 65명의 환자가 사망하였다. 이중 59명의 환자가 전립선 암으로 인해 사망하였다. 단변량 분석에서 MAB 시작시의 PSA, PSA 최저점, TTN, PSA decline은 질병 의 진행 없는 생존의 유의한 예측인자였다. 또한 PSA 최저점, TTN, PSA decline은 암특이 생존의 유의한 예측인자였다. 다변량 분석에서는 높은 PSA 최저점과 짧은 TTN이 질병의 진행 없는 생존과 암특이 생존이 더 짧을 것임을 예측하는 독립적 예후 인자로 나타났다. 이 두 인자를 결합하여 분석한 결과에서 는 높은 PSA 최저점과 짧은 TTN을 보인 군이 질병의 진행 없는 생존과 암특 이 생존에 있어서 가장 나쁜 결과를 보였다. 결론: 본 연구 결과 전이성 전립선 암으로 MAB를 시행 받은 환자들에서 높은 PSA 최저점과 짧은 TTN을 보이는 경우 질병 진행의 위험성 및 불량한 생존율 과 연관이 있었다. 더욱이 이 두 인자는 결과에 있어서 상승적 효과를 가지는 것 으로 나타났다. 핵심어: 예후, 전립선 종양, 전립선특이항원