저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Inflammasome activation in THP-1
target cells triggered by pathogenic
Naegleriafowleri
by
Jong-KyunYoo
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Inflammasome activation in THP-1 target
cells triggered by
pathogenic Naegleriafowleri
by
Jong-KyunYoo
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for the
Degree of Doctor of Philosophy of Biomedical Sciences
Supervised by
Ho-Joon Shin, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertationof
Jong-KyunYoois approved.
SUPERVISORY COMMITTEE
Sun Park Kyongmin Kim Tai-Soon Yong Suk-Yul Jung Ho-Joon ShinThe Graduate School, Ajou University
June 19th, 2015
i
-ABSTRACT-Inflammasome activation in THP-1 target cells
triggered by pathogenic Naegleriafowleri
Naegleriafowleri,also known as the brain-eating amoeba, causes acute primary
amoebic meningoencephalitis. During swimming and other recreational water activities, N. fowleritrophozoites penetrate the nasal mucosa and invade the olfactory bulbs, resulting in intense inflammatory reactions in the forebrain tissue. To investigate what kinds of inflammasome molecules are expressed in target cells due to N.
fowleriinfection,human macrophage cells (THP-1 cells) were co-cultured with N. fowleritrophozoites under the non-contact system, and consequently IL-1β production
was estimated. By western blotting and ELISA analysis, the caspase-1 activation and IL-1β production from THP-1 cells and the culture supernatant were observed 3 hrafter co-cultivation. In addition, the increased expression of ASC and NLRP3, which consist of inflammasome complex, was also observed 3 hr post co-cultivation. To confirm the caspase-1 activation and IL-1β production via NRLP3 inflammasome inTHP-1 cells triggered by N. fowleritrophozoites, several inhibitors were pretreated on THP-1 cells.
Results of the inhibition assay show that CA-074 (cathepsin B
inhibitor),Glybenclamide (NRLP-3 molecule inhibitor) and Z-VAD-FMK (caspase-1 inhibitor) reduced the caspase-1 activation and IL-1β production from THP-1 cells. This study suggests that N. fowleriinfection induces the NLRP3-inflammasome
ii
complex, which activates the caspase-1and subsequentlyproduces the IL-1β, thus resulting in inflammation.
---Keywords: Naegleriafowleri;PAM, caspase-1; inflammasome; IL-1β; NLRP3
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF FIGURES ... v
I. INTRODUCTION ... 1
II. MATERIALS AND METHODS ... 4
A. Naegleriafowlericulture and lysate preparation ... 4
B.Culture of macrophage THP-1 cells ... 4
C. Non-contact culture system... 4
D.Cell extraction from THP-1 cells ... 7
E.SDS-PAGE and Western blotting for detection of inflammasome molecules ... 7
F. IL-1β cytokine measurement by ELISA... 8
G. Measurement of caspase-1 by ELISA ... 9
H. Inhibition assay ... 10
I. Statistical analysis ... 10
III. RESULTS ... 11
A. IL-1β production in THP-1 cells co-cultured with N. fowleri ... 11
iv
C. NLRP3 and ASC expression in THP-1 cells co-cultured with N. fowleri ... 17
D. Inhibition of IL-1β production in THP-1 cells co-cultured with N. fowleri ... 19
E. Inhibition of caspase-1 activation in THP-1 cells co-cultured with N. fowleri ... 21
IV. DISCUSSION ... 23
V. CONCLUSION ... 28
REFERENCES ... 29
v
LIST OF FIGURES
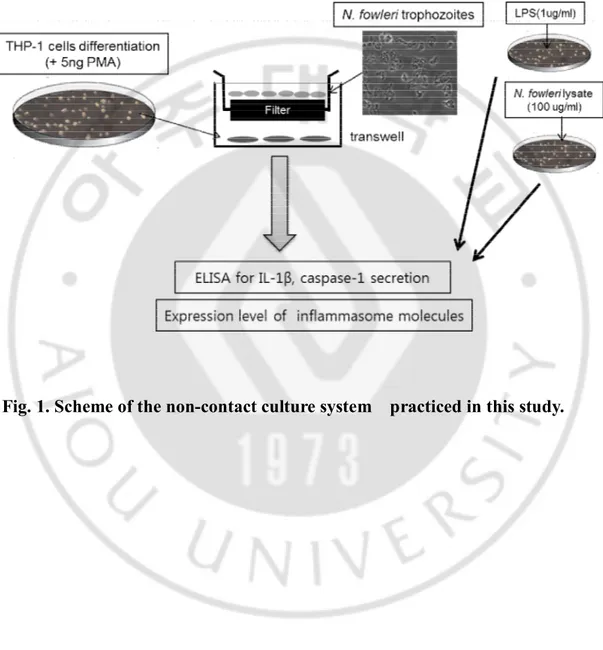
Fig. 1.Scheme of the non-contact culture system practiced in this study ... 6 Fig. 2.IL-1β expression in THP-1 cells co-cultured with N. fowleritrophozoites
in a non-contact system ... 12 Fig. 3.IL-1βsecretion fromTHP-1 cells co-cultured with N. fowleritrophozoites ... 13 Fig. 4.Caspase-1 expression from THP-1 cells co-cultured with N. fowleri
trophozoites in a non-contact system ... 15 Fig. 5.Caspase-1 secretionfrom THP-1 cells co-cultured with N. fowleri
trophozoites ... 16 Fig. 6.NLRP3 and ASC expression inTHP-1 cells co-cultured with N. fowleri
trophozoites ... 18 Fig. 7.Inhibition assay for IL-1β production in THP-1 cells cultured with
N. fowleri ... 20
Fig. 8.Inhibition of caspase-1 activation from THP-1 cells co-cultured with
N. fowleri ... 22
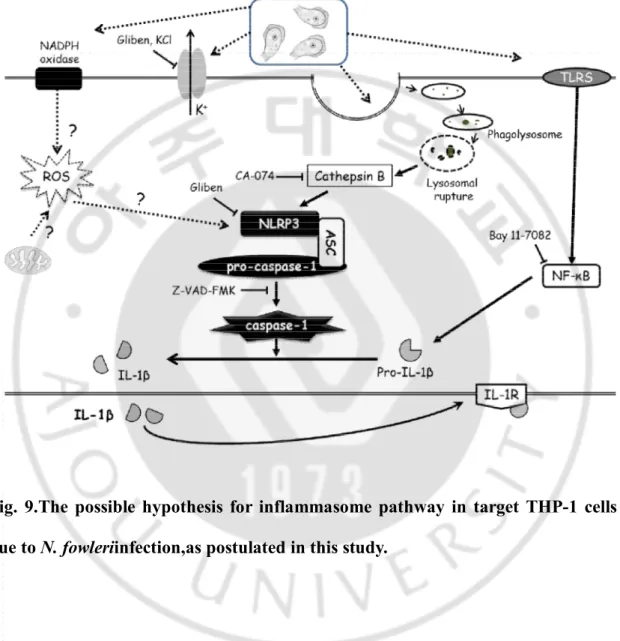
Fig. 9.The possible hypothesis for inflammasome pathway in target THP-1 cells due to N. fowleriinfection,as postulated in this study. ... 27
Inflammasome activation in THP-1
target cells triggered by pathogenic
1
I.INTRODUCTUIN
Pathogen Naegelria fowleri has been labelled as the “brain-eating amoeba”.It has a worldwide distribution, and causes an acute primary amoebic meningoencephalitis (PAM), an acute and severe central nervous system (CNS) disease in experimental animals and humans (Cater, 1970; Culberson, 1971; Visvesvara GS et al., 2007), and is becoming a serious issue in subtropical and tropical countries as a Neglected Tropical Disease (NTD) (De Jonckheere, 2011; Siddiqui and Khan, 2014) . Depending on the environmental conditions, the life cycle of N. fowlerihas three stages: cyst, trophozoite, and flagellate. Thermophilic N. fowleri trophozoites grow between 30 - 37ºC, and can tolerate up to 45ºC (Visvesvara GS et al., 2007). N. fowleri has also been found in moist soil, lakes, rivers and hot springs, swimming pools, and in warm water discharge pools from industrial plants (Culbertson, 1971; De Jonckheere, 2011) .
PAM cases in children occurs through swimming and other recreational water activities; the amoebae flourish in the water during the hot summer, and their presence has increased due to global warming (Heggie 2010; Sifuentes et al., 2014 ). After attaching to the nasal mucosal, N. fowleri trophozoites penetrate the olfactory epithelium and invade the olfactory bulbs, resulting in an intense inflammatory reaction in the forebrain tissue,with increased eosinophils and neutrophils surrounding the amoebae (Marciano-Cabral et al., 2007). PAM is a fulminant infection and leads to death within 1 to 2 weeks from the onset of symptoms. With 95% mortality, early
2
intervention with amphotericin B treatment has resulted in recovery in a few cases (Visvesvara GS et al., 2007).
Under contact-dependent culture conditions, mouse microglial cells co-cultured with
N. fowleritrophozoites secreted the pro-inflammatory cytokines, i.e. tumor necrosis
factor-alpha (TNF-α), IL-1β, and IL-6 (Oh et al., 2005). In addition, N. fowleriinduced the host innate defense mechanisms, such as mucin secretion (MUC5AC) and local inflammation (IL-8 and IL-1β),in respiratory epithelial cells via ROS production (Cervantes-Sandoval et al., 2009). As a contact-independent mechanism, target microglial cells treated with N. fowlerilysate showed a primary immune response via strong cytopathic changes and pro-inflammatory cytokine release (Lee et al., 2012). In addition, IL-1β and IL-6 activated inflammatory responses of astrocytes against the N.
fowleri infection via the modulation of MAPKs and AP-1 (Kim et al. 2013). These data
suggest a major role of inflammation in tissue damage due to N. fowleri infection. In the innate immune system, various pathogens were associated with various molecular patterns via intracellular pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), membrane-associated TLRs (Toll-like receptors) and cytosolic nucleotide-binding oligomerization domain (NOD)-(Toll-like receptors (NLRs) which activate the transcription of many genes, including chemokines and cytokines, such as IL-1β (Cooney et al., 2010; McCoy et al., 2010;Kersse et al., 2011). In the induction of pyroptosis, a form of proinflammatory necrotic cell death which is induced by infection with pathogenic bacteria, the caspase-1 is responsible for the proteolytic processing and secretion of IL-1β and IL-18 (Travassos et al., 2010).
3
During infection with pathogenic microorganisms, the processing of bioactive IL-1β (and that of IL-18) depends on activation of caspase-1 by protein complexes termed the inflammasomes (Martinon et al., 2002). The inflammasome is a multi-protein complex activating caspase-1 and releasing active IL-1β,and is composed of the NLR family, especially members of the NLRP (NOD-like receptor containing pyrin domain) family including NLRP1 and NLRP3, which is composed of the adaptor molecule ASC (Apoptosis-Associated Speck-Like Protein) and the effector molecule caspase-1(Shaw et al., 2008; Netea et al., 2010). To date, some kinds of inflammasomes have been identified in various viral and bacterial infection models, but very little is known about their role in the infection of eukaryotic pathogens.
The activation of the inflammasome in intracellular pathogenic infections, such as malaria, leishmaniasis, trypanosomiasis, amoebiasis and toxoplasmosis etc., has been investigated (Shio et al., 2009; Lima-Junior et al., 2013; Gonçalves et al., 2013; Mortimer et al.,2013; Ewald et al., 2014), but the role of the inflammasome in pathogenic N. fowleri infection has not been addressed yet. In this present study, to investigate what kinds of inflammasome-related molecules may be expressed in target cells due to N. fowleri infection, human macrophage cells were co-cultured indirectly with N. fowleritrophozoites under a non-contact system, and consequently the active IL-1β release was estimated.
4
II. MATERIALS AND METHODS
A. Naegleriafowlericulture and lysate preparation
N. fowleritrophozoites (Carter NF69; ATCC No. 30215) were axenically cultured at
37°C, in Nelson’s medium containing 10% fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA) and penicillin/streptomycin (P/S; Gibco BRL) (Willaert, 1971).
The N. fowleri lysate was prepared by the freezing-thawing method (Said-Fernandez and Lopez Revilla, 1982),followed by ultracentrifugation at 12,000 rpm for 2 hrat 37°C. After centrifugation, the supernatants were collected and used for treatment, as the lysate.
B. Culture of macrophage THP-1 cells
Human macrophage THP-1 cells (an acute monocytic leukemia cell line) were cultured in RPMI 1640 medium (Gibco BRL) containing 10% FBS, at 37°C in a 5% CO2 humidified incubator.
5
C. Non-contact culture system
THP-1 cells (1x106cells/ml/well) were seeded in 6-well tissue culture plates
(Corning, Tewksbury, MA, USA) and differentiated into adherent macrophages by culturingovernight in complete RPMI 1640 medium containing 5 ng/ml Phorbol 12-myrisate 13-acetate (PMA; Sigma, St Louis, MO, USA). A non-contact culture system was set up according to the previously described method (Kim et al., 2008). Briefly, a transwell insert (Corning, Tewksbury, MA, USA) with a membrane pore size of 0.2 μm was placed in a 6-well plate containing differentiated THP-1 cells (1x106cells/ml/well)
with 2 ml of RPMI1640 medium. The N. fowleritrophozoites (1x107cells/ml/well) were put in thetranswell insert and co-cultured with the THP-1 cells for 3, 12 and 24 hr, at 37°Cin a 5% CO2 incubator. For a control group, the differentiated THP-1 cells (1x106
cells/well) were co-cultured with lipopolysaccharide (LPS; Sigma) (1 μg/ml) or N.
6
7
D. Cell extractionfrom THP-1 cells
THP-1 cells were co-cultured with N. fowleritrophozoites in a non-contact culture system for 3, 12 and 24 hr (treatment groups), and with either LPS (1 μg/ml) or N.
fowleri lysate (100 μg/ml) for 12 hr as the control groups. After cultivation, the THP-1
cells were pelleted in a tube and suspended in 100 μl of Cell lysis buffer (Intron Biotechnology, Seongnam, Korea);the tube was gently rocked at 4°C for 20 min. This lysate was then centrifuged at 13,000 rpm for 20 min at 4°C,and the supernatant was collected and stored at -20°C, until further use. Protein concentration was estimated by Bradford assay,with BSA (bovine serum albumin) as a standard.
E. SDS-PAGE and Western blotting for detection of inflammasome molecules
The protein samples were suspended in reducing sample buffer (62.2 mMTris, pH 6.8; 10% glycerol; 10% 2–mercaptoethanol; 3% SDS (sodium dodecyl sulfate) and 0.1% bromophenol blue) and boiled for 5 min. Samples were then subjected to 12% SDS-PAGE (polyacrylamide gel electrophoresis). For western blotting, proteins were transferred onto PVDF (polyvinylidenedifluoride) membrane(Millipore Bedford, MA, USA) at 250 mA for 2 hr; the transfer was done on ice. The proteins in PVDF membrane were blocked with 3% BSA in PBS (phosphate buffered saline, pH 7.4)
8
containing 0.05% Tween-20 (Sigma, St Louis, MO, USA) for 2 hr, on a shaker kept at room temperature. The membranes were incubated overnight with the primary antibodies, at 4°C. The primary antibodies used were rabbit polyclonal anti-IL-1β, rabbit polyclonal caspase-1, rabbit polyclonal ASC, or rabbit polyclonal anti-NLRP3 antibodies (all at 1:1,000 dilution) (Abcam, Cambridge, MA, USA), and beta-actin (1:50,000 dilution; Sigma). After washing three times with 0.05% PBST for 15 min, the membraneswere incubated with the secondary antibody, peroxidase-conjugated goat anti-rabbit whole IgG (1:5,000 dilution) (Abcam), for 2 hr at room temperatures. After washing three times with 0.05% PBST for 15min, the membranesweretreated with the enhanced chemical luminescence (ECL) solution (Intron, Daejon, Korea) and then exposed to X-ray film (Konica, Tokyo, Japan).
F. IL-1β cytokine measurement by ELISA
IL-1β levels in culture supernatants from THP-1 cells co-cultured with N.
fowleritrophozoites in the non-contact culture system, or after treatment with N. fowleri lysate (100 μg/ml) or LPS (1 μg/ml) (positive controls), were determined using
the ELISA kit for human IL-1β detection,according to the manufacturer’s instruction(R&D systems, Minneapolis, MN, USA). Briefly, 200 μl of the culture supernatant was put into each well of a 96-well culture plate, andthe plate was
9
incubated at room temperature for 2hr. The supernatants from each well were discarded. Wells were washed thrice with wash buffer (400 μl each), after which 200 μl of IL-1β conjugate solution was added into each well, and the plate was incubated for 1 hr at room temperature. After another three washes, 200 μl of substrate solution was added into each well, and incubated for 20 min at room temperature. The reaction was stopped by adding stop solution;theO.D value of each well wasmeasuredat 450 nm, using a Microplate reader, and the levels of IL-1β were estimated.
G. Measurement of caspase-1 by ELISA
To determine the activated caspase-1 levels in supernatants from THP-1 cells co-cultured with N. fowleritrophozoites in a non-contact culture system,ortreated with N.
fowleri lysate (100 μg/ml) or LPS (1 μg/ml), we used the Quantikine ELISA
kit,according to the manufacturer’s instruction(R&D systems, Minneapolis, MN, USA). Briefly, 100 μl of culture supernatant was put into each well of 96-well plate and incubated for 90 min at room temperature. The sample was discarded and plates were washedthree times with wash buffer, following which 100 μl of Caspase-1 antiserum solution was added to each well and incubated for 30 min at room temperature. Washing was repeated three times, and 200 μl of substrate solution was put in each well and further incubated for 20 min at room temperature. The reaction was stopped
10
by adding stop solution,theO.D value wasmeasured using a Microplate reader at 450 nm, and levels of caspase-1 were evaluated.
H. Inhibition assay
THP-1 cells were pre-incubated for 60 min with either 130 mMKCl (potassium channel inhibitor) (Sigma, St Louis, MO, USA), 50 μMGlybenclamide (Gliben; ATP-dependent potassium channel inhibitor; NLRP3 inhibitor) (Invitrogen, San Diego, CA, USA), 24 μM CA-074 Me (cathepsin B inhibitor) (Calbiochem, San Diego, Ca, USA), 10 μM Z-VAD-FMK (caspase-1 inhibitor)(Invitrogen), or10 μM Bay 11-7082 (NFκB inhibitor) (Calbiochem),after which they werecocultured with N. fowleritrophozoites for 6 hr in a non-contact culture system. The caspase-1 activation and IL-1β secretion from the culture supernatants were measured by ELISA.
I. Statistical analysis
All experiments were performed at least three times. Statistical differences between groups or samples were determined with Student′s t-test. The difference was considered significant when P< 0.05.
11
III. RESULTS
A. IL-1β production in THP-1 cells co-cultured with N. fowleri
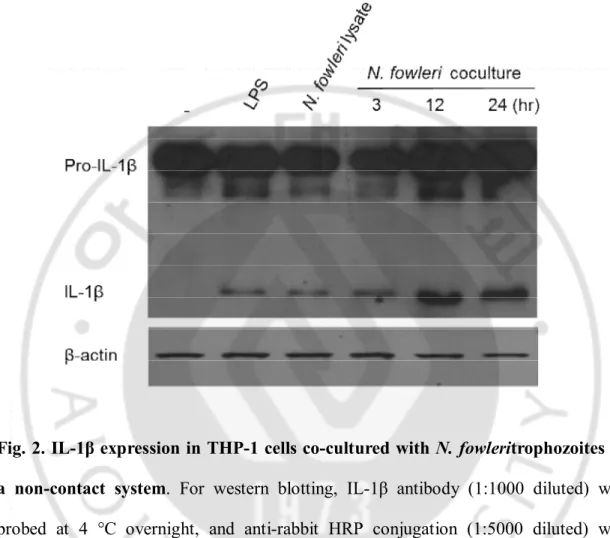
In the non-contact culture of THP-1 cells with N. fowleritrophozoites, the IL-1β expression via pro-IL-1β cleavage wasdemonstrated by Western blot at 3, 12 and 24hr post cultivation;IL-1β levels were similar to that of the control groups (treatment withN.
fowlerilysate and LPS (a non-specific positive control)) (Fig. 2). The expression levels
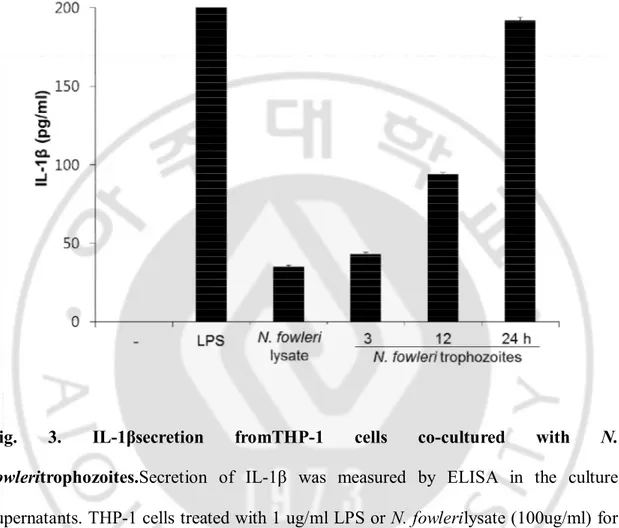
of IL-1βincreasedwiththe culture time. Results of ELISA analysis with culture supernatants in the non-contact culture also showed that the IL-1β production increased in a time-dependent manner (Fig. 3).
12
Fig. 2. IL-1β expression in THP-1 cells co-cultured with N. fowleritrophozoites in a non-contact system. For western blotting, IL-1β antibody (1:1000 diluted) was
probed at 4 °C overnight, and anti-rabbit HRP conjugation (1:5000 diluted) was probedfor 2 hr at RT. Lane 1, control (THP-1 cell lysate); lane 2, THP-1 + LPS (1 ug/ml); lane 3, THP-1 + N. fowlerilysate (100 ug/ml); lane 4, 5 and 6, THP-1 + N.
13
Fig. 3. IL-1βsecretion fromTHP-1 cells co-cultured with N.
fowleritrophozoites.Secretion of IL-1β was measured by ELISA in the culture
supernatants. THP-1 cells treated with 1 ug/ml LPS or N. fowlerilysate (100ug/ml) for 12 hr. Untreated THP-1 cells and LPS treatment were used as anegative control and positive control, respectively. THP-1cells co-cultured with N. fowleritropozoites for 3, 12 and 24 hrin a non-contact system induced the secretion of IL-1β in a time-dependent manner (P<0.05).
14
B. Caspase-1 activation in THP-1 cells co-cultured with N. fowleri
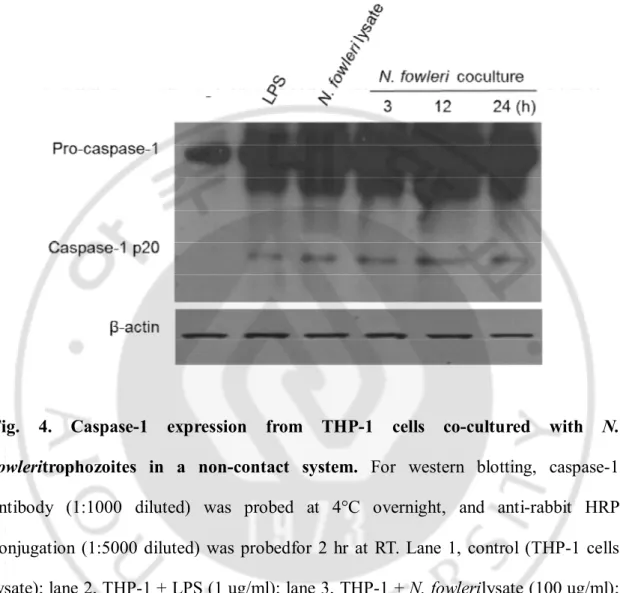
In THP-1 cells co-cultured with N. fowleritrophozoites in a non-contact system, thecaspase-1 activation (activate form p20) wasdemonstrated by Western blot at 3, 12 and 24hr post cultivation, which was similar to the treatment withN. fowlerilysate and LPS (a non-specific positive control) (Fig. 4). To estimate the release of the activated caspase-1 from THP-1 cells co-cultured with N. fowleritrophozoites, the released caspase-1 levels were also evaluated in the culture supernatants by ELISA; analysisrevealed increasing levels in a time-dependent manner(Fig. 5).
15
Fig. 4. Caspase-1 expression from THP-1 cells co-cultured with N.
fowleritrophozoites in a non-contact system. For western blotting, caspase-1
antibody (1:1000 diluted) was probed at 4°C overnight, and anti-rabbit HRP conjugation (1:5000 diluted) was probedfor 2 hr at RT. Lane 1, control (THP-1 cells lysate); lane 2, THP-1 + LPS (1 ug/ml); lane 3, THP-1 + N. fowlerilysate (100 ug/ml); lane 4, 5 and 6, THP-1 + N. fowleritrophozoites for 3 , 12 and 24hr , respectively.
16
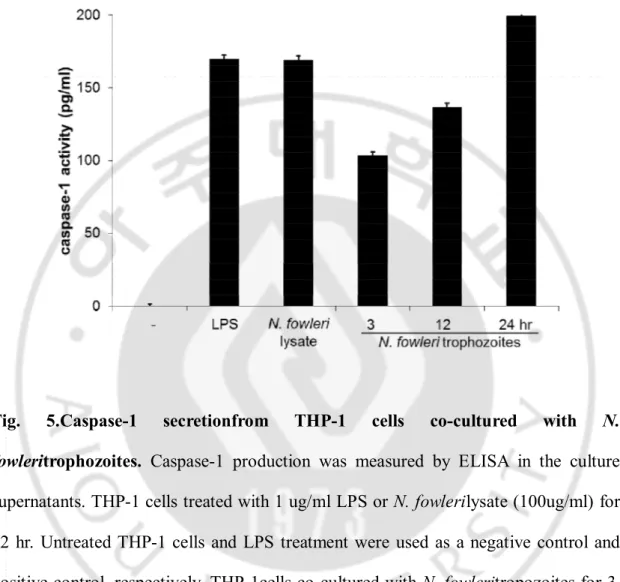
Fig. 5.Caspase-1 secretionfrom THP-1 cells co-cultured with N.
fowleritrophozoites. Caspase-1 production was measured by ELISA in the culture
supernatants. THP-1 cells treated with 1 ug/ml LPS or N. fowlerilysate (100ug/ml) for 12 hr. Untreated THP-1 cells and LPS treatment were used as a negative control and positive control, respectively. THP-1cells co-cultured with N. fowleritropozoites for 3, 12 and 24 hrin a non-contact system induced the production of caspase-1 in a time-dependent manner (P<0.05).
17
C. NLRP3 and ASC expression in THP-1 cells co-cultured with N. fowleri
In order to observe what types of inflammasomeareformed in caspase-1 activation and IL-1β secretion, the expression levels of NLRP3 and ASC molecules were observed in THP-1 cells co-cultured with N. fowleritrophozoites in a non-contact system. By Western blot analysis, NLRP3 and ASC expression were seenat 3, 12 and 24hr post cultivation, which was similar to the expression seen when treatedwithN.
18
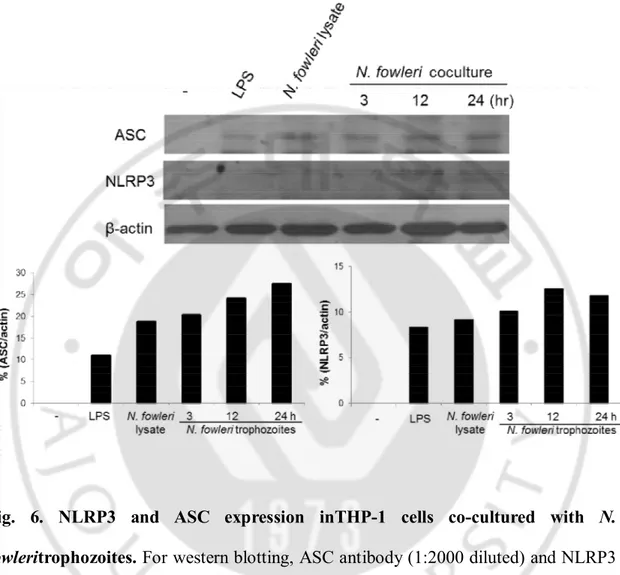
Fig. 6. NLRP3 and ASC expression inTHP-1 cells co-cultured with N.
fowleritrophozoites. For western blotting, ASC antibody (1:2000 diluted) and NLRP3
antibody (1:500 diluted) were reacted at 4°C overnight, and anti-rabbit HRP conjugation (1:5000 diluted) was reacted for 2 hr at RT. Lane 1, control (THP-1 cell lysate); lane 2, THP-1 + LPS (1 ug/ml); lane 3, THP-1 + N. fowlerilysate (100 ug/ml); lane 4, 5 and 6, THP-1 + N. fowleritrophozoites for 3, 12 and 24 hr, respectively.
19
D. Inhibition of IL-1β production in THP-1 cells co-cultured with N. fowleri
In order to observe whether the IL-1β production is inhibited by signaling inhibitors, THP-1 cells pre-treated with the five inhibitors were co-cultured with N.
fowlerilysate and trophozoites in a non-contact system (Fig. 7). In THP-1 cells cultured
with N. fowlerilysate, the production of IL-1βwas remarkably inhibited after treatments of KCl(potassium channel inhibitor), CA-074(cathepsin B inhibitor), Glybenclamide(Gliben; NLRP3 inhibitor), Z-VAD-FMK(caspase-1 inhibitor) and Bay 11-7082 (NFκB inhibitor) (each P<0.05) (Fig. 7A). Also, when THP-1 cells pre-treated with the five inhibitors were co-cultured with N. fowleritrophozoites in a non-contact system, the IL-1β productionwas noticeably inhibited by treatments of KCl, CA-074, Glybenclamide, Z-VAD-FMK and Bay 11-7082 (each P<0.05) (Fig. 7B).
20
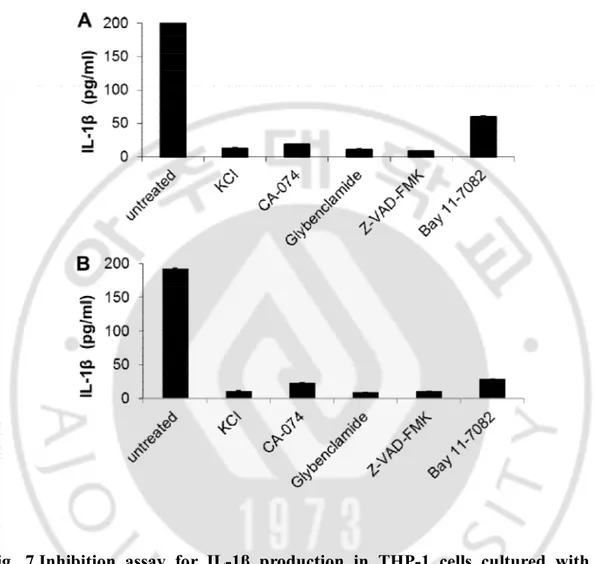
Fig. 7.Inhibition assay for IL-1β production in THP-1 cells cultured with N.
fowleri. THP-1 cells were pretreated with 10 µM Z-VAD-FMK, 130 mMKCl, 50 µM
Glybenclamide, 25 µM CA-074 and 10 µM Bay 11-7082 for 1 hr, and then co-cultured with N. fowlerilysate (A) andtrophozoites (B) for 6 hr. The inhibition of IL-1βproduction was measured by ELISA.
21
E. Inhibition of caspase-1 activation in THP-1 cells co-cultured with N. fowleri
In order to observe whether the expression of caspase-1 is inhibited by signaling inhibitors, THP-1 cells pre-treated with the five inhibitors were co-cultured with N.
fowlerilysate and trophozoites in a non-contact system (Fig. 8). In the non-contact
culture with N. fowlerilysate, the expression of caspase-1 from THP-1 cells was significantly inhibited by treatment of Glybenclamide and Bay 11-7082 (each P<0.05) (Fig. 8A),whereas theinhibition of caspase-1 by treatment of KCl,CA-074 and Z-VAD-FMK was relatively less. In addition, when THP-1 cells pre-treated with the five inhibitors were co-cultured with N. fowleritrophozoites in a non-contact system, the expression of caspase-1 was significantly inhibited by treatment of KCl, Glybenclamide, Z-VAD-FMK, and Bay 11-7082 (each P<0.05) (Fig. 8B),butthe inhibitionof caspase-1 expression by CA-074 treatment was relatively less.
22
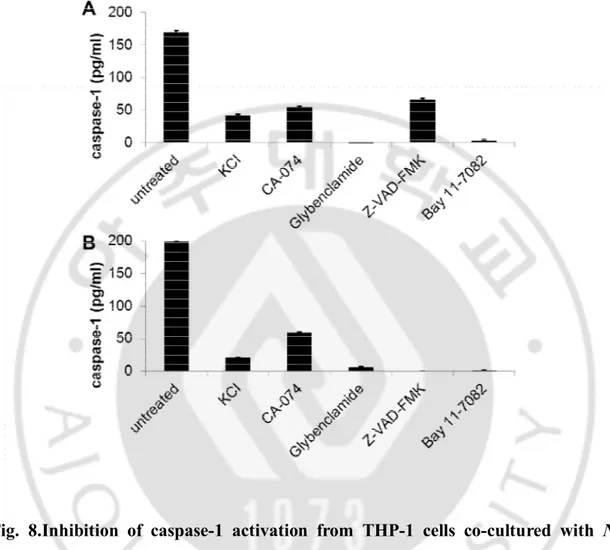
Fig. 8.Inhibition of caspase-1 activation from THP-1 cells co-cultured with N.
fowleri. THP-1 cells were treated with 10 µM Z-VAD-FMK, 130 mMKCl, 50 µM
Glybenclamide, 25 µM CA-074 and 10 µM Bay 11-7082 for 1 hr, and then co-cultured with N. fowlerilysate (A) andtrophozoites (B) for 6 hr. The inhibition of caspase-1 activation wasmeasured by ELISA.
23
IV. DISCUSSION
Development of PAM after invasion of the CNS by N. fowleritrophozoites, is a prominent feature of fatal N. fowleri infection in humans and experimental animals. The major route of invasion for N. fowleri infection is the nasal cavity. Amoebic trophozoites attachto the nasal mucosa, invade the olfactory nerve, and cause inflammation in the brain (Marciano-Cabral et al., 2007; Visvesvara GS et al., 2007). In the PAM-tissue, severe inflammatory responses and hemorrahagic necrosis were revealed as major pathologic findings. Further evidence was shown in experiments where the primary culture rat microglial cells coculturedwith N. fowleri trophozoites undergo necrosis and some apoptosis, and were shown to produce the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 (Oh et al., 2005). Also, the RNase protection assay demonstrated that IL-8 mRNA expression was markedly increased in human astrocytes (CRT-MG cell line) cocultured with N. fowleri lysate (Kim et al., 2012). The adhesion of amoebae to the host cells is a criticalfirst step in the pathogenesis of N. fowleri infection. Previously, we reported that pathogenicity ofN.
fowleri would be a complex processes which involves contact-independentpathways as
well as contact-dependent pathways (Kim et al., 2008). In contact-dependent mechanisms, Nfa1 protein and actin protein had been characterized and identifiedin N.
fowleri infections(Oh et al., 2005; Sohn et al., 2010). In addition, excretory-secretory
proteins (ESPs) released from N. fowlerihavealso been identified, which may play an important role in itspathogenicity (Kim et al., 2009).
24
In the host inflammatory responsewhen infected by pathogenic microorganisms, the activation of caspase-1 by protein complexes,namely the inflammasomes, was observed,along with the expression of the bioactive IL-1β (Martinon et al., 2002). In general, several nucleotide-binding, NLRs and PYHIN proteinsfrom inflammasomes, such asNLRP1, NLRP3 and NLRC4, which are composed of the adaptor molecule ASC protein, induce the caspase-1activation by functioningof various stimulators. Afterrecognizing such stimulators, inflammasomes areassembled, and caspase-1 causes the secretionof IL-1β and IL-18(Shaw et al., 2008; Netea et al., 2010). In addition, while NLRP3 ligands have been well identified, there is little known about the upstream mechanisms of regulation in NLRP3 activation. Some mechanisms that have been reported includeefflux of potassium, increased intracellular calcium, ROS generation and lysosome disruption(Shio et al., 2009). The IL-1β production and inflammatory response induced by Plasmodium Hz (hemozoin) are dependent on NLRP3, ASC and caspase-1 activation, and tyrosine kinase signaling pathways are known to regulate the NLRP3 inflammasome (Shio et al., 2009). In the Leishmaniaamazonensis infection, the NLRP3 inflammasome is engaged in host response, and the activation of this molecular platform has a crucial role in the restriction of the parasite replication, both in macrophages and in vivo, with notable IL-1β production (Lima-Junior et al., 2013). The NLRP3 inflammasome controls T. cruziparasitemia by inducing NO production via a caspase-1-dependent, IL-1R-independent pathway (Gonçalves et al., 2013). With inflammasome formation, other protozoa, such as Entamoebahistolytica and
25
NLRP family and caspase-1 activation, and IL-1β production (Mortimer et al.,2013; Ewald et al., 2014)
Previously,we reported that both N. fowleri lysates and trophozoites can trigger the production of inflammatory mediators, such as IL-1β and IL-6, via theactivation of signaling cascades involving MAP kinase familymembers and transcription factor (AP-1) in astrocytes (Kim et al., 2013). In this study, to observe the role of upstream signaling in the activation of theNLRP3inflammasome that results in IL-1β production against theN. fowleri infection, THP-1 cells were co-cultured with N.
fowleritrophozoites under a non-contact system, and subsequentlythe IL-1β production
was estimated.In the THP-1 cells co-cultured with N. fowleritrophozoitesunder the non-contact culture, the activation of caspase-1 and the production of IL-1βincreased in a time-dependent manner,as also the formation and activation of the inflammasome complex. This was confirmed by the formation of NLRP3/ASC inflammasome complex in the THP-1 cells. In addition, THP-1 cells co-cultured with N.
fowleritrophozoitesunder the non-contact culture did not showed the formation of
NLRP1 and NLRP4 inflammasome by Western blot,when probed with an anti-NLRP1 and NLRP4 antibody (data not shown).
The NLRP3 inflammasomeis activated in response to several stimuli, such aspore-forming toxins, noninfectious crystals,potassium efflux, ROS and lysosomal disruption. The assembly of NLRP3inflammasome requires a potassium efflux, and is impaired by inhibitors of potassium transporters, lysosomalcathepsins and ROS.After this activation, caspase-1 induces the processing and secretion of IL-1β. In this study, to
26
observe whether the casepase-1expression and production of IL-1βis inhibited by signaling inhibitors, THP-1 cells were pre-treated with six different inhibitors and co-cultured with N. fowlerilysate and trophozoites in a non-contact system. The activation of caspase-1 from THP-1 cells was markedly inhibited by treatment of three inhibitors, namelyKCl (potassium channel inhibitor), Glybenclamide (ATP-dependent potassium channel inhibitor), and Bay 11-7082 (NFκB inhibitor).The production of IL-1βfromTHP-1 cells pre-treated with Cyt D (phagocytosis inhibitor), KCl, CA-074 (cathepsin B inhibitor), Glybenclamide, Z-VAD-FMK (caspase-1 inhibitor), and Bay 11-7082 was remarkably inhibited. The possible hypothesis for inflammasome pathway in host cells due to N. fowleriinfection is shown in Fig. 9. In our hypothesis, further studies are required to understand whether the NLRP3inflammasome activation is triggered through ROS and NADPH oxidase. A recent study has revealed that NADPH oxidase may play a role in inducing ROS, and that ROS production is required to prime NLRP3inflammasome (Bauernfeind et al., 2011). Moreover, what constituents of the N. fowlerilysate ortrophozoitestrigger the signal pathway will be evaluated in furtherstudies. Our study also showed that the activation of caspase-1 and the production of IL-1β from THP-1 cells were markedly inhibited by treatment of inhibitors, such as Cyt D, KCl, CA-074, Glybenclamide, Z-VAD-FMK, and Bay 11-7082. To summarize, our findings identified that N. fowleriinfection induces caspase-1 activation through the NLRP3-inflammasome formation withsubsequent IL-1β secretion, which consequently causes the inflammation.
27
Fig. 9.The possible hypothesis for inflammasome pathway in target THP-1 cells due to N. fowleriinfection,as postulated in this study.
28
V. CONCLUSION
Pathogenic N. fowleri, a brain-eating amoeba associated with swimming and other recreational water activities, results in intense inflammatory reactions in the forebrain tissue:a condition known as acute PAM. To investigate what kinds of inflammasome-related molecules are expressed in target cells due to N. fowleriinfection,human macrophage THP-1 cells were co-cultured with N. fowleritrophozoites under the non-contact system using a transwell, and consequently IL-1β production was estimated.
In THP-1 cells co-cultured with N. fowleritrophozoites in a non-contact system, the activation of caspase-1 and production of IL-1β were evaluated by Western blot and ELISA analysis. Also, the formation of NLRP3/ASC inflammasome complex in THP-1 cells induced the caspase-1 activation and consequent IL-1β secretion. In addition, both, the activation of caspase-1 andthe production of IL-1β from THP-1 cells,wereinhibited by pretreatment of THP-1 cells with inhibitors, such as KCl, CA-074, Glybenclamide, Z-VAD-FMK, and Bay 11-7082.
In conclusion, this study suggests that N. fowleriinfection induces caspase-1 activation through the NLRP3-inflammasome formation,withsubsequent IL-1β secretion, which consequently causes the inflammation.
29
REFERENCES
1. BauernfeindF, Bartok E, Rieger A, FranchiL, NunezG, Hornung V (2011) Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 187: 613-617.
2. Carter RF (1972) Primary amoebic meningoencephalitis appraisal of present knowledge. Trans R Soc Trop Med Hyg. 66: 193-213.
3. Cervantes-Sandoval I, Serrano-Luna JJ, Meza-Cervantez P, Arroyo R, Tsutsumi
V, Shibayama M (2009)Naegleriafowleri induces MUC5AC and
proinflammatory cytokines in human epithelial cells via ROS production and EGFR activation. Microbiology. 155: 3739–3747.
4. Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A (2010) Nod2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 16:90–97.
5. Culbertson CG (1970) Pathogenic Naegleria and
Hartmannella(Acenthamoeba).Ann N Y Acad Sci. 174: 1018-1022.
6. De Jonckheere JF (2011) Origin and evolution of the worldwide distributed pathogenic amoeboflagellateNaegleriafowleri. Infect Genet Evol.11: 1520–1528. 7. Ewald SE, Chavarria-Smith J, Boothroyd JC (2014)NLRP1 Is an Inflammasome
30
8. Heggie TW (2010) Swimming with death: Negleriafowleri infections in recreational waters. Travel Med Inf Dis. 8: 201–206.
9. Gonçalves VM, Matteucci KC, Buzzo CL, Miollo BH, Ferrante D, Torrecilhas AC, Rodrigues MM, Alvarez JM, Bortoluci KR (2013) NLRP3 Controls
Trypanosomacruzi Infection through a Caspase-1-Dependent IL-1R-Independent
NO Production. PLOS Neglected Tropical Diseases. 7(10):e2469.
10. Kersse K, Bertrand MJM, Lamkanfi M, Vandenabeele P (2011) NOD-like receptors and the innate immune system: Coping with danger, damage and death. Cytokine Growth F R. 22:257–276.
11. Kim JH, Song AR, Sohn HJ, Lee J, Yoo JK, Kwon D, Shin HJ (2013) IL-1beta and IL-6 activate inflammatory response of astrocytes against Naegleriafowleri infection via the modulation of MAPKs and AP-1. Parasite Immunol. 35(3-4): 120-18.
12. Kim JH, Kim DS, Shin HJ (2008) Contact-independent cell death of human microglial cells due to pathogenic Naegleriafowleritrophozoites. Korean J Parasitol. 46:217-221.
13. Kim JH, Yang AH, Sohn HJ, Kim D, Song KJ, Shin HJ (2009) Immunodominant antigens in Naegleriafowleri excretory–secretory proteins were potential pathogenic factors.Parasitol Res 105:1675-1681.
14. Lee YJ, Park JE, Kim JH, Sohn HJ, LEE J, Jung SY, Shin HJ (2012) Naegleria
fowleri lysate induces strong cytopathic effects and pro-inflammatory cytokie
31
15. Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva ALN, Mineo TWP, Gutierrez FRS, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zambon DS (2013) Inflammasome-derived IL-1β production induces nitric oxide– mediated resistance to Leishmania. Nat Med. 19(7):909-917
16. Marciano-Cabral F, Cabral GA (2007) The immune response to Nagleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Microbiol. 51(2): 243-259.
17. Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 10: 417–426.
18. McCoy AJ, Koizumi Y, Higa N, Suzuki T (2010) Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated b
19. y aerolysin and type III secretion system during Aeromonas veroniiinfection. J Immunol. 185: 7077–7084.
20. Mortimer L, Moreau F, Cornick S, Chadee K (2013). Gal-lectin-dependent contact activates the inflammasome by invasive Entamoebahistolytica. Mucosal Immunol.7:829-841
21. Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JWM, Joosten LAB (2010) IL-1beta processing in host defense: Beyond the inflammasomes.PLoSPathog. 6(2): e1000661
22. Oh YH, Jeong SY, Kim JH, Song KJ, Kim K, Park S, Sohn S, Shin HJ (2005) Cytopathic changes and pro-inflammatory cytokines induced by Naegleria
32
fowleri trophozoites in rat microglial cells and protective effects of an anti-Nfa1
antibody. Parasite Immunol. 27(12): 453-459.
23. Shaw MH, Reimer T, Kim YG, Nunez G (2008) NOD-like receptors (NLRs): bona fide intracellular microbial sensors. CurrOpinImmunol. 20: 377–382.
24. Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M (2009) Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoSPathog. 5(8): e1000559.
25. Siddiqui R, Khan NA (2014) Primary amoebic meningoencephalitis caused by
Naegleriafowleri: An old enemy presenting new challenges. PLoSNegl Trop Dis.
8: 1–8.
26. Sifuentes LY, Choate BL, Gerba CP, Bright KR (2014) The occurrence of
Naegleriafowleri in recreational waters in Arizona. J Environ Sci Health.
49:1322-1330.
27. Sohn HJ, Kim JH, Shin MH, Song KJ, Shin HJ (2010) The Nf-actin gene is an important factor for food-cup formation and cytotoxicity of pathogenic
Naegleriafowleri. Parasitol Res 106:917-924.
28. Travassos LH, Carneiro LAM, Ramjeet M, Hussey S, Kim Y-G, Magalhaes JG, Yuan L, Soares F, Chea E, Bourhis LL, Boneca IG, Allaoui A, Jones NJ,Nuñez G, Girardin SE, Philpott DJ (2010) Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 11:55–62.
33
29. Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic
free-living amoebae:Acanthamoebaspp.,Balamuthiamandrillaris,
34 -국문요약-
병원성 파울러자유아메바에 의해 유도되는
THP-1표적세포내
inflammasome 활성
아주대학교 대학원 의생명과학과 유종균 (지도교수: 신 호 준) 자연환경에서 널리 분포하고 있는 파울러자유아메바(Naegleriafowleri)는 치명적인 원발성아메바성수막뇌염을 일으킨다. 파울러자유아메바의 병인기전은 식 세포작용처럼 표적세포에 직접적으로 접촉하여 세포사멸을 일으키는 접촉성병인기 전(contact-dependent mechanism)과 표적세포와 접촉하지 않은 상태에서 아메 바의 분비물질에 의해 세포사멸이 야기되는 비접촉성병인기전(contact-independent mechanism)으로 나뉜다. 이전의 연구에서 파울러자유아메바와 혼합 배양된 표적세포들로부터 면역조절에 관여하는 다양한 사이토카인들(1β, IL-4, IL-6, IL-8, IL-18)이 분비하는 것을 확인한바 있어 inflammasome(염증조절 복합체)의 활성에 대한 구체적인 연구가 요구되었다. 선천성면역 특히, 염증반응에 서 중심적인 역할을 하는 inflammasome은 NLR, ASC 및 caspase-1의 결합으로 구성되며, 궁극적으로 caspase-1을 활성화시켜 pro-IL-1β를 IL-1β로 전환하 여 세포 밖으로 분비하는데 관여하는 복합단백질 결합체라 할 수 있다. 파울러자유 아메바의 감염 시 유도되는 염증반응은 아메바성수막뇌염의 중요한 면역병리현상 인데 그와 관련하여 유도되는 inflammasome에 대해서는 아직 보고된 바 없다. 따 라서 본 연구에서는 파울러자유아메바를 표적세포와 비접촉적으로 배양하였을 때,35
표적세포 내에서 유도되는 inflammasome 형성 관련 인자들의 활성에 대해서 알아 보고자 하였다. 파울러자유아메바영양형을 0.2um pore 사이즈를 갖는 transwell을 이용하여 표적세포인 THP-1세포(사람 대식세포의 한 분리주)와 비접촉배양상태 를 유지하여 배양하였다. 배양 후 3시간 후부터 표적세포내 및 배양액에 IL-1β가 생섬됨을 western blot과 ELISA를 통해 확인할 수 있었다. 또한, inflammasome 구성 단백질로 예상된 ASC, NLRP3 및 caspase-1의 발현이 배양 후 3시간 후부 터 활성화 되는 것을 western blot을 통해 확인할 수 있었다. 한편 NLRP3-inflammasome의 활성 억제를 관찰하기 위해 여섯가지의 특이 억제제들 즉, cytochalasin D (phagocytosis inhibitor), Z-VAD-FMK (caspase-1 inhibitor), KCl (K+ channel inhibitor), Glybenclamide (ATP-dependent K+ channel inhibitor), CA-074 (cathepsin B inhibitor), Bay 11-7082 (NF-κB inhibitor) 를 표적세포에 전처리하여파울러자유아메바와 함께 비접촉적으로 배양하였다. 억제 제에 처리된 표적세포로부터 IL-1β와 caspase-1의 발현이 처리하지 않은 대조 군에 비해서 크게 감소되는 것을 ELISA를 통해서 확인할 수 있었다. 따라서 본 실 험 결과, 파울러자유아메바에의한 감염시표적세포내에서 NLRP3-inflammasome 의 발현이 유도되며 뒤이어, caspase-1을 활성화 시켜 궁극적으로 IL-1β를 분 비하여 염증반응을 유도하는 것으로 생각된다. Keywords: 파울러자유아메바, 아메바성수막뇌염, 비접촉성 병인기전, 염증조절복합체, caspase-1 활성, IL-1β 생성