저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Makorin 1, Drosophila orthologue of human mkrn3
is required for oogenesis
by
Seongsu Jeong
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Makorin 1, Drosophila orthologue of human mkrn3
is required for oogenesis
by
Seongsu Jeong
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for
The Degree of Master of Biomedical Sciences
Supervised by
Eun Young Kim, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation of
Seongsu Jeong is approved.
SUPERVISORY COMMITTEE
Eun Young Kim
Byung Gon Kim
Myung Ae Lee
The Graduate School, Ajou University
December, 16th, 2016
-ABSTRACT-
Makorin 1, Drosophila orthologue of human mkrn3 is
required for oogenesis
Precocious puberty is puberty occurring at an unusually early age. According to the recent study, a family with precocious puberty is associated with a mutation in the makorin 3( mkrn3) gene.
To determine whether a change in mkrn3 causes similar effects on
Drosophila as it does in mammals and to test in vivo and find the
mechanism, we chose mkrn1 in drosophila because it is a orthologue of
mkrn3.
We made mkrn1 deletion mutant flies by imprecise excision of p -element. To determine the effect of this mutant line on growth and maturation. We checked various aspect of fly. mkrn1exS flies has longer pupation time and pupa length than control flies. Also, we found that
mkrn1exS is much more heavier and consumed food than male and control female fly.
Altogether, these results reveal that mkrn1exS affects drosophila growth and maturation.
When we crossed the mutant fly we were able to find that female homozygous mutant drosophila lose their fertility. In the case of ovaries from control flies, vitellogenesis occurs, resulting in the formation of
yolk and eggs. In the case of ovaries from mkrn1exS, however, vitellogenesis does not occur, resulting in infertility.
To find out why vitellogenesis does not occur, we checked the InR/TOR signaling pathway. We have confirmed that the mkrn1exS has high activity of InR/TOR signaling pathway. However, abnormal InR/TOR signaling pathway does not seem to be the reason why vitellogenesis does not occur because it is still working.
Also, we checked the Notch signaling pathway. As a result, it was found that mkrn1exS has an abnormal Notch signaling pathway. In addition,.we found that the number of follicle cells expressing cyclin B is higher in mkrn1exS. From these results, we thought that vitellogenesis does not occur because of the abnormality of Notch signaling pathway, which is essential for controlling the cell cycle of the follicle cell. This result is supported by the fact that the cell cycle regulator, Cyclin B, is abnormal. However, it is not yet clear whether this is the direct cause of infertility.
Taken together these results, mkrn1 is required for drosophila oogenesis. However, exact mechanism is unknown yet.
Keyword: Drosophila, oogenesis, makorin, precocious puberty, vitellogenesis, growth, maturation
TABLE OF CONTENTS
-ABSTRACT-………. i
TABLE OF CONTENTS………. iii
LIST OF FIGURES……… v
LIST OF TABLES………... vi
I. INTRODUCTION……….. 1
II. MATERIALS AND METHODS……….. 4
A.Excision fly strain……… 4
B.Ovary dissection………... 5
C.Genomic DNA extraction………. 5
D.RT-PCR……….. 6
E.TUNEL staining……… 6
F. Immunoblot………... 7
G.Immunohistochemistry………. 8
H.Pupation time measurement……… 9
I. Feeding assay………... 10
III. RESULT……….. 11
A.Deletion mutation of MKRN1 gene……… 11
B.mRNA and protein expression pattern in MKRN1 gene deletion mutant……… 15
C. mkrn1exS affects Drosophila growth and maturation……… 17
D. mkrn1exS lose their fertility……… 20
F. mkrn1exS has abnormal InR/TOR signaling pathway………. 27
G. mkrn1exS has abnormal Notch signaling pathway……… 31
H. A large number of follicle cells express Cyc B in mkrn1exS …..35
I. Abnormal expression of cytoskeletal protein in mkrn1exS ………37
DISCUSSION ………39
CONCLUSION ……….…….42
REFFERENCE ………..44
LIST OF FIGURES
Figure 1. Deletion mutant of MKRN1 gene ...14
Figure 2. mRNA and protein expression patterns in MKRN1 gene deletion mutant ...16
Figure 3. mkrn1exS affects Drosophila growth and maturation ...18
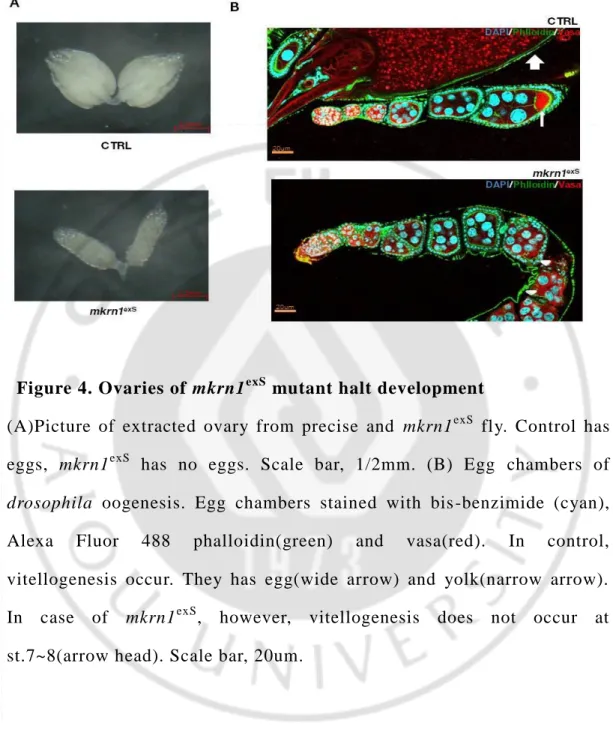
Figure 4. Ovaries of mkrn1exS mutant halt development ...24
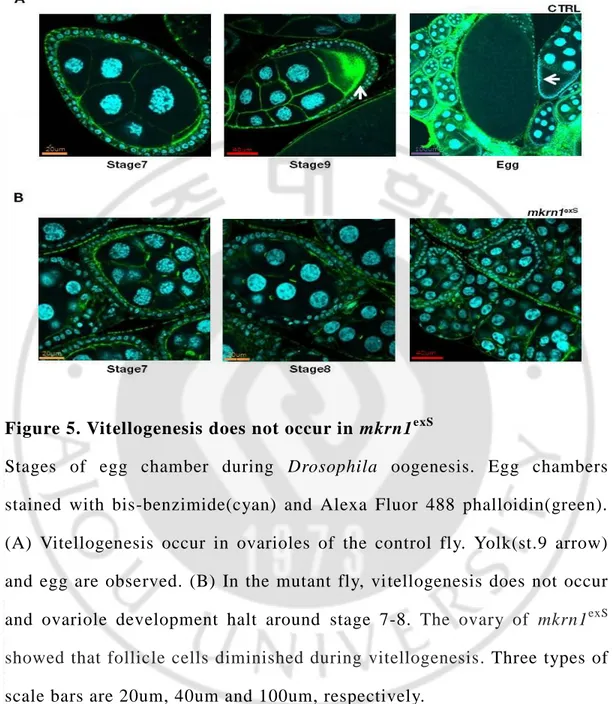
Figure 5. Vitellogenesis does not occur in mkrn1exS ...25
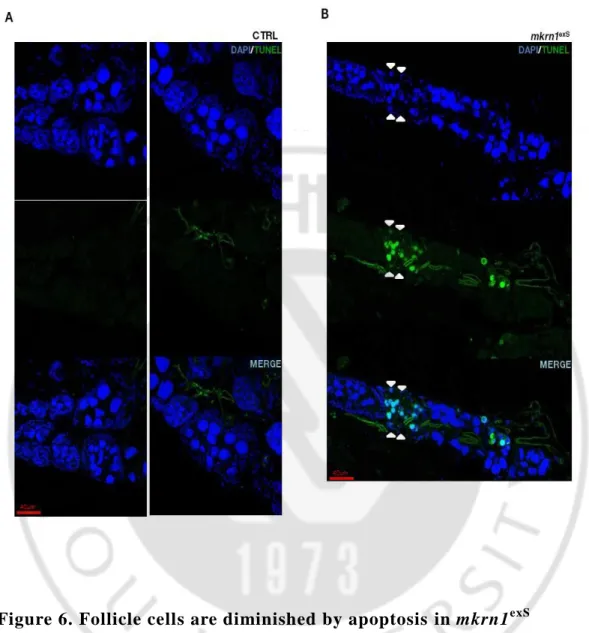
Figure 6. Follicle cells are diminished by apoptosis in mkrn1exS ...26
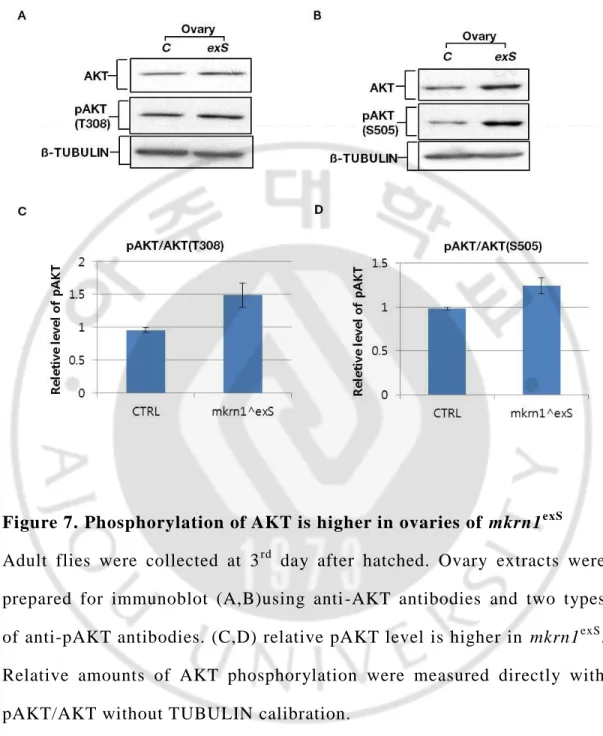
Figure 7. Phosphorylation of AKT is higher in ovaries of mkrn1exS ...29
Figure 8. mkrn1exS flies has low PTEN expression level ...30
Figure 9. mkrn1exS flies has abnormal CUT expression pattern ...33
Figure 10. Hindsight expression level is higher in mkrn1exS ...34
Figure 11. The number of follicle cells expressing CyclinB is high inmkrn1exS ...36
LIST OF TABLES
Table 1. Drosophila orthologues of human mkrn3……….13 Table 2. Sterility test …...……….21
I. INTRODUCTION
Growth and sexual maturation are tightly coordinated during the development. Precocious puberty is a disorder that causes precocious maturation and early cessation of growth. Recent studies have identified makorin RING-finger protein 3 (MKRN3) associated with precocious puberty. (Ana paula et al., 2013).
Insect life is similar to other animal s, and there are unique stages that represent embryonic development, growth, maturation, and breeding. In
Drosophila, this stage is very similar to the four developmental states
that are morphologically distinct, such as embryos, larvae, pupae, and adults. (Robertson, C.W., 1936).
Oogenesis is the production of eggs. Development of several stages of immature oocytes is necessary. Drosophila oogenesis is a famous model
for researching developmental and cell biology. (Valeria Cavaliere et al., 2005.). In drosophila melanogaster, there are about 16 ovarioles per ovary and there are two ovaries. Oogenesis of drosophila occurs in each ovariole. Ovarioles has 14 distinct stages in the development of oocyte, and egg chamber surrounded by follicle cells. Follicle cells supply nutrients to develop eggs for growth and maturation..
Vitellogenesis is the process of yolk formation via nutrients being deposited in the oocyte. It serves as a check point on the adequate nutrient and accurate developmental gene expression.
In Drosophila oogenesis, the most important point is vitellogenesis. Vitellogenesis is the process of yolk formation in the oocyte. In insects, it starts when insects has sufficient nutrition and stimulated by hormones such as juvenile hormone and ecdysone. Drosophila oogenesis is controlled by Drosophila insulin-like peptides(DILPs). That is mediate the response to nutrition. (D. Drummond-Barbosa, et al., 2001.) Insulin signaling is well-known to play an important role in regulation growth and metabolism. (Taha and Klip, 1999).
The insulin receptors are all required for normal growth. (Efstratiadis, 1998) In Drosophila, when the nutrition is sufficient, Embryonic stem cells and somatic stem cells have high cell division rates. However, when the insulin signaling is reduced, high division rates are also reduced, and vitellogenesis is blocked. (D. Drummond-Barbosa, et al., 2001.)
When the flies grow up in normal food, the egg chambers are formed, and then eggs of all egg stages are formed (st.1 -14) into ovarioles. The two main stages of egg chamber development can be distinguished by the follicle cell nuclear cycle (mitotic cycle and endo-cycle). During the mitotic phase (st.1-6), the follicle cells are immature and express the transcription factor cut. (Sun and Deng, 2005). Then, between st .6 and st.7, follicle cells switch cell cycle. Follicle cells exit the M phase and enter the E phase. (L-Schier and St. Johnston, 2001; Deng et al., 2001; Tamori et al., 2013). At this time, Hindsight expression is induced,
hindsight inhibits cut expression. (Patrick J et al., 2014). This
development is known as the M/E switch. This switch is controlled by the Notch signaling. (Shcherbata et al., 2004; Deng et al., 2001; L-Schier and St Johnston, 2001; Schaeffer et al., 2004).
In Drosophila melanogaster, there is a representative of each mitotic cyclin type and the type of cyclin required depends on the cell cycle. (Knoblich and Lehner, 1993; Jacobs et al., 1998). Drosophila cyclinB is expressed in mitotic cells. (Jacobs et al., 1998). When CycB accumulates, it undergoes mitosis.(Reber et al., 2006).
In this study, we found that mkrn1 affects the growth and maturation of
drosophila. In mkrn1exS, vitellogenesis does not occur, and it is thought that it is caused by abnormal InR/TOR pathway or/and abnormal Notch signaling pathway. InR/TOR pathway was more active than the control, but the signal did not reduce or disappear. Therefore, we thought that this is not the reason that vitellogenesis does not occur. However, Notch signaling was abnormal. It seems that the M/E switch does not work normally because the cyclin B accumulates a lot. We thought that notch signaling is one of the reasons why vitellogenesis does not occur. However, it is not yet clear whether this is the direct cause of vitellogenesis does not occur and we do not know what mechanism of
II. MATERIALS AND METHODS
A. Excision fly strain
We made mkrn1 excision fly strain using p-element. We used p-element insertion line, p{EPGY2}mkrn1EY14602, which is a transposon that usually does not jump around the gene, but when it meets transposage, it takes along a random part of the gene with it. So, we also use d transposage line, Dr/TM3,p{△2-3}, which is a transposable element insertion line.
We crossed the male fly with a p-element in the MKRN1 gene and a female fly with a transposage. This crossing resulted in an offspring that had the p-element with its transposage, which we then crossed with another female that had a balancer chromosome(MKRS/TM6B), eliminating the p-element as a result. To determine when excision of the genes was successful, we created primers and did PCR using genomic DNA as templates. The primer sequences were as follows:
forward, 5’-CCCGCCTTTTCCATAATCGTGTCA-3’ ; reverse, 5’-TTCATGCGCGGCTGGTCTATCG-3’ ; reverse, 5’-TCATTGCCGCGTCATTATTAGGAG-3’.
B. Ovary dissection
Flies incubated in food containing yeast for 24hours before dissect. Female mutant flies were collected and anaesthetized using CO2. Female
flies were submerged into 95% EtOH and transferred to 1X PBS. Ovaries were dissected by squeezing the body using pair of forceps.
C. Genomic DNA extraction
Collect 1 anesthetized flies in EP tube and freeze at –80oC. Grind fly in 100ul Buffer A(100mM Tris-HCL, ph7.5, 100mM EDTA, 100mM NaCl, 0.5% SDS). After then incubate at 65oC for 30min. After the incubation, add 200ul LiCl/KAc(Mix 1 part 5M KAc : 2.5 parts 6M LiCl) solution on ice for 10min. Spindown for 15minutes at RT. Transfer supernatant into a new tube, avoiding floating crud. Add 200ul Isopropanol and invert, spindown for 15min at RT. Aspirate away supernatant and wash with 70% EtOH 140ul and spindown for 15min at RT. Remove EtOH and dry for 10min. Finally, resuspend in 25ul of TE buffer.
D. RT-PCR
RNA was isolated from ovaries using TRI reagent (Molecular research Center, Inc.). 700ng of total RNA was reverse-transcribed with primer using reverse transcriptase (Gen DEPOT), and did PCR using cDNA as templates. The mkrn1 primer sequences were as follows:
forward, 5’-TGTGCTCATGTGGATTGGTGT-3’ ; reverse, 5’-ACGCGGATAGTAACTTGAGGGTAG-3’. and the GAPDH primer sequences were as follows: forward, 5’-ACCGACTTCTTCAGCGACAC-3’ ; reverse, 5’-GAGTTCGGTTACTCCAACCG-3’.
E. TUNEL staining
Female adult fly ovaries were dissected in PBS, and at least twenty to thirty ovaries were analyzed for TUNEL staining. Ovaries were fixed in one part Buffer B(100mM KH2PO4/K2HPO4(pH6.8), 450mM KCl,
150mM NaCl, 20mM MgCl26H2O), 1 part 36% formaldehyde, 4 parts
dH2O and an equal volume heptane. And rinsed with PBT(0.1% Triton
X-100). Fixed ovaries treated with proteinase K(Sigma Aldrich) for 15min. After then, wash with PBT and postfix ovaries in 2% PFA. After
the fixation, TUNEL staining is performed using TUNEL st aining kit(Millipore ApopTag, S7110).
After several washes with PBT, ovaries were incubated with 0.5% PBT containing bis-benzimide for 10 min, and ovaries were transferred onto slides and mounted with mounting medium(0.1M phosphate buffer containing 50% gl ycerol). Confocal images were obtained with an LSM510 Confocal Microscope(Zeiss) and processed with Zen software(Zeiss).
F. Immunoblot
For protein extracts from flies, flies were coll ected at 24hours after hatching. Heads, bodies and ovaries were isolated, and protein extracts were prepared using 0.1M HEMG buffer(10mM HEPES pH7.5, 50mM KCL, 10% Glycerol, 5mM Tris-HCl pH7.5) with the fresh addition of 5mM EDTA, 1mM DTT, 0.1% Triton X-100, Protease inhibitor (Sigma), 1mM Na3VO4, 0.25mM NaF. Protein extracts from each organs were
resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and target protein were identified by immunoblotting. Immunoblots were probed using primary antibodies at the following dilutions: (1)Anti -MKRN1 (Bethyl laboratory INC.), 1:1000; (2)anti -pAKT(T308, Cell Signaling
Tech.), 1:1000; (3)anti-pAKT(S473, Cell signaling Tech.), 1:1000; (4)anti-PTEN(DSHB), 1:1000 (5)anti-tubulin,beta(DSHB), 1:1000, (6)anti-Actin(Sigma Aldrich), 1:1000. Quantification of immunoblots was performed with NIH ImageJ software.
G. Immunohistochemistry
Female adult fly ovaries were dissected in PBS, and at least twenty to thirty ovaries were analyzed for each protein. Ovaries were fixed in 4% paraformaldehyde and rinsed with PBS containing 0.5% Triton X-100. Fixed ovaries were incubated for 30min to several hours in a blocking solution comprised of PBT solution (PBS containing 0.5% Triton X -100) containing 5% horse serum. Primary antibodies were directly added to the blocking solution and incubated overnight at 4℃. The following antibodies and final dilution were used: (1)anti -CUT(DSHB) 1:100, (2)anti-Hindsight(DSHB) 1:100, (3)anti-CyclinB(DSHB) 1:100, (4)anti-Vasa(Santa cruz) 1:100.
Subsequently, ovaries were washed with PBT and blocking contain ing secondary antibodies was added and incubated overnight at 4 ℃. The secondary antibodies used were Alexa555-conjugated anti-mouse IgG (Sigma), final dilution of 1:100. After several washes with PBT, ovaries
were incubated with 0.5% PBT containing bis -benzimide for 10 min, final dilution were used 1:1000. After then, if we required mem brane staining, ovaries were incubated with 0.5% PBT containing Alexa Flour 488 phalloidin(Life technology) for 30 min, final dilution of 1:200.
After several washes with PBT, ovaries were transferred onto slides a nd mounted with mounting medium(0.1M phosphate buffer containing 50% glycerol). Confocal images were obtained with an LSM710 Confocal Microscope(Zeiss) or LSM510 Confocal Microscope(Zeiss) and processed with Zen software(Zeiss).
H. Pupation time measurement
Transfer the larvae from the vial to a 5% sucrose solution. Collect the 2nd instar larva and transfer to normal food vial. 12hour later, collect the 3rd instar larva and transfer to normal food vial. Observe that it becomes a pupa. All cultures were conducted 25 ℃.
I. Feeding assay
Flies were collected after 1day from hatching. Collect homozygote flies and incubate normal food for 1day. After then transfer flies to blue -color food(5% sucrose/1% agar diets containing 1% FD&C Blue No.1) for 2 hours. After the feeding, the flies were frozen. Fly bodies wer e homogenized in PBS and centrifuges for 30min. The supernatants were transferred to a new tube and, following a 30min spin at 13,000rpm, were transferred to cuvettes. Absorbance was measured at 625nm. (Amita sehgal et al., 2008.)
III. RESULT
A. Deletion mutation of MKRN1 gene
The role of MKRN3 in pubertal initiation was first described in 2013. (Abreu et al. 2013). Recently, other investigators have also reported MKRN3 defects associated with precocious puberty. (Settas et al., 2014.) After we realized that the mutation of MKRN3 gene causes precocious puberty through previous study, we searched MKRN3 gene for research in drosophila to do further study on this topic, especially growth and maturation.
In Drosophila melanogaster, 4 genes including MKRN1 gene were identified as human MKRN3 gene orthologues. (table 1) We decided to do experiment with MKRN1 gene first among four genes. The MKRN1 gene is located on the left arm of the 3rd chromosome, and has many features of proteins like zinc finger, and is known for its ubiquitin protein ligase activity. Using drosophila, to understand the role of
human mkrn3 in the control of puberty timing, we generated a loss
-of-function Drosophila mutants for mkrn1 and named as mkrn1exS. To make
mkrn1 mutant fly, we used the p-element system to randomly excise mkrn1 in 3rd chromosome.
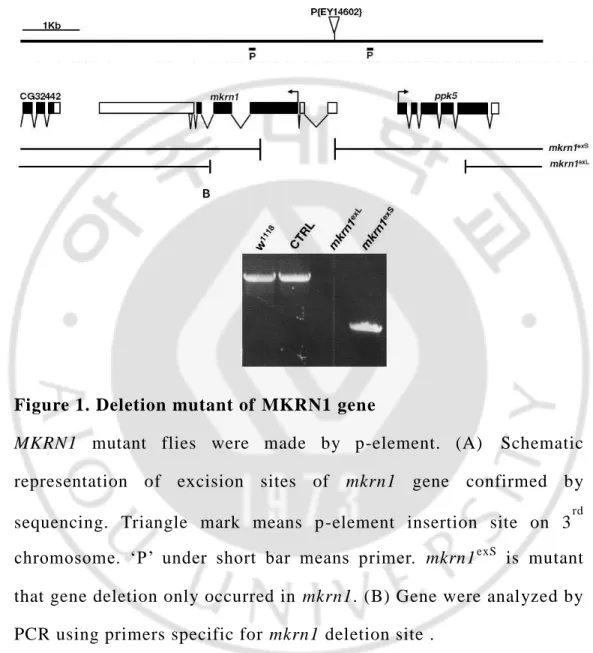
Through DNA sequencing, we were also able to determine that excision occurred in this specific site. (Figure. 1A) It had only excised MKRN1
gene. After then, we conducted to PCR to confirm the deletion of MKRN1 gene. On mutation of mkrn1, we observed that mkrn1exS PCR product size is smaller than control. (Figure. 1B)
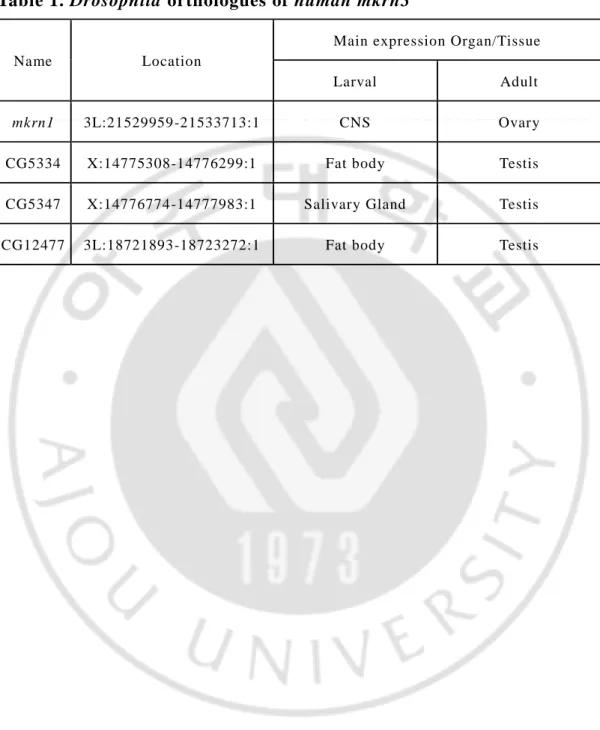
Table 1. Drosophila orthologues of human mkrn3
Name Location
Main expression Organ/Tissue
Larval Adult
mkrn1 3L:21529959-21533713:1 CNS Ovary
CG5334 X:14775308-14776299:1 Fat body Testis CG5347 X:14776774-14777983:1 Salivary Gland Testis CG12477 3L:18721893-18723272:1 Fat body Testis
Figure 1. Deletion mutant of MKRN1 gene
MKRN1 mutant flies were made by p-element. (A) Schematic
representation of excision sites of mkrn1 gene confirmed by sequencing. Triangle mark means p-element insertion site on 3rd chromosome. ‘P’ under short bar means primer. mkrn1exS
is mutant that gene deletion only occurred in mkrn1. (B) Gene were analyzed by PCR using primers specific for mkrn1 deletion site .
B. mRNA and protein expression pattern in MKRN1 gene
deletion mutant
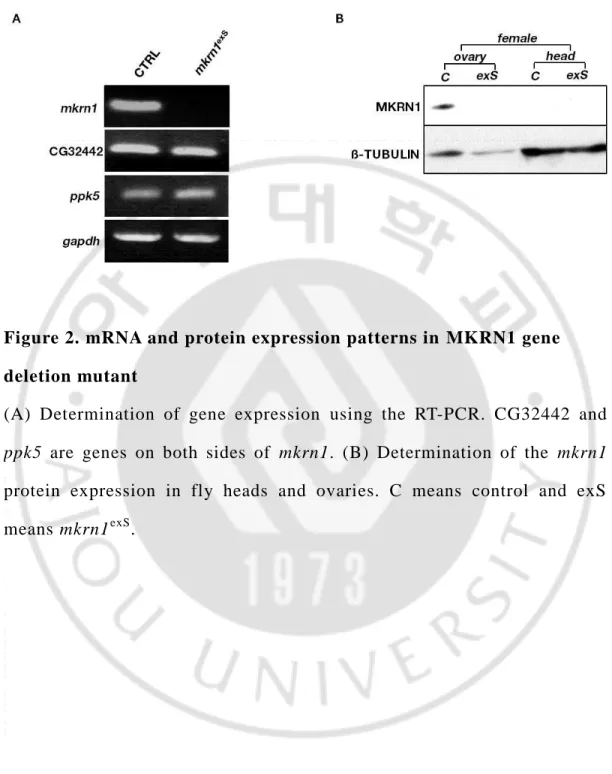
To analyze the expression of MKRN1, we conducted to RT-PCR and immunoblotting. When we performed the RT-PCR using cDNA as templates, we could found that MKRN1 mRNA expressed only in control female. In case of mutant fly, MKRN1 mRNA is not found. In addition, other genes on both side of MKRN1, ppk5 and CG32442, were found that they are normal. (Figure. 2A)
To confirm protein expression, we performed the immunoblotting using female ovary, we could find MKRN1 expressed only in control female ovary. In case of control head and mutant female head and ovary, MKRN1 is not found. Overall, these observations indicate that MKRN1 gene and protein are not expressed in mkrn1exS and they are particularly expressed in female ovary. (Figure. 2B)
Figure 2. mRNA and protein expression patterns in MKRN1 gene deletion mutant
(A) Determination of gene expression using the RT-PCR. CG32442 and
ppk5 are genes on both sides of mkrn1. (B) Determination of the mkrn1
protein expression in fly heads and ovaries. C means control and exS means mkrn1exS.
C.
mkrn1exS affects Drosophila growth and maturationTo determine whether changes in the MKRN1 gene affect growth and maturation, we looked for condition that demonstrated the differences between normal and abnormal growth and maturation in drosophila. We found that mass, length, and pupation time is important in determining whether growth and maturation of drosophila was normal or abnormal.
First, we checked the pupation time. Interestingly, mkrn1exS has longer pupation time than control. The mkrn1exS took about ~58hr, and the control took about ~50hr. (Figure. 3A)
Second, we measured the pupa length in the same condition. Pupa length of mkrn1exS is longer than control. Control pupa length is 3.56mm and mkrn1exS pupa length is 3.68mm on average. (Figure . 3B)
Finally, we checked fly weight and food intake. Generally female fly is heavier than male fly. Our data also show the same result. However, comparing female, mkrn1exS is heavier than control. Also, we found that
mkrn1exS much more consumed food than male and control female fly. (Figure. 3C,D)
Altogether, these results reveal that mkrn1exS affects drosophila growth and maturation.
Figure 3. mkrn1exS affects drosophila growth and maturation
(A)Pupation time is measured at 25oC. Control and mkrn1exS measured with 62 and 39 flies, respectively. From 3rd instar larva to pupa, control took 50.9hr and mkrn1exS took 57.6hr on average.(p<0.05). (B) Control and mkrn1exS measured with 76 and 74 flies, respectively. Control pupa length is 3.56mm and mkrn1exS pupa length is 3.68mm on average.(p<0.05). (C) From the left side of the graph, 50, 29, 28, 34 flies were used respectively. (D)After incubating in normal food for one day, it was transferred to blue dye-mixed food, incubated for two hours. Then grind flies and measure the absorbance. (Absorbance is measured at 625 nm.). From the left side of the graph, 100flies were used respectively.
D.
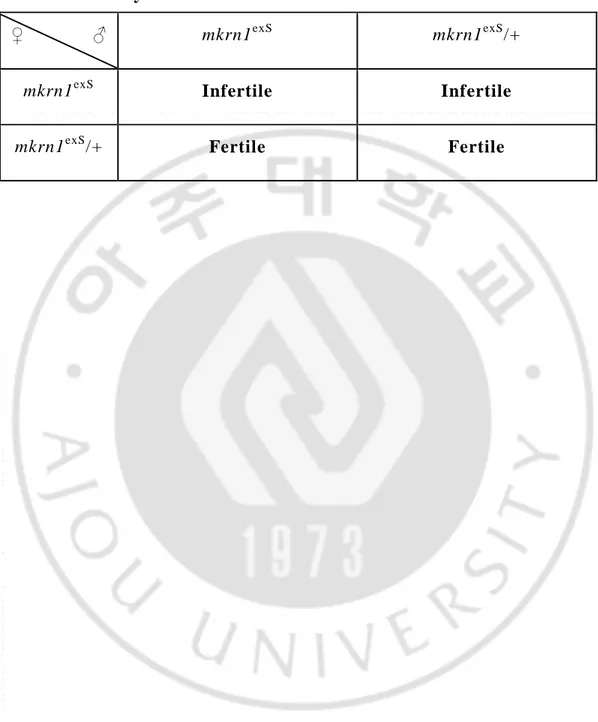
mkrn1exS lose their fertilityTo determine another effect of this mutant line on growth and maturation, we crossed homozygous male and female and found that they were sterile. So, we performed a sterility test. When we crossed homozygous male and female, we saw no eggs, even after three days, which was usually enough time to see presence eggs in normal circumstances. In the crossing of heterozygous male and homozygous female, we got the same results as when we crossed homozygous male and female. However, in the case of crossing homozygous male and heterozygous female, eggs were present, and larva was observed. (Table. 2)
Thus, we concluded that male homozygous drosophila’s fertility is not affected by the excision of mkrn1, but female homozygous drosophila lose their fertility.
Table 2. Sterility test
♀ ♂ mkrn1exS mkrn1exS/+
mkrn1exS Infertile Infertile
E. Ovaries of
mkrn1exSmutant halt development
To find out the reason for female drosophila lose their fertility, we conducted to dissect ovaries from female control and mkrn1exS.
Ovaries from mkrn1exS is much smaller than control. (Figure. 4A) So, we performed the staining in order to find the exact stage that ovaries were blocked development. We found the reason why mkrn1exS ovary is too small. Because they has no eggs. In control ovary, we could observe egg and yolk. However, mkrn1exS ovaries had no egg and yolk. (Figure. 4B) To check more accurately, we observed specific stages of egg chambers more magnified. The reason we had discovered is that vitellogenesis does not happen in mkrn1exS. In the case of ovaries from control flies, vitellogenesis occurs, resulting in the formation of yolk and eggs. (Figure. 5A) In the case of ovaries from mkrn1exS flies, however, vitellogenesis does not occur and he ovary of mkrn1exS showed that follicle cells diminished during vitellogenesis should occur. (Figure. 5B)
TUNEL staining is a method for detecting DNA fragmentation that results from apoptotic signaling cascades. We conducted a TUNEL staining, because we assumed that the follicle cells were broken by apoptosis. According to the results, we could be seen that TUNEL staining is not performed in case of control fly. That is, follicle cells were not diminished. (Figure. 6A) However, in the case of mkrn1exS, follicle cells were stained when they were broken, and we found that TUNEL
stain was occurred even in nurse cell. (Figure. 6B)
Thus, we concluded that cause of female mutant fly infertility is due to vitellogenesis does not occur, at that time follicle cells are diminished.
Figure 4. Ovaries of mkrn1exS mutant halt development
(A)Picture of extracted ovary from precise and mkrn1exS fly. Control has eggs, mkrn1exS has no eggs. Scale bar, 1/2mm. (B) Egg chambers of
drosophila oogenesis. Egg chambers stained with bis -benzimide (cyan),
Alexa Fluor 488 phalloidin(green) and vasa(red). In control, vitellogenesis occur. They has egg(wide arrow) and yolk(narrow arrow). In case of mkrn1exS, however, vitellogenesis does not occur at st.7~8(arrow head). Scale bar, 20um.
Figure 5. Vitellogenesis does not occur in mkrn1exS
Stages of egg chamber during Drosophila oogenesis. Egg chambers stained with bis-benzimide(cyan) and Alexa Fluor 488 phalloidin(green). (A) Vitellogenesis occur in ovarioles of the control fly. Yolk(st.9 arrow) and egg are observed. (B) In the mutant fly, vitellogenesis does not occur and ovariole development halt around stage 7-8. The ovary of mkrn1exS showed that follicle cells diminished during vitellogenesis. Three types of scale bars are 20um, 40um and 100um, respectively.
Figure 6. Follicle cells are diminished by apoptosis in mkrn1exS
TUNEL stain in egg chamber during drosophila oogenesis. Egg chambers stained with bis-benzimide(blue) and Anti-Digoxigenin(green). (A) In control fly, TUNEL-positive cells are not present. (B) In the mutant fly, TUNEL-positive cells are seen within the follicle cells and nurs e cells.(arrow head). TUNEL stain of nurse cell is not indicated separately. Scale bar, 40um.
F.
mkrn1exShas abnormal InR/TOR signaling pathway
There are many reasons why vitellogenesis is not occur. The first reason we guessed is insulin signaling pathway is abnormal. Because progression through vitellogenesis requires insulin signaling. (A. C. Spradling et al., 2001; C. Chen et al., 1996.) Compl ete loss of drosophila insulin receptor function results in complete block in vitellogenesis.
So, we checked the AKT phosphorylation using fly ovary lysate. In
drosophila, there are two main AKT phosphorylation site. One is
Threonine 308 the other is Serine 505 site. T308 site is downstream of PIP3 and PDK1 the other S505 site is downstream of TOR pathway. In result, we found that relative pAKT level is different between control fly ovary and mkrn1exS fly. In the case of mkrn1exS, two types of relative pAKT is higher than control. (Figure. 7)
To find out why the pAKT level is higher, we checked PTEN. This is because PTEN suppresses the change from PIP2 to PIP3 and restores PIP3
to PIP2.As a result, PTEN expression level is low in mkrn1exS. (Figure. 8)
In general, the loss-of-function mutants of foxo show a phenotype as opposed to mkrn1exS. So, we created a double mutant that contains
foxo△94 and mkrn1exS. The result is only a phenotype of mkrn1exS. (Data is not shown here.) This shows that foxo mutants could not rescue
mkrn1exS.
changes in PTEN. We thought that abnormal InR/TOR pathway is not the cause of infertility and mkrn1 enhance PTEN stability in drosophila ovary. Because the InR/TOR signal did not decrease or disappear.
Figure 7. Phosphorylation of AKT is higher in ovaries of mkrn1exS
Adult flies were collected at 3rd day after hatched. Ovary extracts were prepared for immunoblot (A,B)using anti -AKT antibodies and two types of anti-pAKT antibodies. (C,D) relative pAKT level is higher in mkrn1exS. Relative amounts of AKT phosphorylation were measured directly with pAKT/AKT without TUBULIN calibration.
Figure 8. mkrn1exS has low PTEN expression level
Adult flies were collected at 3rd day after hatched. Ovary extracts were prepared for immunoblot (A)using anti -PETN antibodies, anti-ACTIN and anti-ß -tubulin antibodies. (B) relative PTEN level is higher in control. Relative amounts of PTEN were measured directly without TUBULIN or ACTIN calibration.
G.
mkrn1exShas abnormal Notch signaling pathway
The second reason we guessed is Notch signaling pathway is abnormal. In drosophila, follicle cell has two main phases during oogenesis. One is mitotic phase the other is endocycle phase. (Sun and Deng, 2005). Follicle cells switch their cell cycle, this transition is M/E switch. This is under the control of Notch signaling pathway. (Schaeffer et al., 2004; Lopez S and S. Johnston, 2001; Shcherbata et al., 2004; Deng et al., 2001).
First, we checked the CUT protein. We performed CUT staining using anti-CUT antibody. Result shows that CUT of ovaries from control fly is disappeared at st.7 but CUT of ovaries from mkrn1exS fly is disappeared at st.6 clearly. (Figure. 9A,B) In detail, CUT expression level and pattern are different between control and mkrn1exS. In control fly, CUT still remain at st.6 but level is lower than st.5. However, mkrn1exS fly CUT is not expressed at st.6. (Figure. 9C)
Second, we checked the Hindsight protein. We performed Hn t staining using anti-Hnt antibody. Results show that stages being Hnt expressed are not different. (Figure. 10A,B) In both control and mkrn1exS, we could see that the expression begins at the stage where the CUT disappears. This result shows that the Hnt expression inhibit CUT expression.
However, there was no difference in expression pattern, but there was a difference in expression level. We could find that mkrn1exS Hnt is more
expressed. (Figure. 10C) We thought that the difference in Hnt expression level between control and mkrn1exS is the cause of the difference in expression pattern of CUT.
Thus, we concluded that mkrn1exS has abnormal Notch Signaling Pathway. Therefore, we thought that the M / E switch will not operate normally.
Figure 9. mkrn1exS flies has abnormal CUT expression pattern
Egg chambers stained with bis-benzimide(cyan) and anti-CUT(red). In normal flies, CUT expressed up to st.6. (A) In case of control, the CUT is normally expressed. (B)However, mkrn1exS flies were not normally expressed. It is not observed in st.6, which should be expressed. (C) It is a graph to quantify the relative intensity. In case of control, the expression level in st.6 decreased but is still expressed. In mkrn1exS, CUT is not expressed in st.6. (A,B) Scale bar, 20um.
Figure 10. Hindsight expression level is higher in mkrn1exS
Egg chambers stained with bis-benzimide(cyan) and anti-Hindsight(red). In normal flies, Hindsight begins to appear from st.6. (A,B)Hindsight expression pattern is not different between CTRL and mkrn1exS. (C) It is a graph to quantify the relative intensity. In the mkrn1exS egg chambers, Hindsight expression level is higher than CTRL egg chambers. (A, B) Scale bar, 20um.
H. A large number of follicle cells express Cyc B in mkrn1
exSThrough previous experimental results, we thought that the cell cycle of the follicle cell would not be normal. So , we checked Cyclin B.
We performed Cyclin B staining using anti-Cyclin B antibody. From the results, we could see that the follicle cell is expressed Cyc B up to the st.5 before follicle cells goes to endocycle. However, we found that the number of Cyc B expression follicle cells is significantly different. It could be seen that a large number of cells express Cyc B in mkrn1exS than control. (Figure. 10A,B) So, we counted the number of follicle cells in the egg chamber and counted the number o f follicle cells expressing Cyc B. As a result, we could confirm that mkrn1exS follicle cell express Cyc B more than control follicle cell in the stage before endocycle, although there is no difference in st.6 in which endocycle begins. (Figure. 10C)
Thus, we concluded that mkrn1exS has an abnormal cell cycle regulator, cyclin B, which may cause M/E switch not to operate normally. However, it is not yet clear whether this is the direct cause of vitellogenesis does not occur.
Figure 11. The number of follicle cells expressing CyclinB is high in mkrn1exS
Egg chambers stained with bis-benzimide(cyan) and anti-Cyclin B(red). (A,B)CyclinB expression pattern is not different between CTRL and
mkrn1exS. (C) It is a graph that counts the number of follicle cells. (A, B) Scale bar, 20um.
I. Abnormal expression of cytoskeletal protein in
mkrn1exSWhile we were doing various experiments, we found interesting phenomena.
First, we found abnormalities in ACTIN expression in ovary lysate. In the case of mkrn1exS, the amount and size of ACTIN were larger than control. (Figure. 12A)
Second, we found that the expression of TUBULIN in the ovary lysate is abnormal. In the case of mkrn1exS, the amount of TUBULIN was slightly smaller than control. (Figure. 12B)
Thus, we concluded that the expression of cytoskeletal protein in
Figure 12. Abnormal Expression of cytoskeletal proteins in mkrn1exS
Adult flies were collected at 3rd day after hatched. Ovary extracts were prepared for immunoblot using anti-ACTIN antibodies and anti-ß-TUBULIN antibodies. (A) Determination of actin protein expression in fly ovaries. (B) Determination of the ß-tubulin protein expression in fly ovaries. C means control and exS means mkrn1exS.
DISCUSSION
Recent studies have shown that mutations in the makorin3(mkrn3) gene cause precocious puberty. Precocious puberty occurs when the maturation is abnormally early age.
In order to investigate the effect of mkrn3 on mammals and to conduct in vivo experiments, the experiment was carried out using mkrn1, a orthologous of human mkrn3.
The mkrn1 gene was non-specifically removed using a P-element to create a mutation. (Figure. 1) In order to determine whether the mutation affects growth and maturation as well as humans, we examined the various states of drosophila to see what changes in their growth and maturation. In case of mkrn1exS, pupation time took longer than the control and pupa length was longer. The mkrn1exS females were heavier and consumed much food than the other flies . (Figure. 3) After then, we found that the mkrn1 mutant female lost reproductive ability. (Table . 2)
From these results, we found that mkrn1 mutation affects the growth and maturation of female drosophila and causes loss of reproductive capacity.
The loss of reproductive capacity is due to the cause that vitellogenesis does not occur in mkrn1exS. (Figure. 5) When vitellogenesis does not occur, the follicle cell breaks down. (Figure. 6)
The InR/TOR signaling pathway was checked through phosphorylated AKT and PTEN. As a result, mkrn1exS has abnormal InR/TOR signaling pathways. InR/TOR signaling pathway is raised by changes in PTEN (Figure 7,8). In addition, when we created a double mutant that contained foxo△94 and mkrn1exS, the foxo mutant could not rescue
mkrn1exS. This confirms that the downstream of the pAKT is not the cause of infertility.
Therefore, we thought that abnormal InR/TOR pathway is not the cause of vitellogenesis does not occur. This is because vitellogenesis does not occur when the InR/TOR pathway is reduced. (D. Drummond-Barbosa, et al., 2001.)
In Drosophila melanogaster, when the Notch signaling pathway is activated, it inhibits the expression of CUT protein by Hindsight. (Patrick J et al., 2014). In the case of control flies, the CUT protein is expressed in the st.6 of egg chamber, but mkrn1exS is not expressed in st.6. (Figure. 9)
In the case of Hindsight, there is no difference in expression pattern but there is a difference in expression level. More amounts of Hnt are expressed in mkrn1exS. (Figure. 10) At this time, ovary follicle cells are transformed from the mitotic phase to the endocycle phase. (Patrick J et al., 2014).
Since the cell cycle is changed by the operation of the M/E switch, Cyclin B, a cell cycle regulator, was confirmed. The result shows that
Cyclin B is expressed until st.5, which is the pre -stage of M/E switch operation. However, it can be seen that an abnormally large number of follicle cell is expressed Cyc B in mkrn1exS. (Figure. 11). Thus, we concluded that mutant drosophila does not have a normal cell cycle regulator, which may cause the M/E switch not to occur normally.
However, it is not yet clear whether this is the direct cause of vitellogenesis does not occur.
We could find an interesting phenomenon during the experiment. It is an abnormal expression of the cytoskeletal protein. First, the ACTIN size was larger in mkrn1exS and the expression amount was larger. In the case of TUBULIN, the mutant expresses a relatively small amount. (Figure 11). Through this result, we thought that this is not a direct cause of vitellogenesis does not occur. However, we thought that the reason for the abnormality of ACTIN, which is related to follicle cell migration (Tânia Ferreira et al., 2014), and tubulin, which is needed to form polar cells (Alexandre D et al., 2012), is that vitellogenesis did not occur.
Altogether, these results reveal that mkrn1exS affects Drosophila growth. And mkrn1 is required for drosophila oogenesis. However, exact
CONCLUSION
We have successfully generated mutants of mkrn1 by P-element excision mutagenesis. MKRN1 protein strongly expressed in ovary. (Figure. 1,2)
To determine the effect of this mutant line on growth and maturation, we crossed male and female of homozygous and heterozygous mutant each other. We were able to conclude that male homozygous drosophila's fertility is not affected by the excision of mkrn1, but female homozygous
mkrn1 mutants lose their fertility. (Table. 2)
To determine whether a change in mkrn1 mutation causes similar effects on drosophila as it does in mammals. We checked pupation time, pupa length and weight. mkrn1exS has longer pupation time than control, and has longer pupa length than control. mkrn1exS female fly is heavier than other flies. Also, we found that mkrn1exS much more consumed food than other flies. (Figure. 3)
Vitellogenesis does not occur in mkrn1exS flies, resulting in the defects in the development of egg chambers. Ovaries from mkrn1exS flies demonstrated diminished follicle cells around the time when vitellogenesis normally occur. (Figuer. 4,5)
To test if insulin signaling is affected in mkrn1 mutants, we have examined relative level of pAKT. In mkrn1exS, AKT phosphorylation higher than control. This result is because PTEN decreased in mkrn1exS.
CUT and Hindsight are related to Notch signaling. We observed that patterns of CUT expression is different between control and mkrn1exS. In case of control, the expression level in st.6 decreased but is still expressed. In mkrn1exS, CUT is not expressed in st.6. (Figure. 9)
There is no difference in Hnt expression pattern, but expression level is different. In the case of mkrn1exS, expression level is higher than control. (Figure. 10)
We checked the Cyclin B, a cell cycle regulator. There is no difference in Cyclin B expression pattern. However, in mkrn1exS, more follicle cells expressed Cyclin B than control. (Figure. 11)
In addition, we found that the expression of the cytoskeletal protein was abnormal. In the case of mkrn1exS, the amount of ACTIN and size are larger than control. Also, the expression amount of TUBULIN is slightly smaller than control. (Figure. 12)
Altogether, these results reveal that mkrn1exS affects Drosophila growth. And mkrn1 is required for drosophila oogenesis. However, exact
REFFERENCE
[1] Alexandre D. Baffet, Béatrice Benoit, Jens Januschke, Jennifer Audo, Vanessa Gourhand, Siegfried Roth, and Antoine Guichet . “Drosophila tubulin-binding cofactor B is required for microtubule network formation and for cell polarity.” MboC Article. Vo.23, 2012.
[2] Ana Paula Abera, Anderew Dauber, Delanie B. Macedo, Sekoni D. Noel, Vinicius N. Brito, John C. Gill, Priscilla Cukier, Iain R. Thompson, Victor M. Navarro, Priscila C. Gagliardi, Tania Rodrigues, Cristiane Kochi, Carlos Alberto longui, Dominique Beckers, Francis de Zegher, Luciana R. Montenegro, Berenice B. Mendonca. “Central precocious Puberty Caused by Mutations in the Imprinted Gene MKRN3.” N Eng J. 368(26):2467-75, 2013.
[3] Andersen DS, Colombani J, Léopold P. “Coordination of organ growth: principles and outstanding questio ns from the world of insects.” Trands Cell Biol. 23(7):336-44, 2013.
[4] Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. “An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control.” Curr Biol. 11(4):213-21, 2001.
[5] Chen C, Jack J, Garofalo RS. “The Drosophila insulin receptor is required for normal growth.” Endocrinology. 137(3):846-56, 1996.
Léopold P. “A nutrient sensor mechanism controls Drosophila growth.” Cell. 114(6);739-49, 2003.
[7] Daisaku Hiraoka, Sawako Hori-Oshima, Takeshi Fukuhara, Kazunori Tachibana, Eiichi Okumura, Takeo Kishimoto. “PDK1 is required for the hormonal signaling pathway leading to meiotic resumption in starfish oocytes.” Developmental Biology. 276: 330–336, 2004.
[8] Daniela Drummond-Barbosa, Allan C. Spradling. “Stem Cells and Their Progeny Respond to Nutritional Changes during Drosophila Oogenesis.” Dev B. 231:265-278, 2001.
[9] Deng WM, Althauser C, Ruohola-Baker H. “Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells.” Development. 128(23)4737-46, 2001.
[10] Edgar BA. “How flies get their size: genetics meets physiology.” Nat Rev Genet, 7(12):907-16. 2006.
[11] Efstratiadis A. “Genetics of mouse growth.” Clin Orthod Res. 1(2):102-6, 1998.
[12] Géminard C, Rulifson EJ, Léopold P. “Remote control of insulin secretion by fat cells in Drosophila.” Cell Metab. 10(3):199 -207, 2009. [13] Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. “Nutrient -dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila.” Curr Biol. 12(15):1293-300, 2002.
B3 is required for female fertility and is dispen sable for mitosis like Cyclin B.” Genes Dev. 12: 3741-3751, 1998.
[15] Kanyan Xu, Xiangzhong Zheng, Amita sehgal. “Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila.” Cell Metab. 8(4):289-300, 2008.
[16] Knoblich, J. A., and C. F. Lehner. “Synergistic action of Drosophila cyclins A and B during the G2 -M transition.” EMBO J. 12: 65–74, 1993. [17] Patrick Jouandin, Christian Ghiglione, Stéphane Noselli. “Starvation induces FoxO-dependent mitotic-to-endocycle switch
pausing during Drosophila oogenesis” The company of Biologists. 141:3013-3021, 2014.
[18] “Precocious Puberty". KidsHealth. Retrieved 2013.
[19] Puig O, Marr MT, Ruhf ML, Tjian R. “Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway.” Genes Dev. 17(16)2006-20, 2003.
[20] Rameh LE, Cantley LC. “The role of phosphoinositide 3 -kinase lipid products in cell function.” J Biol Chem. 274(13):8347-50, 1999. [21] Reber, A., C. F. Lehner, and H. W. Jacobs. “Terminal mitoses require negative regulation of Fzr/Cdh1 by Cyclin A, preventing premature
degradation of mitotic cyclins and String/Cdc25. ” Development. 133: 3201–3211, 2006.
[22] Robertson, C.W. “The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological
changes.” J. Morphol. 59, 351–399. 1936.
[23] Schaeffer D. “Gerontopsychiatry and nursing science. Similarities and differences.” Z gerontol Geriatr. 37(4):307 -15 German, 2004.
[24] Shcherbata HR, Althauser C, Findley SD, Ruohola -Baker H. “The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell -cycle transitions.” Development. 131(13):3169-81, 2004.
[25] Sun J, Deng WM. “ Notch-dependent downregulation of the homeodomain gene cut is required for the mitoticcycle/endocycle switch and cell differentiation in Drosophila follicle cells.” Development. 132(19)4299-308, 2005.
[26] Taha C, Klip A., “The insulin signaling pathway.” J Member Biol. 1;169(1):1-12, 1999.
[27] Tamori Y, Deng WM. “Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia.” Dev Cell. 28;25(4):350-63, 2013.
[28] Tânia Ferreira, PedroPrudêncio, RuiGonçaloMartinho. “Drosophila protein kinase N(Pkn)is a negative regulator of actin–myosin activity during oogenesis.” Dev Biol. 394:277-291, 2014.
[29] Valeria Cavaliere, Alessandra Donati, Anita Hsouna, Tien Hsu, Giuseppe Gargiulo “dAKT Kinase Controls Follicle Cell size During Drosophila Oogenesis.” NIH public Access, 232(3): 845-854, 2005. [30] Wang X, Proud CG. “Nutrient control of TORC1, a cell -cycle
regulator.” Trands Cell Biol. 19(6):260-7, 2009.
[31] Wang XQ, Lo CM, Chen L, Ngan ES, Xu A, Poon RY. “CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency.” Cell Death Differ. 1–11, 2016.
[32] Wigby S, Slack C, Grönke S, Martinez P, Calboli FC, Chapman T, Partridge L. “Insulin signalling regulates remating in female Drosophila.” Proc Biol Sci. 278(1704):424-31, 2011.
-국문요 약 -
초파리의 난형성에 필수적인 인간 mkrn3의 이종
상동유전자인 mkrn1
아주대학교 대학원 의생명과학과 신경과학전공 정 성 수 (지 도 교수 : 김 은 영 ) 최근의 연구에 따르면 makorin 3 (mkrn3) 유전자의 돌연변이는 성조숙증을 일으킨다고 한다. 성조숙증은 충분한 성장이 일어나기 전 성숙이 비정상적으로 이른 시기에 발생하는 것이다. 우리는 mkrn3가 포유류에서 미치는 영향에 대한 기작을 찾고 생 체 내 실험을 진행하기 위하여 mkrn3의 이종상동유전자인 초파리 mkrn1을 이용하여 실험을 진행 하였다. P-element를 이용하여 mkrn1 유전자를 비특이적으로 제거하여 돌연변이를 만들었다. 이 돌연변이가 성장과 성숙에 어떤 변화가 있는지 확인해보기 위하여 초파리의 다양한 상태를 확인해 보았다. mkrn1 돌연변이 초파리의 경우 번데기가 되는 기간이 정상 초파리 보다 오래 걸렸고 번데기의 길이도 더 길었다. 그리고 mkrn1 돌연 변이 암컷은 다른 파리들에 비해 더 무겁고 식이섭취량도 많은 것 을 알 수 있었다계속해서 실험을 진행하기 위하여 동형접합 돌연변이 유전자를 가 진 암수 파리를 서로 교배하였다. 그 결과 자손이 나타나지 않는 현상을 발견하였다. 그 후 생식 능력을 확인하여 mkrn1 돌연변이 암컷은 생식능력을 잃게 되는 것을 확인하였다. 생식능력을 잃어버리는 원인은 동형접합 돌연변이 초파리 암컷의 경우 난황 형성 과정이 일어나지 않기 때문이다. 난황 형성 과정은 발생단계에서 중요한 역할을 하는데, 영양분과 발달 유전자를 확인 하는 시점이 된다. 만약 InR/TOR 신호전달 경로와 발달 유전자가 정상적이지 않다면 초파리에서 난황 형성이 일어나지 않는다. 인산 화된 AKT를 이용하여 InR/TOR 신호전달 경로를 확인한 결과 mkrn1 돌연변이 초파리의 InR/TOR 신호전달 경로는 대조군에 비 해 활성화 되어 있었다. 이는 PTEN 단백질이 mkrn1 돌연변이에서 대조군보다 부족하기 때문이다. 하지만 InR/TOR 신호전달 경로의 활성이 감소되거나 사라지지 않기 때문에 난황형성이 일어나지 않 는 원인은 아니라고 생각된다. 초파리에서는 Notch 신호전달 경로가 활성화되면 Hindsight(Hnt) 가 발현되어CUT 단백질 발현을 억제한다. 정상초파리의 경우 난형 성 과정 중 6단계에서 여전히 CUT 단백질이 발현되고 있지만 mkrn1 돌연변이 초파리의 경우 5단계 까지만 발현되고 6단계에서 는 발현되지 않는다. Hnt의 경우 mkrn1 돌연변이 초파리에서 더 강하게 발현하는 것을 확인 할 수 있다. 이는 mkrn1 돌연변이 초 파리의 경우 Notch 신호전달 경로가 정상적이지 않다는 것을 의미 한다.
Notch 신호전달 경로는 초파리의 follicle 세포의 세포주기에 변화 를 주는 역할을 한다. 비정상적인 Notch 신호전달 경로를 가지고 있는 돌연변이 초파리에서 세포주기 조절인자가 어떻게 변화하는지 확인하기 위하여 CyclinB(CycB)를 확인해 보았다. CycB는 mkrn1 돌 연변이 초파리에서 더 많이 발현하는 것을 확인 할 수 있다. 이는 mkrn1 돌연변이 초파리의 경우 세포주기 조절인자가 비정상적으로 발현되고 있는 것을 의미한다. 이는 mkrn1 돌연변이 초파리에서 난 황형성이 일어나지 않는 원인이 될 수 있다고 생각한다. 하지만 여 전히 작용 기작을 모르기 때문에 직접적으로 작용하는지는 아직 알 수 없다. 또한 mkrn1 돌연변이 초파리에서 세포뼈대 단백질의 발현이 정상 적이지 않은 것을 확인 하였다. 이 현상은 난황형성 과정이 일어나 지 않는 원인이 아니라 난황형성 과정이 일어나지 않기 때문에 발 생하는 현상이라 생각한다. 결과를 종합하면 mkrn1 돌연변이 초파리의 경우 성장과 성숙과정 에 변화가 발생한다. 그리고 mkrn1은 초파리의 난형성에 필수적이 다. 하지만 아직까지 정확한 기작은 알 수 없다. 핵심어: 초파리, 난형성, makorin, 성조숙증, 난황형성, 성장, 성숙