저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Two Sides of The Same Coin in The
Levodopa Treatment of Parkinson's Disease
by
Jin Young Shin
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Two Sides of The Same Coin in The
Levodopa Treatment of Parkinson's Disease
by
Jin Young Shin
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the
Degree of Ph. D. in Biomedical Sciences
Supervised by
Young Hwan Ahn, M.D., Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation of
Jin Young Shin is approved.
SUPERVISORY COMMITTEE
Young Hwan Ahn
Phil Hyu Lee
Eun Hye Joe
In Soo Joo
Eun Young Kim
The Graduate School, Ajou University
December, 23rd, 2010
i
-ABSTRACT-
Two Sides of The Same Coin in The Levodopa Treatment of
Parkin-son's Disease
Parkinson’s disease (PD) is a progressive neurodegenerative movement disorder. PD is a characterized by selective loss of dopaminergic neurons in the pars compacta of the substan-tia nigra. Levodopa (L-dopa) and dopamine agonists have been most commonly used for symptomatic treatment. Since its initial introduction in 1967, L-dopa has been shown to be the most effective pharmacological agent. Thus, L-dopa has been regarded as the gold stan-dard treatment of PD.
First, there are discrepancies between clinical and experimental data with respect to the neuroprotective effects of these drugs on dopaminergic neurons. My study demonstrated that both L-dopa and PPX had comparable neuroprotective properties for dopaminergic neurons in MPTP- treated PD animal models, through modulation of cell survival and apoptotic pathways. Second, the CSF total homocysteine (Hcy) concentration was higher in patients with PD before L-dopa treatment than in controls, and Hcy concentration increased after administration of L-dopa. Elevation of the Hcy concentration in plasma has been implicated in neurodegeneration in patients with stroke, dementia, Alzheimer disease, and Parkinson disease. However, the underlying cellular mechanisms by which elevated Hcy can promote
ii
neuronal death is not clear. The present study investigated the effects of Hcy on neural pro-genitor cells (NPCs) both in vitro and in vivo. I found that Hcy export from astrocytes was induced by the COMT substrates L-dopa. So, I have examined the role of NMDA receptor-mediated activation of the extracellular signal-regulated kinase-mitogen-activated protein (ERK-MAP) kinase pathway in Hcy - dependent neurotoxicity on NPCs in isolated from the postnatal subventricular zone (SVZ) and in PD animal model.
Key words : Parkinson’s Disease (PD), Levodopa (L-dopa), Pramipexole (PPX),
iii
TABLE OF CONTENTS
ABSTRACT ···i
TABLE OF CONTENTS ···iii
LIST OF FIGURES ··· vi
I. INTRODUCTION ··· 1
PART. A Neuroprotective effects of L-dopa on dopaminergic neurons is comparable to pramipexole in MPTP- treated animal model of Parkinson’s Disease. ··· 2
PART. B Increased level of homocysteine induced by levodopa inhibits neurogenesis by mediating NMDA receptor signal cascade in MPTP- treated animal model of Parkinson’s disease: comparison with pramipexol. ···4
II. MATRIALS AND METHODS ···6
1. Animals and drugs administration.···6
2. Cell culture. ···7
2.1 Neural progenitor Cells (NPCs) culture. ···7
2.2 Cotical astrocytes culture and drug treatment. ···8
2.3 Co-Cultures of Neural progenitor cells and astrocytes treated with L-dopa or PPX. ···9
3. Preparation of plasma and rat brain samples. ···9
iv
5. Measurements of Hcy. ···10
6. BrdU administration. ···11
7. Immunohistochemistry and Immunocytochemistry. ···11
8. Stereological cell counts. ··· 13
9. Total RNA Extraction and Reverse Transcriptive PCR (RT-PCR). ···14
10. Flow cytometric measurement of cell death using Annexin-V/PI. ···15
11. Caspase -3 activity assay. ···15
12. Western blot analysis. ···16
13. Statistical analysis. ···17
III. RESULTS ···18
PART. A Neuroprotective effects of L-dopa on dopaminergic neurons is comparable to pramipexole in MPTP- treated animal model of Parkinson’s Disease. ···18
1. Effects of L-dopa and PPX administration on GSH levels in MPTP- treated mice. ···18
2. Effects of L-dopa and PPX administration on ERK phosphorylation in MPTP- treated mice. ···20
3. Effects of L-dopa and PPX administration on JNK phosphorylation in MPTP- treated mice. ···20
4. Effects of L-dopa and PPX administration on apoptosis related proteins (Bax, cytochrome c, and Bcl-2). ···23
v
5. Effects of L-dopa and PPX administration on survival of dopaminergic neurons in
MPTP- treated mice ···26
PART. BIncreased level of homocysteine induced by levodopa inhibits neurogenesis by mediating NMDA receptor signal cascade in MPTP- treated animal model of Parkinson’s disease: comparison with pramipexol. ···29
1. The phenotype and proliferative capacity of cultured NPCs from the SVZ. ···29
2. mRNAs Expression and Immunodetection of NMDA Receptor Subunits in cultured NPCs.···31
3. Increased level of homocysteine in astrocytes culture media after L-dopa treatment.···31
4. Increased apoptosis in NPCs after L-dopa treatment ···34
5. Effects of L-dopa treatment on regulation of ERK-MAP kinase signaling. ···37
6. L-dopa treatment leads to increase level of homocysteine in both plasma and brain. ···39
7. L-dopa treatment leads to decrease neurogenesis in the SVZ zone. ···41
IV. DISCUSSION ···44
REFERENCES···56
vi
LIST OF FIGURES
Fig. 1 The level of glutathione (GSH) in the substantia nigra (SN). ···19
Fig. 2 Effects of L-dopa and pramipexol (PPX) administration on ERK phosphorylation in MPTP- treated mice ···21
Fig. 3 Effects of L-dopa and pramipexol (PPX) administration on JNK phosphorylation in MPTP- treated mice. ···22
Fig. 4 Effects of L-dopa and pramipexol (PPX) administration on apoptosis related proteins. (Bax, cytochrome c, and Bcl-2). ···24
Fig. 5 Effects of L-dopa and pramipexol (PPX) administration on survival of dopaminergic neurons. ···27
Fig. 6 Most NPCs continue to express a neuronal phenotype and ability to proliferate for several days in vitro. ···30
vii
Fig. 8 Hcy export from astrocytes was induced by the COMT substrates L-dopa. ···33
Fig. 9 Hcy in the synthesis from L-dopa induce NPCs apoptosis in vitro. ···35
Fig. 10 Hcy-mediated regulation of ERK-MAP kinase. ···38
Fig. 11 Measurements of Hcy in plasma and brain. ···40
Fig. 12 Representative photomicrograph of BrdU+ and Hematoxylin+ after drugs administration. ···42
1
I.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative movement disorder that is es-timated to affect approximately 1% of the population older than 65 years of age (Lang and Lozano 1998; Lang and Lozano 1998) and characterized by the selective loss of dopaminer-gic neurons in the pars compacta of the substantia nigra (SN). The pathomechanism underly-ing the selective vulnerability of SN neurons is still unclear, although mitochondrial dysfunc-tion resulting in complex I loss is recognized as an important final pathway in the pathogene-sis of PD (Schapira, Gu et al. 1998; Vila, Ramonet et al. 2008). Over 40 years after its intro-duction, L-dopa is still the most effective symptomatic treatment for PD, able to restore do-paminergic striatal stimulation, thus reducing patients’ disability and increasing life expec-tancy (Abbruzzese 2008). Thus, L-dopa has been regarded as the gold standard treatment of PD (Agid 1998). Dopamine agonists have also been commonly used to ameliorate parkin-sonian symptoms. Pramipexole (PPX), a novel DA agonist with preference for D3 receptors,
has been used to treat both early and advanced PD with marked symptomatic efficacy (Hubble, Koller et al. 1995; Molho, Factor et al. 1995).
2
PART. A
Neuroprotective effects of levodopa on dopaminergic neurons is com-arable to
pramipexole in MPTP- treated animal model of Parkinson’s Disease.
Long-term treatment with L-dopa may enhance dopaminergic neuronal cell death and ac-celerate disease progression in vitro and in vivo, because L-dopa can act as a source of free radicals that inhibit the activity of mitochondrial enzymes(Melamed, Offen et al. 1998). In contrast to L-dopa, ample laboratory evidence shows that dopamine agonists provide neuro-protection of dopaminergic neurons through their anti-oxidative effects, mitochondrial mem-brane stabilization, and cytotrophic effects (Iida, Miyazaki et al. 1999; Gu, Iravani et al. 2004; Du, Li et al. 2005). In the clinical setting, however, there is no direct evidence that L-dopa hastens the progression of PD or that L-dopamine agonist has neuroprotective properties in PD. This discrepancy between clinical and laboratory results may be ascribed to insuffi-cient experimental designs, such as an in vitro study without glial cell involvement, pre-treatment with drugs before the emergence of PD pathology, and the lack of a PD animal model that suitably mimics human PD (Emborg 2004; Olanow, Kieburtz et al. 2008). An in-hibitor of mitochondrial complex I, 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP), has been reported to induce dopaminergic neuronal loss in the SN through the activation of the mitogen-activated protein kinase (MAPK) pathway and the mitochondrial apoptosis pathway induced by the generation of reactive oxidative species (ROS) (Saporito, Thomas et al. 2000; Karunakaran, Saeed et al. 2008). MAPK family members, including extracellular
3
signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, play critical roles in cellular responses to a wide range of stimuli. A dynamic balance among these kinas-es is implicated in the regulation of cell death and survival. The activation of ERK may pref-erentially confer a survival or growth advantage to cells, whereas JNK and P38 may be in-volved in neuronal cell death via the activation of apoptosis signaling (Miloso, Scuteri et al. 2008). Several in vitro and in vivo studies have also suggested that the JNK signal transduc-tion pathway may be associated with the pathogenesis of dopaminergic neuronal death in PD (Peng and Andersen 2003).
In this study, to determine whether L-dopa is toxic or dopamine agonist is neuroprotective to dopaminergic neurons, I evaluated the neuroprotective properties of L-dopa and the PPX with a focus on the regulatory effects of the anti-oxidant properties and cell survival or apop-totic signal pathways in the same experimental design, using MPTP- treated PD animals.
4
PART. B
Increased level of homocysteine induced by levodopa inhibits neurogenesis by
mediating NMDA receptor signal cascade in MPTP- treated animal model of
Parkinson’s disease: comparison with pramipexol.
Recent studies have demonstrated that the adult mammalian brain has the potential to gen-erate new neurons and to incorporate them into brain area affected by disease process (Doetsch, Garcia-Verdugo et al. 1997). The neurogenesis that occurs in the subventricular zone and the subgranular zone of the dentate nucleus may act as endogenous repair mecha-nisms, and several factors of neurotransmitters (Hagg 2009), growth factor (Bath and Lee 2010), and disease states are suggested to modulate neurogenesis. Thus, augmenting strategy of neurogenesis might have a great interest in the treatment of neurodegenerative diseases. Growing evidence indicates that neurogenesis in the SVZ and hippocampus is decreased sig-nificantly in patients with PD and animal model of PD (Hoglinger, Rizk et al. 2004; He, Nakayama et al. 2006), and neurochemical deficits of dopamine and direct α-synuclein ac-cumulation in neural precursor cells of the SVZ may influence the neurogenetic systems. Since neural precursor cells in the SVZ express dopaminergic receptors (Winner, Desplats et al. 2009), some human study an in vivo data supported that dopamine enhancing drugs may increase neurogenesis in those areas.
Levodopa treatment is the gold standard therapy in patients with Parkinson's disease (PD), with its controlling motor symptoms, improving quality of life, and prolonging patient's life-
5
expectancy. L-dopa therapy, however, causes increase in serum homocysteine level as the drug is metabolized via catechol O-methyltransferase (Miller, Selhub et al. 2003; Yasui, Nakaso et al. 2003). Indeed, some reports have demonstrated that hyperhomocysteinemia in PD might be associated with increased prevalence of coronary artery disease, hypertrophy of carotid artery, peripheral neurodegeneration, and increased cerebrovascular resistance (Blandini, Fancellu et al. 2001; Kim, Choi et al. 2003; Jakubowski 2004; Boldyrev 2009). In addition, recent in vitro studies indicate that elevated Hcy induces oxidative injury in nerve terminals and involves NMDA receptor stimulation(Lipton, Kim et al. 1997), neuronal nitric oxide synthase (nNOS) activation, and associated free radical formation (Jara-Prado, Ortega-Vazquez et al. 2003). Furthermore, Hcy leads to significant increases in neuronal cell death over basal levels that are dependent on NMDA receptor activation (Poddar and Paul 2009). Until now, there are no studies evaluating a direct cause-effect association between homo-cystein and neurogenesis in the adult brain.
In this study, I examined whether L-dopa treatment related hyperhomocysteine would lead to modulation of neurogenesis via NMDA receptor signal cascade using in vitro and in vivo systems, and I performed additionally comparative analysis of neurogenesis with pramipexol, a dopamine agonist.
6
II.
MATRIALS AND METHODS
1. Animals and drugs administration.
Male C57BL/6J mice (5 weeks old) were allowed to acclimate in a climate-controlled room with a constant 12 h light/dark cycle (12 h on, 12 h off) for a week prior to the start of injections. MPTP was freshly dissolved in normal saline. These mice, 6 weeks of age, were injected subchronically for 5 days with MPTP (25 mg/kg/day as the free base in normal sa-line, i.p, Sigma , St. Louis, MO, USA) (Schober 2004) or vehicle (normal sasa-line, 0.1 ml/10 g, i.p ;control group). Beginning on day 8 after the first MTPT injection, the mice were ran-domly divided into six groups and treated daily with L-dopa (Sinemet®, carbidopa/levodopa, 25/100 mg), PPX (Boehringer-Ingelheim, Germany), or normal saline for 4 weeks, as fol-lows: Group 1, control mice and Group 2, normal saline-treated mice (normal saline, 0.1 ml/10 g, i.p; MPTP- only treatment group) Group 3, L-dopa-treated mice (200mg/kg, twice daily, i.p; MPTP + L-dopa treatment group), Group 4, L-dopa-treated mice (200mg/kg or 20mg/kg, twice daily, i.p; MPTP + L-dopa treatment group) and Group 5, L-dopa- and MK-801- treated mice(200mg/kg or 20mg/kg L-dopa, 10mg/kg MK-801, twice daily, i.p; MPTP + L-dopa/MK-801 treatment group) (Murer, Dziewczapolski et al. 1998; Nicholas 2007), Group 6, PPX- treated mice (1 mg/kg, twice daily, i.p ; MPTP + PPX treatment group) and Group 9, PPX- and MK-801- treated mice (1 mg/kg PPX, 10mg/kg MK-80- treated mice, twice daily, i.p ; MPTP + PPX/MK-801 treatment group) (Anderson, Neavin et al. 2001).
7
Subsequently, the mice were maintained for 2 weeks without any further treatment. To ex-clude locomotor activity-related neuroprotective effects by dopaminergic drugs, the MPTP- treated mice were immobilized in a cast on the first day of randomization, and the mice were maintained for 6 weeks.
2. Cell culture.
2.1 Neuro-progenitor Cells (NPCs) culture.
The procedure that was developed for obtaining SVZ cells for transplantation was utilized to harvest SVZ progenitor cells for tissue culture (Zigova, Betarbet et al. 1996). Embryo of Sprague–Dawley rat was used. The brains were removed from the skull, immediately im-mersed in DMEM/F12 (Gibco, BRL, Grand Island, NY), and hemisected. A parasagittal sec-tion was taken from the medial surface of each hemisphere and a wedge of tissue was micro-dissected from the portion of the telencephalon surrounding the anterolateral aspect of the lateral ventricle that included the anterior part of the subventricular zone (SVZ) (Luskin, Zigova et al. 1997). The individual SVZ was collected in DMEN/F12 and was dissociated using HBSS containing 0.1% trypsin-EDTA (Gibco, BRL, Grand Island, NY). The SVZ cells were mechanically dissociated using several fire-polished Pasteur pipettes and centri-fuged at 700 rpm for 7 min. The pelleted cells were resuspended and expanded in complete media containing DMEM /F-12 mixture (1:1) supplemented with epidermal growth factor and basic fibroblast growth factor (20 ng/mL each; both from PromoCell, Heidelberg,
Ger-8
many), 1% penicillin/streptomycin (Gibco, BRL, Grand Island, NY), 10% fetal bovine se-rum (FBS, Gibco, BRL, Grand Island, NY) (Wegner, Kraft et al. 2009). For experimental NPCs were plated on 6-wll plate at a density of 1x104/cm2 (Corning, NY, USA).
2.2 Cotical astrocytes culture and drug treatment.
Astrocytes were cultured from the cerebral cortices of 1-day-old Sprague-Dawley (SD) rats. The cortices were rinsed twice in minimum essential medium (MEM; Sigma, St Louis, MO, USA) containing 10% FBS and were mechanically triturated. The dissociated cells were plated in T75 flasks (Kim, Park et al. 2009). Briefly, dissected cortices without men-inges were broken into smaller pieces by pipetting in MEM, and trypsin-EDTA was added. After inactivation of typsin-EDTA, cells were centrifuged at 1000rpm for 5 min. The cell pellet was resuspended with fresh medium and cells were grown in T75 flasks. On day 14 after preparation, cells grown in T75 flasks were shaken vigorously at least 3 times to detach microglia cells from astrocytes layer and removed. Cells were cultured for 2 weeks, split onto new dishes when confluent, before they were used for experiments. For experiment, cells plated in insert at a density of 5 x103/cm2 (Corning, NY, USA). Astrocytes were treated
with several dose of levodopa and PPX for 24hr and 72hr. For each condition, Astrocytes viability was decreased in a dose-dependent manner. The astrocytes exposure of PPX were vulnerable compared with levodopa (data not shown). All experiments were performed in 200mM levodopa and 1mM PPX.
9
2.3 Co-Cultures of NPCs and astrocytes treated with L-dopa or PPX.
To test the effects of Hcy, and NPCs were co-cultured without cell contact. Astrocytes treated with L-dopa or PPX were maintained on a Costar transwell insert and NPCs were maintained on the bottom of 6-well in a humidified incubator at 37℃ and 5% CO2 for 24h and 72h. And NPCs were pretreated 10mM MK-801. The NPCs were then collected to assay.
3. Preparation of plasma and rat brain samples.
To measure Hcy, rat brains were isolated and blood samples were collected into EDTA containing tubes (BD, sparks, MD, USA). Plasma and blood cells were separated by cen-trifugation (2000×g for 20 min). Rat brain and plasma were frozen and stored at − 70 °C until analyzed (Parzer and Mannhalter 1991). And all mice were deeply anesthetized with chloral hydrate (0.4 g/kg, i.p) and then perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed, post-fixed overnight in buffered 4% paraformaldehyde, and stored in 30% sucrose solution for 2 days, until they sank. They were then sectioned on a sliding microtome to obtain a 30-mm-thick coronal section. All sections were stored in tissue stock solution (30% glycerol, 30% ethylene glycol, 30% 3rd D.W., 10% 0.2 m PB; pH 7.2; Sigma , St. Louis, MO, USA ) at 4 ℃ until required. For ELISA, animals were killed after seven weeks, and the SN area was rapidly removed from the brains and fro-zen at 70 ℃.
10 4.
Measurements of GSH assay.
For the determination of total GSH, a Bioxytech GSH-420 kit (Oxis Research, Portland, OR, USA) was used. Brain tissues were washed in 0.9% NaCl solution and then homoge-nized in ice-cold precipitation reagent at a ratio 1: 15 (w/v). An aliquot (200 mL) of each brain sample supernatant was transferred to a fresh microcentrifuge tube. The reaction mix-ture (200 mL) and reducing agent were added to each tube and mixed well. Then a chro-mogen or color developer was added. Each reaction was incubated at room temperature (RT) in the dark for at 30 min before the absorbance at 420 nm was measured (Jones, Carlson et al. 1998).
5.
Measurements of Hcy.
Tissue homogenates of striatum were centrifuged (20 min, 14 000 g, 4_C) and the super-natant was transferred to a fresh tube for the dopamine assay using GC-MS. GC–MS analy-ses in both scan and selected ion monitoring modes were performed using an Agilent 6890 gas chromatograph, interfaced to an Agilent 5973 mass-selective detector (70eV, electron impact mode) and installed with an Ultra-2 (5% phenyl–95% methylpolysiloxane bonded phase; 25 m·0.20 mm i.d., 0.11 lm film thickness) cross-linked capillary column (Agilent Technologies, Atlanta, GA, USA). The temperatures of the injector, interface, and ion source were 260, 300, and 230_C, respectively. Helium was used as carrier gas at a flow rate of 0.5–1 mL/min in a constant flow mode. Derivatized extracts were introduced in
split-11
injection mode (10: 1)and the oven temperature was initially at 100℃ for 2 min, and pro-grammed to rise to 205℃ at 5℃/min and finally to 300℃ (3min) at 20℃/min. The mass range scanned was 50–800 U at a rate of 0.42scan/s. All GC–SIM–MS runs were performed in triplicate. The mass range scanned was 50–800 U at a rate of 0.42scan/s. All GC–SIM– MS runs were performed in triplicate.
6.
BrdU administration.
To assess ongoing cell proliferation, all animals were assigned to a BrdU injection regime following a modified protocol described by Kralic et al. (Vicario, Tabernero et al. 1993). Each mouse was injected with BrdU (Sigma, St. Louis, MO, USA) that incorporates into the DNA in the S phase of the cell cycle. BrdU was dissolved in PBS and administered i.p once daily on five subsequent days at a concentration of 50 mg/kg in a 20mg/ml volume.
7. Immunohistochemistry and Immunocytochemistry.
The brain sections and co-cultured cells were rinsed twice in PBS and incubated in 0.2% Triton X-100 for 30 min at room temperature (RT). For BrdU staining this procedure was added, they were incubated in 50% formamide and 2x SSC buffer at 65℃ for 2 hr. And then they were treated with 2N HCL at 37℃ for 30min. After 2N HCL treat, they were rinsed three times in PBS and incubated hydrogen peroxide blocking solution (Thermo, Fremont, CA USA) for 10min at RT. For immune-staining, they were rinsed and incubated in triton
X-12
100 for 30 min at RT. They were rinsed three times with 0.5% bovine serum albumin in PBS for blocking. After blocking, they were incubated overnight at 4℃ with primary antibody. The primary antibodies were used as follows: mouse anti-OX-42 (1: 200 for immunocyto-chemistry; Serotec, Raleigh, NC, USA), mouse anti-GFAP (1: 200 for immunocytochemis-try; Abcam, Cambridge, UK), mouse anti-Ki67 (1: 200 for immunocytochemistry, Chemicon, Billerica, MA, USA), rabbit anti-NMDA2A receptor and NMDA2B receptor (1:200 for im-munocytochemistry, Chemicon, Billerica, MA, USA), mouse anti-BrdU (1: 200 for immu-nohistrochemistry, Roche, IN, USA), mouse anti-tyrosine hydroxylase (TH; 1: 1000 for im-munohistochemistry, Chemicon International, Temecula, CA, USA). After over night, the brain tissues and cells were rinsed three times in 0.5% bovine serum albumin in PBS (10 min/rinse) and incubated with the appropriate biotinylated secondary antibody and avidin– biotin complex (Elite Kit; Vector Laboratories, Burlingame, CA, USA) for 1 h at RT. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, USA) and 0.03% H2O2. For Immunofluorescence labeling,
cells were incubated with goat anti-mouse IgG (AlexaFluor-488, green) and goat anti-rabbit IgG (Alexa Fluor-594, red) secondary antibodies for 1 h at RT. Cell nuclei were counter-stained with DAPI (1: 2000 dilution, Molecular Probes) for 1 h at RT. Processing was stopped with H2O, and sections were dehydrated through graded alcohols, cleared in xylene,
and overslipped in permanent mounting medium (Vector, Vector Laboratories, Burlingame, CA, USA). For Hematoxylin staining, the brain tissues were dried and stained with Mayer’s
13
Hematoxylin (MUTO, Tokyo, Japan). The immune-stained cells were analyzed by bright-field microscopy and viewed using an conforcal laser scanning microscope (Olympus, Tokyo, Japan).
8. Stereological cell counts.
Unbiased stereological estimations of the total number of the stained cells in the SVZ and SN were made using an optical fractionator, as previously described with some modifica-tions (Kirik, Rosenblad et al. 1998). This sampling technique is not affected by tissue vol-ume changes and does not require reference volvol-ume determinations. The sections used for counting covered SVZ and the entire SN, from the rostral tip of the pars compacta back to the caudal end of the pars reticulate. This generally yielded 8–12 sections in a series. Sam-pling was performed with the Olympus C.A.S.T.-Grid system (Olympus Denmark A/S, Denmark), using an Olympus BX51 microscope, connected to the stage and feeding the computer with the distance information in the z-axis. The SN was delineated at 1.25 magni-fication. A counting frame (46%, 40, 1699 mm2) was placed randomly on the first counting
area and systematically moved though all counting areas until the entire delineated area was sampled. Actual counting was performed using a x100 oil objective. Guard volumes (4 mm from the top and 4–6 mm from the bottom of the section) were excluded from both surfaces to avoid the problem of lost cap, and only the profiles that came into focus within the
count-14
ing volume (with a depth of 10mm) were counted. The total number of stained cells was cal-culated according to the optical fractionator formula (West, Slomianka et al. 1991).
9. Total RNA Extraction and Reverse Transcriptive PCR (RT-PCR).
Total RNA was extracted from the NPCs using Trizol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. An equal amount of RNA (approxi-mately 1mg) in each experiment was reverse transcribed using a amfiRivert cDNA Synthesis Premix (GenDEPOT, Barker, TX, USA). Subsequently, 2ml of cDNA was used as a template for RT- PCR analysis in amfiRivert 1-Step RT-PCR Kit. (GenDEPOT, Barker, TX, USA). The PCR reaction was performed using 10 pmol each of the primers for NMDA receptor 1 (NR1; forward 5’-CTG CAA CCC TCA CTT TTG AG-3’, reverse5’-TGC AAA AGC CAG CTG CAT CT-3’), NMAD 2A receptor (NR2A; forward 5’- GAC GGT CTT GGG ATC TTA AC-3’, reverse 5’- TGA CCA TGA ATT GGT GCA GG-3’), NMAD 2B receptor (NR2B; forward 5’- CAA GAA CAT GGC CAA CCT GT-3’, reverse 5’- GGT ACA CAT TGC TGT CCT TC-3’), NMAD 2C receptor (NR2C; forward 5’- TGG AAA CTT CGA CAC TCG GT-3’, reverse 5’- TCC AAA GAG CTG CTC ACG TC-3’) (Sun, Shipley et al. 2000). After an initial denaturation at 94℃ for 5 min, 30 cycles of PCR were performed, consisting of denaturation (30s, 94℃), annealing (1 min, 58℃ [NR1], 55℃ [NR2A, NR2B], 57℃ [NR2C]) extension (1 min, 72℃ followed by a final extension (10 min, 72℃). The PCR products were separated by electrophoresis on 2% agarose gel and stained with
ethi-15
dium bromide. Gels were examined under UV illumination.
10. Flow cytometric measurement of cell death using Annexin-V/PI.
NPCs co-cultured with L-dopa-treated astrocytes were harvested by trypsinization and pel-leted by centrifugation at 1500rpm for 5min. Cell pellets were washed once in ice cold PBS, followed by gentle re-suspension in 100ml of annexin-V binding buffer containing 5ml of FITC-labeled annexin V (Annexin-V; 100mg/ml stock in PBS) and 5ml propidium iodide (PI; 100mg/ml stock in PBS) solution for 15min (BD, San diego, CA, USA). And then add 400ml 1x binding buffer. Samples were immediately kept on ice and analyzed on FACS. Data was acquired and analyzed using Winmdi software. Acquisition gates were wet using the forward and side light scatter of the cells and a minimum of 10,000 events were collected for each sample.
11. Caspase -3 activity assay.
The caspase-3 activity was measured by caspase-3 activity assay kit. (Chemicon, Billerica, MA, USA). Caspase-3 activity was determined by monitoring proteolysis of corresponding colorimeric substrates. NPCs co-cultured with L-dopa-treated astrocytes were collected and washed in ice-cold PBS pH 7.0. NPCs were subsequently lysed in 1x lysis buffer for 10min in ice and the lysates were clarified by centrifugation at 13000rpm. After centrifugation for 10mins, cytosolic extracts of NPCs were transferred to a fresh tube and putted on ice. Then
16
30mg of the caspase-3-specific colorimetric substrate acetyl-Asp-Glu-Val-Asp-7-p-nitroaniline (Ac-DEVD-pNA) was added in cytosolic extracts. They were incubated for 1hr at 37 . The release of℃ DEVD-pNA were quantified at 405nm by ELISA plate reader.
12. Western blot analysis.
For western blotting, NPCs co-cultured with L-dopa-treated astrocytes were washed in ice-cold and lysis buffer containing protease inhibitor (iNtRON Biotechnology, SeongNam, S. Korea). Briefly, 50 mg of protein for each specimen were separated by sodium dodecyl sul-fate polyacrylamide gel electrophoresis and transferred to Hybond-ELC C pure nitrocellu-lose membrane (Amersham, Piscataway, NJ, USA). The membranes were blocked in non-fat milk. Membranes were probed with 1 : 1000 dilutions of the following primary antibodies : rabbit polyclonal ERK1/2 and rabbit polyclonal phosphor-ERK1/2 (Cell Signaling, Danvers, MA, USA). As a secondary antibody, a 1: 2000 dilution of horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibody (Zymed Laboratories, San Francisco, CA, USA) was used. Antigen–antibody complexes were visualized with a chemiluminescence system (Amersham, Piscataway, NJ, USA), followed by exposure to x-ray film (Kodak X-OMAT, Rochester, NY, USA). For semiquantitative analysis, the densities of the im-munoblot bands were measured by computer imaging (Image J; NIH, Bethesda, MD, USA).
17 13.
Statistical analysis.
The group means were compared using the Mann-Whitney U-test for pairs and the Kruskal-Wallis analysis for multiple groups. P values less than 0.05 were considered statisti-cally significant. Statistical analyses were performed using commercially available software (version 12.0; SPSS Inc., Chicago, IL).
18
III. RESULTS
PART. A
Neuroprotective effects of levodopa on dopaminergic neurons is com-parable to
pramipexol in MPTP- treated animal model of Parkinson’s Disease.
1. Effects of L-dopa and PPX administration on GSH levels in MPTP-treated mice. Beginning on day 43 after the first administration of either L-dopa or PPX in MPTP- treated mice, the midbrain was isolated and assayed for GSH. The level of GSH in MPTP- only treated mice was significantly decreased compared with that in control mice. The level of GSH was significantly increased in MPTP- treated mice after PPX administration com-pared with MPTP- only treatment. L-dopa administration also increased the level of GSH in MPTP- treated mice, although not significantly. The level of GSH was not different between L-dopa- and PPX- and MPTP- treated mice (Fig. 1).
19
Fig. 1 The level of glutathione (GSH) in the substantia nigra (SN).
Beginning on day 43 after the first administration of either L-dopa- or PPX- in MPTP- treated mice, the midbrain was isolated and assayed for GSH. The level of GSH in MPTP- treated mice was significantly decreased compared with that in control mice. PPX treatment significantly increased the GSH level in MPTP- treated mice (p < 0.05). L-dopa administra-tion also tended to increase the GSH level in MPTP- treated mice, but without statistical sig-nificance. The data are displayed as the mean (column) ± SEM (bar). The results are repre-sentative of five replications in each group. *p < 0.05, **p < 0.01.
20
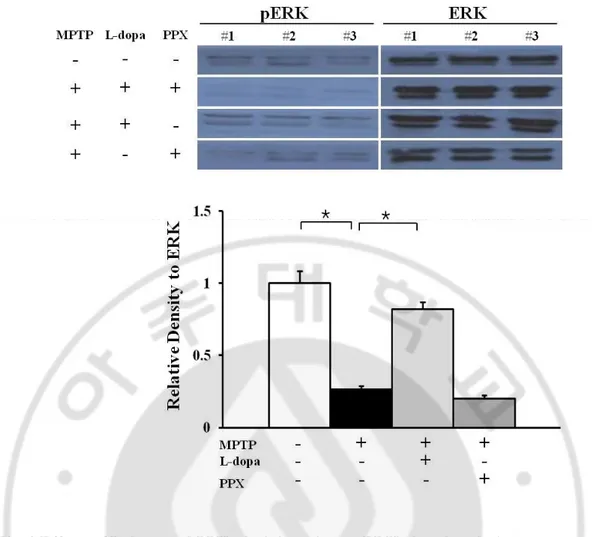
2. Effects of L-dopa and PPX administration on ERK phosphorylation in MPTP- treated mice
.
Compared with control mice, MPTP- treated mice exhibited significantly decreased ex-pression of phosphorylated ERK. The exex-pression of phosphorylated ERK was significantly increased in MPTP- treated mice after L-dopa administration compared with MPTP- only treatment. However, PPX administration did not significantly change the expression of phosphorylated ERK in comparison with MPTP- only treatment (Fig. 2).
3. Effects of L-dopa and PPX administration on JNK phosphorylation in MPTP- treated mice.
The phosphorylated form of JNK was significantly increased in MPTP- treated mice compared with control mice. Both L-dopa and PPX administration led to significantly de-creased expression of JNK phosphorylation in MPTP- treated mice. The degree of reduction in JNK phosphorylation did not differ significantly between L-dopa- and PPX- treated groups (Fig. 3).
21
Fig. 2 Effects of L-dopa and PPX administration on ERK phosphorylation.
The expression of phosphorylated ERK was significantly decreased in MPTP- treated mice compared with control mice. L-dopa administration in MPTP- treated mice signifi-cantly increased ERK phosphorylation compared with the level in MPTP- only treated mice. There was no significant change in ERK phosphorylation in PPX- treated mice. The results are representative of three replications in each group. *p < 0.01.
22
Fig. 3 Effects of L-dopa and PPX administration on JNK phosphorylation in MPTP- treated mice.
JNK phosphorylation was significantly increased in MPTP- treated mice compared with sa-line-treated mice. Both L-dopa and PPX administration in MPTP- treated mice led to signifi-cant and similar decreases in the expression of JNK phosphorylation. The results are repre-sentative of three replications in each group. *p < 0.01.
23
4. Effects of L-dopa and PPX administration on apoptosis related proteins (Bax, cytochrome c, and Bcl-2).
Compared with control mice, MPTP- treated mice showed significantly increased expres-sion of Bax. L-dopa administration significantly decreased Bax expresexpres-sion in MPTP- treated mice compared with the expression in MPTP- only treated mice. PPX administration also significantly reduced Bax expression in MPTP- treated mice. A similar pattern was observed for the expression of cytochrome c in MPTP- treated mice. Both L-dopa and PPX signifi-cantly decreased the expression of cytochrome c, which had been elevated after MPTP treatment. PPX also significantly decreased expression of Bax. There was no statistical dif-ference in the expression level of Bax or cytochrome c between the L-dopa-treated and PPX- treated groups. The expression of Bcl-2 was quite contrast. The administration of L-dopa and PPX significantly increased the expression of Bcl-2, which had been decreased after MPTP treatment. The degree of increased Bcl-2 expression did not differ significantly between the two groups (Fig. 4).
25
Fig. 4 Effects of L-dopa and PPX administration on apoptosis related proteins.
The expression of Bax was significantly increased in MPTP- treated mice compared with saline-treated mice. Both Ldopa and PPX administration in MPTP-treated mice significantly decreased Bax expression to a similar degree, as compared with the expression in MPTP- only treated mice (a). Both L-dopa and PPX significantly attenuated MPTP- induced in-creases in the expression of cytochrome c (b). The expression of Bcl-2 was quite the reverse: the MPTP- induced decrease in Bcl-2 expression was significantly increased after L-dopa or PPX administration (c). The degree of the increase in Bcl-2 expression was not significantly different between the two agents. The results are representative of three replications in each group. *p < 0.01.
26
5. Effects of L-dopa and PPX administration on survival of dopaminergic neurons in MPTP- treated mice.
Brain tissue was harvested from MPTP- treated mice at 2 weeks after 4 weeks of treat-ment with either L-dopa or PPX had been completed. Immunohistochemical analyses showed that both L-dopa and PPX treatment dramatically rescued the decline in the number of tyrosine hydroxylase immunoreactive (TH-ir) and Nissl-stained cells in the SN of MPTP- treated mice (Fig. 5a). Stereological analysis revealed that TH-ir and Nissl-stained cells were significantly decreased in the SN of MPTP- treated mice, which showed an approximately 50% reduction compared with control mice. Both L-dopa and PPX administration in MPTP- treated mice significantly increased the number of TH-ir and Nissl stained cells compared with the number in MPTP- only treated mice (Fig. 5b). The number of TH-ir cells tended to be greater in L-dopa-treated mice than in PPX- treated mice, but the difference was not sta-tistically significant.
28
Fig. 5 Effects of L-dopa and PPX administration on survival of dopaminergic neurons. Immunohistochemical analysis showed that both L-dopa and PPX dramatically attenuated the decline in the number of TH-ir and Nissl-stained cells in the SN of MPTP- treated mice (a). On stereological analysis, TH-ir and Nissl-stained cells in the SN were significantly de-creased in MPTP- treated mice, showing 50% reduction compared with saline-treated mice. After treatment with either L-dopa or PPX in MPTP- treated mice, number of TH-ir cells was significantly increased compared with the number in MPTP- only treated mice (b). The number of TH-ir cells tended to be greater in L-dopa- treated mice than in PPX- treated mice; however, the difference was not statistically significant. The data are displayed as the mean (column) ± SEM (bar). The results are representative of five replications in each group. *p < 0.05, **p < 0.01. Scale bar: 100mm.
29
PART. B
Increased level of homocysteine induced by levodopa inhibits neurogenesis by
mediating NMDA receptor signal cascade in MPTP- treated animal model of
Parkinson’s disease: comparison with pramipexol
1. The phenotype and proliferative capacity of cultured NPCs from the SVZ.
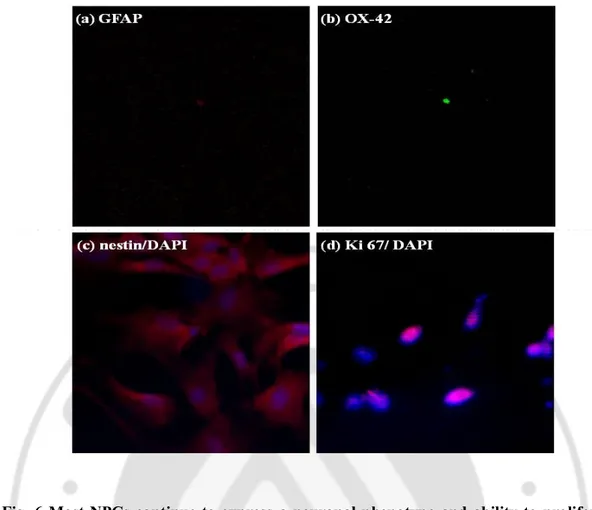
To determine the phenotypic properties of NPCs, the neural stem cell marker of nestin (Wiese, Rolletschek et al. 2004) was used to reveal cells expressing a neuronal phenotype, while astrocytes marker of GFAP and microglia marker of OX-42 were used to reveal the presence of glial cells. When the phenotype of the cultured NPCs was examined in vitro, no GFAP-positive and OX-42-positive cells were observed (Fig. 6A and B) and most NPCs showed nestin-positive (Fig. 6C). In addition, the NPCs were immunostained with Ki67, a proliferation marker (Morimoto, Kim et al. 2009) Fig 6D).
30
Fig. 6 Most NPCs continue to express a neuronal phenotype and ability to proliferate for several days in vitro.
NPCs from SVZ were dissociated, cultured for several days and then double-stained by spe-cific antibody (GFAP; astrocytes marker, OX-42; microglia marker, nestin; neural stem cell marker and Ki67; proliferation marker). They were visualized by a secondary antibody con-jugated to fluorescein with DAPI.
31
2. mRNA Expression and Immunodetection of NMDA Receptor Subunits in cultured NPCs.
Immucytochemistry revealed that NPCs expressed NMDA receptor subunits 2Aand 2B with a strong expression of NMDA receptor subunit 2A (Fig. 7A and B). These results were confirmed by RT-PCR, showing total RNA isolated from NPCs expressed detectable NR1, NR2A, NR2B and NR2C (Fig. 7C).
3. Increased level of homocysteine in astrocytes culture media after L-dopa treatment. To evaluate whether L-dopa introduction would increase the level of homocysteine, I de-termined concentration of homocysteine in astrocytes culture media treated with L-dopa. The extracellular concentration of homocysteine increased linearly with time during incubations with L-dopa and reached higher levels at 72hr after L-dopa treatment. Release of homocys-teine by L-dopa treatment was dependant on the number of astrocytes with maximum level in the highest dose of L-dopa (Fig. 8A and B). However, there were no detectable homocysteine in astrocytes culture media after PPX treatment (Fig 8C and D).
32
Fig. 7 Expression of NMDA Receptor Subunits in cultured NPCs.
Cultured NPCs were expressed NMDA receptor subunits 2A (a), 2B (b). with specific antibodies. And NMDA receptor 1 (NR1; 145bp), 2A (NR2A; 140bp), 2B (NR2B; 229bp), and 2C (NR2C, 220bp) subunits were detected in mRNA levels from NPCs.
33
Fig. 8 L-dopa stimulates the release of Hcy from astrocytes.
Export of Hcy was studied after astrocyte treated with several dose of levodopa and PPX. The extracellular Hcy from astrocytes increased linearly with time during 24h and 72h. Hcy export by levodopa was dose-dependent and sensitive to cell number. However, Hcy was not detected in PPX treated astrocyte culture media.
34
4. Increased apoptosis in NPCs after L-dopa treatment.
To exam whether increased release of homocysteine after L-dopa treatment could induce apoptosis, NPCs were co-cultured with L-dopa- or PPX- treated astrocytes for 24hr and 72hr. Caspase-3 activity in L-dopa- treated NPCs was increased significantly in a time-dependent manner, and this activity was significantly higher than control or PPX- treated NPCs at 72hr (Fig.9A). In addition, flow cytometric assays using annexin V/PI revealed that Annexin-V- and PI- positive cells, a cluster in the right upper quadrant were significantly increased in L-dopa- treated NPCs compared to control or PPX- treated NPCs (Fig. 9B). Quantitative analy-sis calculated by flow cytogram demonstrated a significant increase of apoptotic cell death in NPCs 72hr after L-dopa treatment than in control or PPX treatment (Fig. 9C).
36 Fig. 9 Hcy induce NPCs apoptosis in vitro
caspase-3 activities in NPCs was measured after cocultured with levodopa or PPX treated astrocytes. No change of caspase-3 activity is found in normal NPCs (A). A signifi-cant decrease in caspase-3 occurs at 72h in levodopa- treated NPCs. (n=3/group, *P < 0.005). Folw cytogram shows ongoing cell death by flow cytometric analysis using annexin V/PI. Apoptotic cells were significantly increased in levodopa - treated NPCs compared with con-trol and PPX- treated NPCs. But annexin V positive apoptotic cells were decreased after MK-801 treatment (B). Histogram revealed annexin positive cells (C). (n=4/group, *P < 0.005) Values are means ± SD.
37
5. Effects of L-dopa treatment on regulation of ERK-MAP kinase signaling
To evaluate whether increased levels of homocysteine may modulate in regulation of ERK-MAP kinase signaling pathways through NMDA receptor, NPCs were co-cultured with L-dopa- or PPX- treated astrocytes for 72 hr. The phosphorylated form of ERK was signifi-cantly increased in L-dopa- treated NPCs compared to controls. However, PPX- treated NPCs did not change significantly the expression of phosphorylated ERK in comparison with controls (Fig. 10A and B). Additionally, MK-801, a NMDA antagonist, administration in dopa- treated NPCs significantly decreased phosphorylated ERK compared to only L-dopa- treated NPCs. It was similar expression of phosphorylated ERK in comparison with controls and PPX- treated NPCs.
38 Fg. 10 Hcy mediated regulation of ERK-MAP kinase
Hcy mediated phospholylation of ERK was significantly increased in levodopa-treated NPCs compared with control and PPX-treated NPCs. There was no significant change in ERK phosphorylation in PPX-treated NPCs and control Increasing phophorylation of ERK was down-regulated in MK-801 treatment (A). Histogram revealed relative to ERK ratio (B). The results are representative of three replications in each group. *p < 0.02.
39
6. L-dopa treatment leads to increase level of homocysteine in both plasma and brain Plasma homocysteine levels did not show a significant change in mice with only MPTP administration or in MPTP- treated mice with PPX administration compared to controls. However, plasma homocysteine levels were increased significantly increased in MPTP- treated mice with L-dopa treatment compared to control, MPTP- only or MPTP- and PPX- treated mice (Fig. 11A). The level of homocysteine in the brain was increased significantly in MPTP- treated mice with L-dopa administration compared to controls, whereas the con-centration of homocysteine in the brain was decreased significantly in only MPTP- treated mice or in MPTP-treated mice with PPX compared to controls (Fig. 11B).
40 Fig. 11 Measurements of Hcy in plasma and brain.
The concentration of Hcy increases levodopa-treated group. concentration of Hcy in plasma-treated levodopa was significantly increased compared with contorl and MPTP mice(A). Al-so, levodopa-treated mice was significantly increased compared with control and MPTP mice and pramipexole treated mice in brain tissue (B) (*p <0.05 ; **p < 0.01) The data are presented as mean of 5 determinations .+ S.E.
41
7. L-dopa treatment leads to decrease neurogenesis in the SVZ zone
To investigate the effect of L-dopa treatment on neurogenesis in mice, NPCs
immu-nostained with BrdU were determined in the SVZ. Immunohistochemistry revealed that
BrdU-positive NPCs was significantly decreased in MPTP- treated mice compared to controls, whereas L-dopa or PPX treatment in MPTP- treated mice increased BrdU-positive NPCs compared to only MPTP- treated mice (Fig. 7A. a-d). Stereological analysis revealed that decreased number of BrdU-positive cells in the SVZ of MPTP- treated mice compared to controls was more evident, and the number of BrdU positive cells tended to be greater in PPX- treated mice compared to L-dopa- treated mice (Fig. 7B).
Additionally, I analyzed whether MK-801, a NMDA antagonist would lead to modulate de-creased neurogenesis associated with L-dopa treatment. MK-801 administration in L-dopa- treated PD animal model significantly increased the number of BrdU-positive cells in the SVZ compared to L-dopa- treated PD animal model. However, MK801 administration in PPX- treated mice was not changed (Fig. 7A e-f and B).
43
Fig. 12 Representative photomicrograph of BrdU+ and Hematoxylin+ after drugs ad-ministration.
Neuroprogenitor cells, BrdU positive cells, in SVZ were decreased in MPTP- treated mince than control (A, a, b). Levodopa –treated mice were increased neuroprogenitor cells than MPTP but more increased in levodopa and MK-801- treated mice (A, c, d). Similary, PPX- and PPX-and MK-801-treated mice were significantly increased neuroprogenitor cells in SVZ (A, e, f) But PPX- and PPX- and MK-801 treated mice had no significant. Graph represents the number of BrdU+ cells in SVZ (B).(*p <0.02 ; **p < 0.002) The data are presented as mean of 5 determinations .+ S.E.
44
IV. DISCUSSION
PART. A
Neuroprotective effects of L-dopa on dopaminergic neurons is com-arable to
pramipexole in MPTP- treated animal model of Parkinson’s disease.
Until now, there have been no in vivo data that directly compare neuroprotection between L-dopa and PPX. In this study, to closely mimic the neuroprotective strategies in PD in a clinical setting, I used a subchronic model of MPTP, which is suitable for evaluating the apoptotic cell death pathway, and chronically administrated candidate drugs after the devel-opment of nigral pathology. My study demonstrated that both L-dopa and PPX have neuro-protective properties on dopaminergic neurons in the MPTP- treated animal model of PD, acting through the promotion of cell survival signaling and inhibition of apoptotic signaling. Inhibitory effects on the JNK-related apoptotic pathway were similar between L-dopa and PPX, whereas L-dopa more potently activated ERK, and PPX seemed to exhibit a greater anti-oxidant effect. These results suggest that the neuroprotective effect of L-dopa on dopa-minergic neurons is comparable to that of PPX in an MPTP- treated PD model. MPTP may disrupt the balance between neuronal survival and apoptosis, producing a condition prone to neuronal degeneration. Through activation of the MAPK pathway by production of ROS, MPTP inhibits the activation of the ERK signaling pathway(De Girolamo and Billett 2006) and activates the JNK signaling pathway resulting in the phosphorylation of c-Jun (Saporito,
45
Thomas et al. 2000). Activated JNK can promote the release of cytochrome c from the mito-chondrial inner membrane through a Bax-dependent mechanism, enhancing the formation of apoptosomes (Vila, Ramonet et al. 2008). Additionally, activated JNK can translocate to mi-tochondria, where it can phosphorylate Bcl-2 proteins, thereby inhibiting the anti-apoptotic activity of Bcl-2 (Dhanasekaran and Reddy 2008). As expected, MPTP treatment in my study markedly activated JNK and increased the expression of related apoptotic proteins such as Bax and cytochrome c, concomitantly with decreased Blc-2 expression and signifi-cant inhibition of ERK activation. Ample evidence exists showing that dopamine agonists such as PPX have neuroprotective effects through an antiapoptotic activity, by decreasing the fall in mitochondrial membrane potential, cytochrome c release, and caspase activation in experimental models generating ROS (Gu, Iravani et al. 2004; Karunakaran, Saeed et al. 2008). This effect would seem to be mediated by a mechanism that is either independent of or dependent on dopamine receptors. As expected, PPX treatment in this study decreased the MPTP- induced activation of JNK-related apoptosis, thereby restoring some of the overall balance between neuronal survival and apoptosis, which was disrupted by MPTP treatment. Additionally, in contrast that the neuroprotective activity of PPX has been shown to occur in condition of pretreatment or pre-incubation before the introduction of neurotoxins (Anderson, Neavin et al. 2001; Gu, Iravani et al. 2004; Iravani, Haddon et al. 2006), this study demon-strated that PPX can also exert neuroprotective activity when administered after the onset of pathological changes in the SN. Interestingly, L-dopa treatment in this study decreased
sig-46
nificantly the MPTP- induced activation of JNK-related apoptosis. These results conflict with previous in vitro data demonstrating that L-dopa can be toxic to dopaminergic neurons. Although the exact mechanism is not fully understood, the capacity of L-dopa to undergo oxidative metabolism and generate ROS has been suggested as a possibility (Melamed, Offen et al. 1998). However, several studies using co-cultured neurons and glial cells have shown that glia can buffer ROS and that L-dopa has protective effects on dopaminergic neu-rons, even in high doses (Han, Mytilineou et al. 1996; Mena, Davila et al. 1998). The results of the in vivo administration of L-dopa to PD animal models have been conflicting; (Blunt, Jenner et al. 1993)) suggested a suppressive effect of L-dopa on dopaminergic neurons in the ventral tegmental area ipsilateral to a 6-hydroxydopamine lesions, whereas (Murer, Dziewczapolski et al. 1998) and (Datla, Blunt et al. 2001) demonstrated that chronic admini-stration of L-dopa increased the density of dopaminergic fibers or neurons without toxic ef-fects to remaining nigral dopaminergic neurons. ELLDOPA, a clinical trial to explore L-dopa toxicity in early stages of PD, showed contradictory findings between progression of clinical severity and functional imaging in L-dopa- treated PD patients compared with placebo-treated patients (Fahn, Oakes et al. 2004), suggesting that the potential long-term effects of L-dopa on PD remain uncertain. Overall, my study suggests that the neuroprotective activity of L-dopa, acting through an anti-apoptotic activity, is comparable to that of PPX. The acti-vation of ERK is widely believed to participate in the survival of dopaminergic neurons (Cavanaugh, Jaumotte et al. 2006; Zigmond 2006) although there is also increasing evidence
47
relating its activation with cell death (Canals, Casarejos et al. 2003). There have been few studies regarding the role of ERK signaling in PD models; in vitro studies using MPTP or rotenone have demonstrated that neurotoxins can inhibit ERK activation (De Girolamo and Billett 2006; Chen, Zhang et al. 2008), whereas ERK activation may contribute to dopa-minergic neuronal death in a 6-hydroxydopamine in vitro model (Kulich and Chu 2001). In my study, ERK activation in the midbrain was significantly decreased in MPTP-treated mice compared with control mice, which suggests that ERK activation may be involved in the survival pathway of dopaminergic neurons. Interestingly, ERK activation in MPTP- treated mice was more prominent in L-dopa- treated group compared to MPTP only treatment group as well as PPX- treated group. This is an unexpected finding because dopamine agonists, including PPX, have been known to activate ERK signaling, possibly through the up-regulation of glial cell line-derived neurotrophic factor and brainderived neurotrophic factor (Du, Li et al. 2005; Chen, Zhang et al. 2008). Several in vitro and in vivo studies demon-strated that L-dopa had neurotrophic properties for dopaminergic neurons, thus promoting cell survival and neurite outgrowth, which may be mediated by factors in glial cells that are up-regulated by L-dopa (Han, Mytilineou et al. 1996; Mena, Davila et al. 1997). Mena et al. reported that L-dopa potentiated the neurotrophic response of nerve growth factor, proposing that subtoxic oxidative stress by L-dopa may provide a trophic effect. In this regard, it is spe-culated that L-dopa or L-dopa metabolite-elicited neurotrophic factors may stimulate cell survival-related trophic factors, thus resulting in the activation of ERK. Of the various
anti-48
oxidant systems in the brain, the GSH system is particularly important in controlling the cel-lular redox state and is the primary defense against oxidative stress (Cooper and Kristal 1997). In accordance with previous studies, the present study showed that PPX administra-tion significantly increased the level of GSH compared with the level in MPTP- only treated mice. PPX also has an inhibitory effect on ROS production via decreased turnover of dopa-mine metabolism, because PPX acts on dopadopa-mine autoreceptors. In addition, PPX displays anti-oxidant properties through the direct scavenging of free radicals and the stimulation of cellular GSH peroxidase and catalase (Le, Jankovic et al. 2000). In the L-dopa- treated group, the level of GSH did not changed significantly compared with the level in the MPTP- only treated group; this is in contrast to in vitro studies showing detrimental effects of L-dopa on GSH levels(Spencer, Jenner et al. 1995). This discrepancy may be ascribed to differences in the antioxidant defense environment according to experimental designs(Han, Mytilineou et al. 1996) or to a biphasic effect of L-dopa on GSH, in which GSH synthesis is upregulated in response to mild oxidative damage and reduced in response to severe oxidative damage (Mytilineou, Walker et al. 2003).
Overall, the promotion of cell survival signaling by L-dopa and PPX after MPTP treat-ment led to the neuroprotection of dopaminergic neurons, as evidenced by an immunohisto-chemical analysis indicating that TH-ir neuron survival in the SN after MPTP treatment was significantly increased in both the L-dopa and PPX treatment groups, compared with the MPTP only treatment group. Additionally, increased survival of TH-ir cells by L-dopa and
49
PPX in MPTP- treated mice was also observed in immobilized groups, suggesting that neu-roprotective effect of L-dopa and PPX would not be resulted from enhanced locomotor activ-ity by these drugs. On direct comparison between L-dopa and PPX, there was no significant difference of neuroprotective effect on dopaminergic neurons in MPTP- treated mice. The similarity between the anti-apoptotic properties of L-dopa and PPX and their comparable neuroprotective effects, through ERK activation for L-dopa and via anti-oxidative effect for PPX, may produce similar increases in the survival of dopaminergic neurons for both agents. In summary, my study demonstrated that both L-dopa and PPX had comparable neuroprotec-tive properties for dopaminergic neurons in MPTP- treated PD animal models, through mod-ulation of cell survival and apoptotic pathways. These data may provide in vivo evidence that L-dopa is not toxic but is neurotrophic to dopaminergic neurons in PD. Nevertheless, my data should be interpreted cautiously in clinical implications for L-dopa therapy because the daily dose of L-dopa used in this study is higher as compared with that normally used in PD patients. Future study with the daily dose of L-dopa commonly used in clinical practice would helpful to resolve this issue.
50
PART. B
Increased level of homocysteine induced by levodopa inhibits neurogenesis by
mediating NMDA receptor signal cascade in MPTP- treated animal model of
Parkinson’s disease: comparison with pramipexol.
This is the first study evaluating the effect of L-dopa induced hyperhomocysteinemia on neurogenesis of in vitro and in vivo system with comparative analysis of dopamine agonist. The major findings were (1) hyperhomocysteinemia associated with L-dopa treatment exerts an antiproliferative effect on NPCs in the SVZ, (2) L-dopa treatment induced apoptosis of NPCs is mediated by ERK-MAP kinase signaling pathways through NMDA receptor, and (3) dopamine agonist has more augmenting effects of neurogenesis compared to L-dopa.
There is accumulating clinical evidence that chronic administration of L-dopa in patients with PD lead to increase the homocysteine synthesis (Blandini, Fancellu et al. 2001; Miller, Selhub et al. 2003). Similarly, my data showed that L-dopa treatment increased release of homocysteine from cultured astrocytes as well as concentration of homocysteine in both plasma and brain in MPTP- treated PD animals. Experimental studies indicated that homo-cysteine acts as an excitatory aminoacid by activating NMDA receptors (Lipton, Kim et al. 1997; Poddar and Paul 2009) and thus induce mitochondrial dysfunction, free radicals(Jara-Prado, Ortega-Vazquez et al. 2003) and cytosolic calcium accumulation (Kruman, Culmsee et al. 2000), and apoptotic pathways (Jiang, Gu et al. 2000). Accordingly, preclinical evidence has suggested that L-dopa treatment associated with hyperhomocysteinemia may
51
lead to detrimental effects on dopaminergic neurons as well as on non-dopaminergic neurons in PD models (Huang, Dragan et al. 2005; Imamura, Takeshima et al. 2007). However, whether L-dopa induced hyperhomocysteinemia may contribute to accelerate progression of nigal motor dysfunction and risk of extra-nigal non-motor features in patients with PD is controversial and remains to be determined. In this regard, scientific evidence addressing metabolic consequences of L-dopa treatment on other non-dopaminergic systems, such as neurogenetic system evaluated in the present study, are of great importance to determine so-phisticated therapeutic strategies for patients with PD.
My current in vitro data demonstrated that increased release of homocysteine from L-dopa treated astrocytes had a neurotoxic peroperty on NPCs of the SVZ, and phosphorylation of ERK through NMDA receptor led to induction of apoptosis in NPCs. In my study, NPCs iso-lated from the SVZ express NMDA receptor subunits 2Aand 2B as well as NR1, where the NR2A subunit is known to conveys high affinity for glutamatergic agonists. The role of NMDA receptor in regulating an upstream MAPK superfamily and ERK mediated proapop-totic signals has been extensively investigated. In NMDA receptor mediated neuronal toxic-ity, largely via the NMDAR-mediated influx of extracellular Ca2+, MAPKERK1/2 is known to be rapidly and transiently activated, and be involved in glutamate-induced apoptosis (Jiang, Gu et al. 2000; Haddad 2005). Additionally, my vitro study showed that a NMDA antagonist (MK-801) treatment significantly attenuated L-dopa induced activation of ERK kinase signaling pathways and apoptotic cell death in the NPCs. This result might further
52
support that L-dopa induced hyperhomocysteienemia has an important role in antiprolifera-tive effect on NPCs through NMDA receptor mediated apoptosis.
To evaluate blocking effects of NMDA receptor on the neurogenesis of the SVZ, I ex-tended my study into animal model of PD using MPTP. As expected, the neurogenesis in the SVZ measured by BrdU-positive NPCs cells was decreased significantly in MPTP- treated animals, which is in accordance with previous studies demonstrating that dopamine deple-tion led to decrease neurogenesis in the SVZ of postmortem brain of PD patients and animal model of PD (Hoglinger, Rizk et al. 2004). Interestingly, my in vivo data demonstrated that the treatment of NMDA anagonist in L-dopa- treated PD animals significantly increased neu-rogenesis in the SVZ compared to only L-dopa- treated PD animals. Several evidence have suggested that prolonged NMDA receptor activation might decrease the rate of proliferation and the number of newly generated neurons in the SVZ, although the neurogenetic effects of NMDA receptor is depending on exposing time or dose of NMDA agonists/antagonists (Kitayama, Yoneyama et al. 2003). In this regard, NMDA antagonist might attenuate pro-longed NMDA receptor activation induced by increased homocysteine in the brain after L-dopa treatment, and in turn lead to decrease apoptosis of NPCs in the SVZ. This in vivo re-sults further confirm that L-dopa induced hyperhomocysteienemia would modulate NMDA-dependant neurogenesis.
Another interesting finding is comparative analysis of neurogenesis between L-dopa and dopamine agonists. Recent studies reported that NPCs in the SVZ exhibited dopaminergic
53
receptors and dopaminergic innervations are important for the proliferation of NPCs in the SVZ(Winner, Desplats et al. 2009). Importantly, the neruogenetic effects are mediated by activation of the D2/D3 dopamine receptors, where dopamine receptor activation induces CNTF release into neurogenic niches (Mori, Jefferson et al. 2008; Yang, Arnold et al. 2008). Along with augmenting effects of neurogenesis by dopamine agonist in animal model of PD, O'Sullivan et al. demonstrated a positive impact of chronic L-dopa use on the number of neu-ral stem cells in the SVZ of patients with PD, however, there are no available studies dealing with direct comparison of neurogenetic activity between L-dopa and dopamine agonist in the same experimental design until now. In this study, I found that the number of cultured NPCs and BrdU-positive cells in the SVZ was higher significantly in PPX treatment than in L-dopa treatment, showing that the neurogenetic activity of dopamine agonist is superior to that of L-dopa. This difference of neurogenetic activity may be ascribed to detrimental effects of homocysteine on NPCs by L-dopa treatment. In addition, since PPX, as a D2 receptor family, has a more affinity on D3 dopamine receptor relative to L-dopa, this different property may also contribute to difference of neurogenetic activities, being in favor of dopamine agonist. Indeed, the number of BurU-positive cells in the SVZ tended to be higher in L-dopa treated PD animals compared to only MPTP- treated PD animals, which may imply indirectly that pro-neurogenetic effect through increased dopamine innervations by L-dopa seems to be stronger than anti-neurogenetic effect by L-dopa induced homocysteine. However, further clinical evidence of postmortem study regarding the role of dopamine agonist in modulation