저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Control of Proliferation in Human
Mesenchymal Stem Cells by Secreted
Frizzled Related Protein 1
by
Na Ra Lee
Major of Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Control of Proliferation in Human Mesenchymal
Stem Cells by Secreted Frizzled Related Protein 1
by
Na Ra Lee
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of
The Requirements for The Degree
of Master of Neuroscience
Supervised by
Haeyoung Suh-Kim, Ph.D.
Sung-Soo Kim, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
I
- ABSTRACT -
Control of Proliferation in Human Mesenchymal Stem Cells
by Secreted Frizzled Related Protein 1
Human mesenchymal stem cell (hMSC) is adult stem cell that is useful tool for a various disease. However, it has limitations that lost proliferation and differentiation ability in long-term in vitro culture and occurred senescence. This study would like to confirm the relationship between Wnt signaling and proliferation of hMSCs. When checked mRNA expression of various genes that were related to Wnt signaling, most of genes were decreased gradually as follow passages, except Wnt11. Especially, gene expression of sFRP1, an antagonist of Wnt signaling, was also decreased. To confirm whether sFRP1 affects proliferation of hMSC, MSC/sFRP1 and MSC/shsFRP1 were made and then cell proliferation was measured. Growth kinetics result showed that sFRP1 could affect hMSCs’ proliferation and this could be dependent on concentration.
Meanwhile, Wnt signaling can be divided into canonical and non-canonical pathway depending on -catenin involvement in the pathway. ROR is down-stream gene of non-canonical Wnt pathway and it could be translocated into nucleus to interact with -catenin, resulting in suppression of cell proliferation. This study confirmed that ROR expression and nuclear translocation were increased in late passage of hMSCs. Also, MSC/shROR showed promoted growth kinetics, indicating that ROR could be a negative regulator of hMSCs’ proliferation. To figure out whether sFRP1 inhibits nuclear translocation of ROR to suppress non-canonical Wnt signaling, 10ng/ml sFRP1 was treated to hMSCs and localization of ROR was observed. ICC data showed that nuclear translocation of ROR was inhibited in rhsFRP1 treated hMSCs, suggesting that sFRP1 promotes hMSCs’ proliferation by suppressing non-canonical Wnt signaling
II
TABLE OF CONTENTS
ABSTRACT ... I TABLE OF CONTENTS ... II LIST OF FIGURES ... III LIST OF TABLES ... IV LIST OF ABBREVIATION ... VIII
INTRODUCTION ... 1
MATERIALS AND METHODS ... 6
Culture of human MSCs in vitro ... 6
Differentiation of human MSCs in vitro ... 6
SA--gal staining ... 7
Semiquantitative - (RT-PCR) and quantitative-reverse transcriptase polymerase chain reaction (qRT-PCR) ... 7
Production of virus and Transduction ... 8
Growth kinetics of hMSCs ... 8
Treatment of recombinant human sFRP1 protein ... 9
Immunocytochemistry ... 9
Western blot analysis ... 9
RESULTS ... 11
DISCUSSION ... 24
REFERENCES ... 27
III
LIST OF FIGURES
Fig. 1. Cell surface marker of hMSCs at a various passages ... 15
Fig. 2. Property alteration of hMSCs in long term culture in vitro ... 16
Fig. 3. Canonical Wnt signaling is activated in the early passage of hMSC ... 17
Fig. 4. sFRP1 could affect proliferation of hMSCs ... 19
Fig. 5. Growth of hMSCs is dependent on sFRP1 concentration ... 20
Fig. 6. Down regulation of ROR accelerates proliferation of hMSCs ... 22
IV
LIST OF TABLES
Table 1. PCR Primer Sequence ... V Table 2. Information of Viral vector ... VI Table 3. Antibody List and Used Condition ... VII
V Table 1. PCR Primer Sequence
Gene Sequence Size(bp)
Wnt1 F ACCCAATCCCTCTCCACTCT 214 R GATTCAAGGAAAAGCCACCA Wnt2 F ACTCTCAGGACATGCTGGCT 161 R ACGAGGTCATTTTTCGTTGG Wnt3a F GGACAAAGCTACCAGGGAGT 176 R ACTCGATGTCCTCGCTACAG Wnt4 F CATGCAACAAGACGTCCAAG 121 R AAGCAGCACCAGTGGAATTT Wnt5a F GGGAGGTTGGCTTGAACATA 141 R GAATGGCACGCAATTACCTT Wnt6 F GTCACGCAGGCCTGTTCTAT 141 R CGTCCATAAAGAGCCTCGAC Wnt10a F GGTTGCTCCACACCCTAAAA 208 R ATGATGAAGGGAATGGTGGA Wnt11 F CCCAAGCCAATAAACTGATG 178 R AGGTATCGGGTCTTGAGGTC Fzd2 F GTCCTCAAGGTGCCATCCTA 248 R CAGCCCGACAGAAAAATGAT Fzd6 F ATGAGAGAGGTGAAAGCGGA 143 R TCAGATACACTGCCTGCCTG sFRP1 F GCTCGGCCAGCGAGTACGAC 453 R AGGCTTCGGTGGCATTGGGC DKK1 F CATCAGACTGTGCCTCAGGA 145 R CCACAGTAACAACGCTGGAA
VI Table 2. Information of Viral vector
Type of Virus Viral vector Vector information
Lenti
Control Sigma, SHC002
shsFRP1 Sigma, SHCLNG
shROR Origene, TL320515
Retro
Scrambled shRNA Origene, TR30021
nLacZ pMSCV-puro
VII Table 3. Antibody List and Used Condition
Antibody Host Dilution
W.B (ICC) Company
-catenin Mouse 1:1000 (1:500) BD
Total GSK-3 Mouse 1:5000 BD
Active GSK-3 Mouse 1:1000 BD
Total ROR Rabbit 1:2500 (1:200) Santacruz
-actin Mouse 1:1000 Millipore
VIII
LIST OF ABBREVIATION
hMSCs Human Mesenchymal Stem Cells
Wnt Wingless-related MMTV integration site
Fzd Frizzled receptor
TCF T Cell-specific Factor
LEF1 Lymphoid Enhancer-binding Factor 1
sFRP1 Secreted Frizzled-Related Protein 1
1
INTRODUCTION
Human Mesenchymal Stem cells (hMSCs)
Recently, mesenchymal stem cells have been on the rise to use for therapy of various diseases. Discovery of hMSCs was initiated from isolation of fibroblast-like osteogenic cells in bone-marrow in 1968 (Friedenstein et al., 1968). According to International Society for Cellular Therapy (ISCT), characteristics of MSCs are as follows. ; First, MSCs must be adherent in standard culture condition. Second, they must have highly expressed surface makers CD44, CD73, CD90, CD105 and have lack expression of CD11b/CD14, CD19/CD79, CD45, CD34, HLA-DR. Third, MSCs can differentiate into osteocytes, chondrocytes, adipocytes (Haynesworth et al., 1992; Yoo et al., 1998; Dennis et al., 1999; Dominici et al., 2006). Also, hMSCs could be isolated from several tissues including adipose tissue, lung, liver, bone-marrow, umbilical cord, and circulate system (Dennis et al., 1999; Erices et al., 2000; Campagnoli et al., 2001; Kuznetsov et al., 2001; Zuk et al., 2001; Fan et al., 2005).
There are several reasons that hMSCs are used in treatment of diseases. As explained above, hMSCs can differentiate into several lineages and also secrete molecules for cell proliferation and survival. Another reason is that they can control immune response and have tropism to damaged region (Liechty et al., 2000; Tasso et al., 2012; Galderisi and Giordano, 2014; Wang et al., 2015). So, MSCs can be applied to treat not only bone, cartilage related disease, but also for therapy of Graft Versus Host Disease (GVHD), a various diseases in liver, kidney, lung, heart (Eom et al., 2015; Richardson et al., 2015; Squillaro et al., 2015).
However, a lot of cells are needed to use the cells in therapy. For instance, Zhao et al. administrated bone-marrow derived hMSCs (BM-MSC) at median dose of 1x106/kg once every 1 week to treat GVHD. Maximum 125 doses were administrated, and they got 75% efficiency in MSC treated group (Zhao et al., 2015). But, it had been expected that 1 cell would exist in 3.4x104 cells in bone-marrow in vivo that is not enough to apply for cell therapy (Wexler et al., 2003). So, production of wholesale cells is needed in vitro.
2 Wnt signaling pathway
Wnt proteins are glycoproteins that have 22 or 24 Cysteine residues. It has been known that Wnt signaling plays a key role in development, cell proliferation and differentiation, cell migration and body axis formation. Especially, in stem cell, Wnt signaling could be involved in self-renewal and differentiation (Turashvili et al., 2006; Nusse et al., 2008).
Depending on involvement of -catenin in the signaling pathway, Wnt signaling can divide into canonical Wnt signaling and non-canonical Wnt signaling. Both signaling pathways could be triggered by binding Wnt protein with Frizzled receptor (Fzd) (Bhanot et al., 1996; Oishi et al., 2003; Lu et al., 2004). Classically, it has been known that Wnt1, 2, 3, 3a, 8 and 8b are involved in canonical Wnt signaling and Wnt4, 5a, 5b, 6, 7a and 11 could trigger non-canonical Wnt signaling (Huelsken and Birchmeier, 2001; Huelsken and Behrens, 2002). Briefly introducing about the mechanism of Canonical Wnt signaling, when Wnt protein does not work, -catenin interacts with -catenin degradation complex containing APC (Adenomatous Polyposis Coli), Axin etc., and it is phosphorylated by GSK3-.
Phosphorylated -catenin would be degraded mediated by β-TrCP mediated
ubiquitin/proteasome pathway (Ikeya and Takada, 1998; Liu et al., 2002). In contrast, when Wnt protein interacts with Fzd, Disheveled (Dvl) is hyper-phosphorylated by CKIɛ resulting in degradation of Axin and disassociation of -catenin from APC–Axin–GSK3β complex (Li et al., 1999b). Stabilized -catenin translocates into nucleus to bind to T cell-specific factor (TCF)/lymphoid enhancer-binding factor 1(LEF 1) and regulates gene expression like MYC, DKK1, FGF20 etc, (He et al., 1998; Kramps et al., 2002; Chamorro et al., 2005; Daniels and Weis, 2005).
Non-canonical Wnt signaling is -catenin independent signaling. Non-canonical Wnt signaling could be separated into Wnt/PCP (planar cell polarity), Wnt/JNK (c-Jun N-terminal kinase), Wnt/calcium, and Wnt/Rho signaling. Those pathways are also started by interaction Wnt protein with Fzd and co-receptors resulting in activation of Dvl or intracellular Ca2+ release (Slusarski et al., 1997; Oishi et al., 2003; Lu et al., 2004; Liu et al., 2008). This activates Ca2+ sensitive enzymes such as protein kinase C (PKC), Ca2+ / calmodulin dependent kinase II (CamKII) (Kuhl et al., 2000). As Ca2+ dependent effector, Nemo-like
3
kinase (NLK) activates to phosphorylate to TCF/LEF1 for suppression of canonical Wnt signaling (Ishitani et al., 1999; Ishitani et al., 2003).
In the regulation of Wnt signaling, it could be inhibited by interference of binding ligands with receptors or suppression of intracellular signaling. For the inhibition of intracellular signaling, as explained above, it could be repressed by -catenin degradation complex resulted in -catenin degradation. Another reason for suppression of the signaling is caused by antagonists like Secreted Frizzled-related peptides (sFRPs), Wnt inhibitory factor 1 (WIF-1), Dickkopfs (Dkks) and Sclerostin. Those antagonists can interfere the interaction of Wnt protein with Fzd by binding Wnt proteins or competing Wnt proteins to interact Fzd and co-receptors, so that suppressing activation of Wnt signaling (Kawano and Kypta, 2003).
In MSC, it was known that they express not only Wnt protein, such as Wnt2, Wnt4, Wnt5a, Wnt11, Wnt16 but also various receptors like Fzd 2, 3, 4, 5, 6 and Wnt antagonists (Etheridge et al., 2004). Also, there are reports that canonical and non-canonical Wnt signaling could interact on each other to maintain characterization of MSCs (Baksh et al., 2007; Baksh and Tuan, 2007). Through those evidences, Wnt signaling could regulate self-renewal and differentiation of multiple lineage of MSC (Moldes et al., 2003; De Boer et al., 2004; Cho et al., 2006; Liu et al., 2014).
Secreted Frizzled Related Proteins (sFRPs)
As explained already, Wnt signaling could be regulated by a various antagonists (Kawano and Kypta, 2003). One of them, sFRPs are 32~40kDa of glycoproteins that were founded 5 isoform in human. Among these 5 sFRPs, sFRP1, like as other isoform, has Fzd-type cysteine-rich domain (CRD) and netrin (NTR) domain so that it could bind with Wnt protein or Fzd resulting in inhibition of Wnt signaling (Bhanot et al., 1996; Povelones and Nusse, 2005). Also, through sequence analysis and phylogenic analysis, sFRP1 is structurally closed with sFRP2, 5 and divide withsFRP3, 4 (Bovolenta et al., 2008).
It had been reported that Wnt signaling plays important role in development of cancer. 90% of colorectal cancer have mutations in Wnt signaling related gene such as APC, CTNNB1, AXIN and moreover, Wnt signaling had been involved in development of gastric cancer, liver
4
cancer (Giles et al., 2003). Another reason for occurrence of cancer is due to suppress of Wnt antagonists. For instance, various prostate cancer cell lines showed methylated SFRP2 about 70%, SFRP5 was methylated as 60% in DU145, PC-3 cell (Perry et al., 2013). In addition, MDA-MB-231, breast cancer cell line, showed that over-expression of sFRP1 could inhibit Wnt signaling resulting in down-regulation of cyclin D1 and up-regulation of p21. This caused suppression of cell proliferation and metastasis (Matsuda et al., 2009). Moreover, there are reports that sFRP1 has anti-cancer effect (Ko et al., 2002; Shih et al., 2007; Ren et al., 2013).
However, it is not always that sFRP1 inhibits Wnt signaling and suppression of cell growth. Even cancer, there are the cells that highly express sFRPs (Sathi et al., 2009; Qu et al., 2013; Ma et al., 2015), and currently, there are some reports that sFRPs could have biphasic effect on Wnt signaling depending on expression dose resulting in different regulation of cell proliferation (Uren et al., 2000; Xavier et al., 2014; Ren et al., 2015). Those results suggested that sFRPs should be applied in Wnt signaling activated cancer cells as anti-cancer drug.
Retinoic acid Orphan-related Receptor (ROR)
RORs are nuclear receptors as transcriptional factors. It was discovered that there are 3 ROR proteins. ; ROR, , and (Giguere et al., 1994; Hirose et al., 1994). Among them, ROR is also called as NR1F1 (nuclear receptor subfamily 1, group F member 1). In human, 4 isoforms of ROR were identified and these isoforms were generated by alternative splicing. ROR has total 4 domain including N-terminal (A/B) domain, DNA-binding domain containing 2 zinc fingers, hinge domain, and C-terminal ligand-binding domain. In ROR isoforms, DNA-binding domain and ligand-binding domain are conserved (Jetten et al., 2001). It has been known that ROR could regulate circadian clock, metabolism, development, immune response and recently, there are reports that ROR has anti-cancer effect on several cancer (Meyer et al., 2000; Ko and Takahashi, 2006; Du and Xu, 2012; Halim et al., 2012).
5
Specially, Lee et al. showed that ROR is down-stream gene of Wnt5a triggered non-canonical Wnt signaling and it could be phosphorylated and activated by PKC at Serine 35 residue in colorectal cancer cell line. Activated ROR translocated into nucleus and bound with -catenin resulting in inhibition of transcriptional regulation of -catenin (Lee et al., 2010). This suggested that non-canonical Wnt signaling could cross-talk with canonical Wnt signaling as other reports (Baksh et al., 2007; Ueno et al., 2007; Abdul-Ghani et al., 2011; Shen et al., 2014)
This study showed the role of sFRP1 in hMSCs’ proliferation. qRT-PCR and RT-PCR results showed that most of Wnt signaling related molecules were decreased as follows passages including sFRP1, except Wnt11. Also, Growth kinetics confirmed that sFRP1 affects proliferation of hMSCs and which could be dependent on its concentration. Meanwhile, nucleus translocation of ROR was decreased after treatment of sFRP1. Through those results suggested that sFRP1 could inhibit non-canonical Wnt signaling for promotion of hMSCs’ proliferation.
6
MATERIALS AND METHODS
Culture of human bone marrow derived mesenchymal stem cell in vitro
Human bone marrow derived mesenchymal stem cells (hMSCs) was used, which was isolated according to this reference and frozen (Lee, 2013). Frozen cells were thawed in 37℃ water and transferred 15ml conical tube containing pre-warmed growth media (10% fetal bovine serum (FBS, Hyclone), 1% penicillin/streptomycin (P/S) in 500ml Dulbeco"s Modified Eagle"s Medium (DMEM, Hyclone)). For remove dimethyl surfoxide (DMSO), cell containing tube was centrifuged in 500g for 5 minute (min). After suction supernatant, a proper volume of growth media was added and the cells were re-suspended. Then, the cells were counted using tryphan blue dye exclusion method and seeded the cells in culture dish. Plated cells were incubated in 37℃, 5% CO2 condition for about 16hrs. After then,
subculture was proceeded to expand cells.
For subculture the cells, Hanks' Balanced Salt Solution (HBSS, Sigma, pH 7.2) was added to wash FBS after suction of growth media in culture dish. Then, 0.25% Trypsin/EDTA was added to detach the cells from culture dish and transferred the cells to 15ml conical tube. After centrifugation in 500g for 5 min, supernatant was eliminated and growth media was added at a proper volume. Using tryphan blue dye exclusion method, the cells were counted and plated at a proper number.
Differentiation of hMSCa in vitro
Osteocytes and adipocytes were differentiated using the method that explained in this reference (Lee, 2013). In the case of chondrocytes differentiation, 2x105 cells in 15ml conical tube were centrifuged in 500g for 5 min. Removed growth media completely, chondrocyte differentiation media was gently added (Gibco, A10071-01). The differentiation was proceeded in 37℃, 5% CO2 condition and the media was exchanged every 3 days.
Staining of chondrocytes was proceeded as followed. Differentiated chondrocytes were washed by 1x phosphate buffer saline (PBS). Then, 4% paraformaldehyde (PFA) was added to fix the cells for 30min. After fixation, the cells were washed 1x PBS for 1 time, distilled
7
water (DW) for 2 times. Dehydration was proceeded as followed. ; 70% ethanol (EtOH), 20min 80% EtOH, 20min 90% EtOH, 20min 95% EtOH. 20min 100% EtOH, 10min, 3 times Xylene, 1min, 3 times. After paraffin embedding and making slides, deparafination process was proceeded to stain the cells.
To stain chondrocytes, alcian blue (3% acetic acid, 0.5% alcian blue) and nuclear fast red (5% aluminum sulfate, 1% nuclear fast red) solutions were used. Added alcian blue and nuclear fast red solution, the slides were washed in DW for 10min. Then, dehydration procedure was followed as like this. ; 70% EtOH 80% EtOH 90% EtOH 95% EtOH 100% EtOH, 3 times. Each step was proceeded for 1min. After mounting, the slides were saved at room temperature (RT).
SA--gal staining
SA--gal staining procedure was followed as this reference (Lee, 2013)
Semiquantitative - (RT-PCR) and quantitative-reverse transcriptase polymerase chain reaction (qRT-PCR)
Collection of the cells was explained as above. Washed 1x PBS as 1 time, a proper volume of RNA zol B was added. Then, chloroform was added as 10% of total volume and mixed. Samples were centrifuged in 4℃, 12000rpm condition for 25min, after saving those in ice for 10min. Collected supernatant, isopropyl alcohol was added as same volume and saved at -70℃ for over 2hrs. After that, samples were centrifuged in 4℃, 12000rpm condition for 25min and removed supernatant. Collected RNA pellet was washed by 75% EtOH and
dissolved byDEPC-DW.
cDNA was made using 1st-strand synthesis kit (Roche). Reverse transcription was proceeded as followed. ; 25℃, 10min 42℃, 1hr 70℃, 15min 4℃, forever. RT-PCR, qRT-PCR were performed using this cDNA. -actin and GAPDH were used as housekeeping gene. Primer of each gene and PCR condition were specified in Table. 1.
8 Production of virus and Transduction
Retrovirus and lentivirus were used for efficient experiment. Each transgenes were mixed with suitable packaging and envelop vector as 4 : 2 : 1 ratio and transfected to 293T cells using PEI (0.01mg/ml). PAM3, 8.9 were used as packaging vectors to make retrovirus, lentivirus. Media was exchanged after 4hr of transfection and collection of supernatant was performed at 2, 3 days later after transfection. Collected media was centrifuged in 500g for 10 min at RT and filter through 0.45m of syringe filter. Filtered supernatant was concentrated as followed reference (Kutner et al., 2009). Briefly explaining about concentration process, virus supernatant was mixed with PEG 6000, 4M NaCl, 1x PBS (final concentration ; 8.5% PEG 6000, 0.3M NaCl). Mixture was centrifuged in 4℃ at 7000g for 10 min after incubation in 4℃ for 1.5 hrs. Supernatant was removed and pellet was dissolved with 50mM Tris-HCl (pH 7.4). Virus was divided into several vials and stored in -70℃. After titration, virus was transduced into hMSCs using 8g/ml polybrene. 4hrs after transduction, media was added and the cells were incubated in 37℃, 5% CO2
condition for 4 days. Then, selection procedure of the transduced cells was proceeded using 2g/ml of puromycin for 1 week. Viral vector information was specified in Table. 2.
Growth kinetics of hMSCs
There are 2 kinds of methods to measure growth rate of hMSCs. First method was using subculture. hMSCs were plated as 5x104 in 100 culture dish. When culture dish was filled with cell about 80%, the cells were counted by tryphan blue exclusion method and subcultured as explained above.
The other method was like this. ; the cells were plated as 4x103` in 24 well plate and counted every 2 days using tryphan blue dye exclusion method. Culture media was exchanged every 2 days.
9 Treatment of recombinant human sFRP1 protein
To confirm the effect of sFRP1 dependent on concentration in the growth of hMSCs, recombinant human sFRP1 protein (rhsFRP1) were treated to hMSCs at a various concentration. The cells in P7 were seeded at 2x103 in 24 well plates and incubated in 37℃, 5% CO2 condition, overnight. Then, rhsFRP1 was treated as these concentrations. ; 0, 1, 3,
10, 30, 100, 300ng/ml. 3 samples were prepared in one concentration to triplicate experiment, and media was exchanged every 2 days.
Immunocytochemistry (ICC)
Cover glasses were put in 24 well plates and coated by 0.1mg/ml of PDL in 37℃, 5% CO2
condition for overnight. Coated cover glasses were washed 3 times by HBSS and the cells were seeded 3x104 cells. After incubation in 37℃, 5% CO2 condition, the cells were washed
by 1x PBS and fixed by 4% PFA for 10 min. 10% Normal goat serum (NGS, 1% NGS, 1% BSA, 0.1% Triton X-100, 1x PBS) was treated for 1 hr for blocking and 1st antibodies were incubated in 4℃ overnight. 1x PBS was treated in negative control. To wash the antibodies, PBS-T (0.1% Triton X-100 containing 1x PBS) was used at 3 times for 5 min each. Then, 2nd antibodies were treated for 2 hrs in dark at RT and washed by PBS-T at 3 times for 5 min each. At the last of washing step, Hoechst staining was proceeded as 1:10000 ratio and fluorescence were observed after mounting. Used condition of antibodies was specified in Table. 3.
Western blot analysis
To verify protein expression in cytosol and nucleus, protein was extracted by using 2 kinds of extraction buffers ; CE buffer (10mM HEPES (pH 7.9), 10mM KCl, 0.1mM EDTA, 0.3% NP-40, 1x protease) for cytosol protein extraction, BL buffer (10mM Tris-HCl (pH 8.0), 0.4M LiCl, 0.5mM DTT, 0.01% PMSF, 1x Protease) for nucleus protein extraction. The cells were collected by trypsinization and centrifugation. Removed supernatant, pellet was washed by cold 1x PBS and re-collected. To separate cytosol protein, CE buffer containing 0.3% NP-40 was added and the cells were centrifuged in 4℃, 3000rpm for 5 min after re-suspension. Supernatant was collected in new e-tube and remained pellet was washed twice by CE buffer
10
without NP-40 (centrifugation in 4℃, 3000rpm for 5 min).
For extraction nuclear protein, the pellet was re-suspended in BL buffer and saved at RT for 5 min. Then, the samples were centrifuged in 4℃, 12000rpm for 47 min and supernatant was collected. Extracted proteins were quantified by Bradford assay and loading samples were made by adding 5x SDS dye. Condition of used antibodies was specified in Table. 3.
11
RESULTS
Characterization and alteration of properties in long term culture in vitro
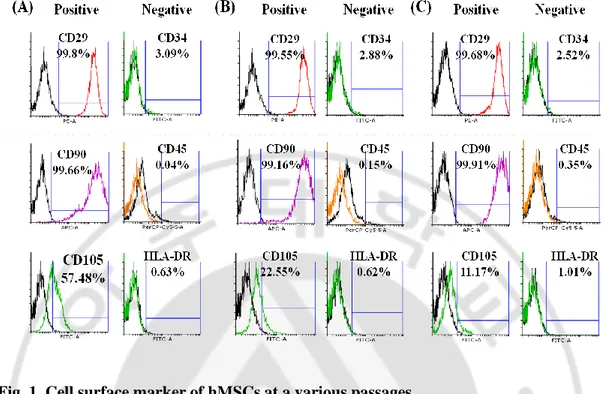
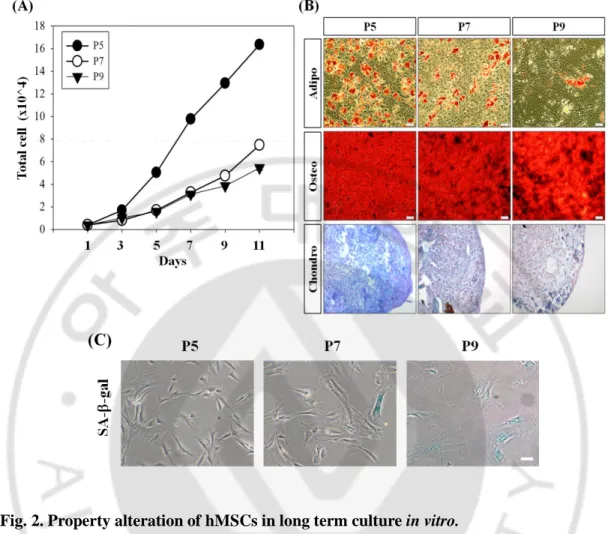
To characterize hMSCs that cultured in long term in vitro, cell surface markers were confirmed. As already known, CD29, CD90, CD105 were positive and CD34, CD45, HLA-DR were negative compared with isotype control. Most of the markers had no significant change between passages, but not CD105. CD105 was gradually decreased as follow passages (Fig. 1). Meanwhile, growth kinetics and differentiation data showed that proliferation and differentiation ability of hMSCs were decreased gradually followed by long term culture in vitro (Fig. 2. A, B). Also, SA--gal positive cells were more appeared in late passage indicating senescence was proceeded (Fig. 2. C).
Canonical Wnt signaling might be involved in regulation of hMSCs’ proliferation
It has been known that Wnt signaling could regulate proliferation of various cell types, and especially, control prolifereation and differentiation of stem cells (Reya et al., 2000; Reya et al., 2003; Chen et al., 2004; Dravid et al., 2005; Anton et al., 2007; Cai et al., 2007; Toyama et al., 2010; Chen et al., 2013; Kim et al., 2013; Bi et al., 2014; Cheng et al., 2014; Liu et al., 2014; Shen et al., 2014). For hMSC, it has been reported that canonical Wnt signaling could regulate proliferation of the cells (Boland et al., 2004; Pavlaki et al., 2014; Zhu et al., 2014; Jeoung et al., 2015). qRT-PCR and RT-PCR were performed to check mRNA expression of Wnt family. The result showed that most of Wnt family mRNA expressions were decreased gradually as follow passages, except Wnt11 (Fig. 3. A).Also, it was confirmed that mRNA expression of sFRP1, Wnt signaling antagonist, was decreased gradually (Fig. 3. B). Meanwhile, ICC and western blot results showed that expression and accumulated amount of -catenin in nucleus were higher in early passage of hMSCs (Fig. 3. C, D). For active GSK3- which triggered -catenin degradation by phosphorylation (Mosimann et al., 2009), it was increased in late passage of the cells (Fig. 3. C).
12 sFRP1 could affect to proliferation of hMSCs
It has been known that sFRP1, an antagonist of Wnt signaling, inhibits canonical Wnt signaling, induces apoptosis and controls cell cycle resulting in suppression of cell proliferation (Ko et al., 2002; Shih et al., 2007; Renstrom et al., 2009; Wu et al., 2012). Previous data was confirmed that activation of canonical Wnt signaling and sFRP1 expression were decreased gradually as follow passages (Fig. 3). Through those results, I hypothesized that sFRP1 could regulate hMSCs’ proliferation.
To confirm sFRP1 effect on proliferation of the cells, recombinant human sFRP1 protein (rhsFRP1) was treated, growth of the cells was measured. 10ng/ml of rhsFRP1 treated hMSCs had higher proliferation rate compared with 100ng/ml sFRP1 treated sample (Fig. 4. A). To check this again, sFRP1 over-expressing hMSCs (MSC/sFRP1) were made using retrovirus and nLacZ transduced hMSCs were used as control (MSC/nLacZ). After transduction, expression of sFRP1 was verified by RT-PCR (Fig. 4. B) and growth kinetics was measured before and after puromycin treatment. Transduction efficiency was confirmed by X-gal staining and ICC, those were about 38.7%, 40.8% for MSC/nLacZ, MSC/sFRP1 each in P8 (data not shown). When the cells cultured with non-transduced cell, the condition existing MSC/sFRP1 had higher growth rate (Fig. 4. C). But, the result was reversed when cultured puromycin selected cell (Fig. 4. D).
Lower concentration of sFRP1 could accelerate proliferation of hMSC
Based on Fig. 4, it was expected that sFRP1 could regulate the cell proliferation depending on concentration. A various concentrations of rhsFRP1 were treated to normal P7 hMSCs and growth of the cells was measured. Proliferation of the cells was promoted in lower concentration (from 1ng/ml to 10ng/ml) of rhsFRP1 but there was no significant in higher concentration (Fig. 5. A). To suppress autocrine effect of sFRP1, sFRP1 shRNA lentivirus was used to down-regulate its expression. Among various shRNA constructs, #172 construct showed the most efficient knock-down of sFRP1 (Fig. 5. B). Growth kinetics of MSC/shsFRP1 was measured after selection of the cells with puromycin. Proliferation of MSC/shsFRP1 was completely retarded (Fig. 5. C). To confirm that whether sFRP1 rescues this suppressed proliferation of MSC/shsFRP1, sFRP1 concentration dependent assay was
13
performed in sFRP1 down-regulated hMSCs. Even if the cell was in early passage, P5, MSC/shsFRP1 could not proliferate well comparing with control. Also, treatment of low concentration rhsFRP1 accelerated MSC/shsFRP1s growth. However, in control cells, there was no effect in low concentration treated sample while control cells in high concentration of rhsFRP1 showed significantly down-regulated growth (Fig. 5. D, E). These results confirmed that lower concentration of sFRP1 could restore proliferation of the cells.
ROR could be negative regulator in proliferation of hMSCs
Many reports showed that non-canonical Wnt signaling has a potential to suppress cell proliferation (Toyama et al., 2010; Bi et al., 2014; Cheng et al., 2014; Liu et al., 2014; Shen et al., 2014). It was also suggested that this signaling could cross-talk with canonical Wnt signaling (Shen et al., 2014; Lee et al., 2010)
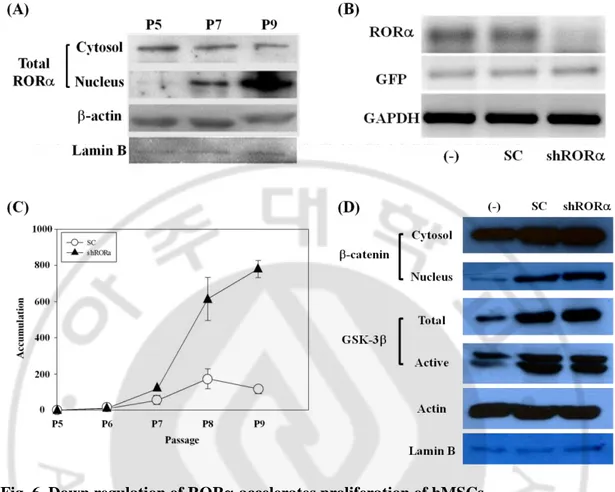
Retinoic acid receptor-related orphan receptor alpha (ROR) is on down-stream of non-canonical Wnt signaling triggered by Wnt5a. It also has been known that ROR could inhibit cell proliferation regarded to anti-cancer target (Odawara et al., 2009; Lee et al., 2010; Du and Xu, 2012; Xiong et al., 2012). So it was expected that non-canonical Wnt signaling could suppress hMSCs’ proliferation and ROR would be involved in this regulation. In ROR expression in various passages, it was higher in late passage of the cells as well as nuclear translocation increased (Fig. 3. D, Fig. 6. A). To figure out the relationship between ROR and proliferation of hMSCs, ROR shRNA lentivirus was used to down-regulate ROR expression (Fig. 6. B). After sorting transduced cells by puromycin, growth kinetics was measured. MSC/shROR could grow well compared with control (Fig. 6. C). Meanwhile, western blot analysis was performed to confirm whether this accelerated proliferation was due to activation of canonical Wnt signaling triggered by knock-down of ROR. The result showed that accumulation level of -catenin in nucleus was higher in MSC/shROR and active GSK-3 level was also decreased. (Fig. 6. D). These results indicated that ROR could be a negative regulator of canonical Wnt signaling resulting in suppression of cell proliferation.
14
sFRP1 could inhibit translocation of ROR into nucleus
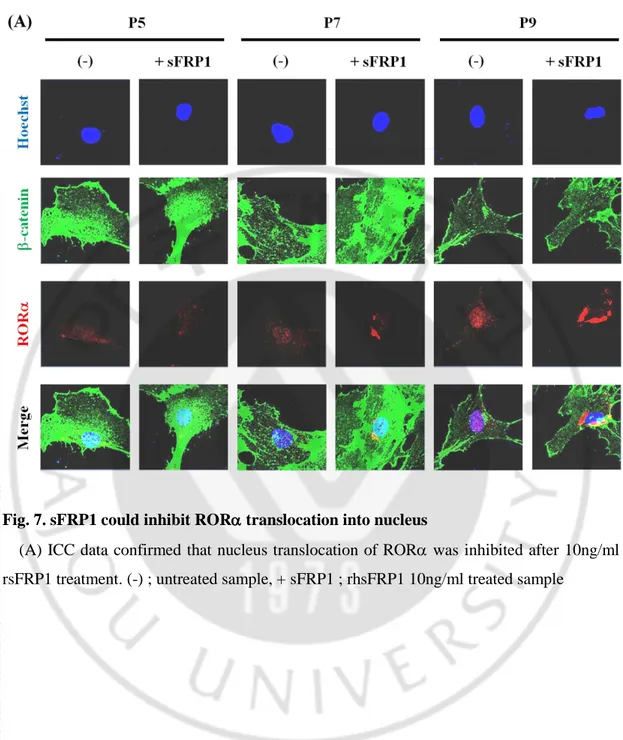
Lee et al.(2010) suggested that phosphor Ser35 ROR by Wnt5a/PKC signaling translocate into nuleus and bind to -catenin resulting in suppression of canonical Wnt signaling. Previous data showed that sFRP1 expression was higher in early passage of the cells and ROR expression and translocation into nucleus were gradually increased as follow passages (Fig. 3). Based on those results, I hypothesized that sFRP1 could repress translocation of ROR into nucleus. ROR localization was observed after treatment of 10ng/ml rhsFRP1 to hMSCs. ICC data showed that there was no effect in early passage of the cells. However, in P7 of hMSC, inhibition of ROR translocation and increased -catenin expression were confirmed. For P9, there was no significant increase of -catenin expression but ROR expressed surrounding nucleus (Fig. 7. A).
15
Fig. 1. Cell surface marker of hMSCs at a various passages
(A), (B) and (C) showed cell surface marker of hMSCs at P5, 7 and 9 respectively. There was almost no change in several markers but not CD105. CD105 was gradually decreased as follow passages.
16
Fig. 2. Property alteration of hMSCs in long term culture in vitro.
(A) Growth kinetics of hMSCs decreased gradually as follow passages. (B) hMSCs lost their differentiation ability as passages go on. Adipocytes (Adipo) and osteocytes (Osteo) were stained by Oil red-O and Alizarin red S respectively. Chondrocytes (Chondro) could be visualized by Alcian blue staining. Scale bar = 50m. (C) SA-ß-gal staining confirmed that more aged cells appeared in late passage of hMSCs. Scale bar = 100m.
18
Fig. 3. Canonical Wnt signaling is activated in the early passage of hMSCs
(A) Through qPCR, almost mRNA expressions of Wnt family were higher in early passage of hMSCs and decreased gradually, except Wnt 11. (B) mRNA level of sFRP1 was confirmed by RT-PCR (n=3). Its expression was decreased gradually as follow passages. (C) Western blot results showed that -catenin was more accumulated in nucleus at early passage of hMSCs and active GSK-3 expression was more increased in late passage of the cells. (D) ICC data showed that -catenin was more accumulated in nucleus at early passage of hMSCs and ROR expression and translocation into nucleus were increased gradually as follow passages.
19 Fig. 4. sFRP1 could affect proliferation of hMSCs
(A) Growth kinetics of hMSC in the presence of rhsFRP1 protein. (B) sFRP1 over-expressing hMSCs was made by using retrovirus. RT-PCR was used to verify sFRP1 expression. RNA was extracted from MSC/sFRP1 at P8. (C) After transduction of sFRP1 retrovirus, the cells were cultured to measure growth kinetics without puromycin selection. (D) Growth kinetics of MSC/sFRP1 was measured after puromycin selection.
21
Fig. 5. Growth of hMSCs is dependent on sFRP1 concentration
(A) sFRP1 concentration dependent assay in P7 normal hMSCs. (B) To verify knockdown efficiency of shRNA lentivirus of sFRP1, shsFRP1 lentivirus was transduced into hMSCs (MSC/shsFRP1). #172 was chosen as the most effective shsFRP1 lentivirus construct through confirmation of sFRP1 expression using RT-PCR. SC ; scrambled shRNA, (-) ; normal hMSCs. (C) Growthh kinetics of MSC/shsFRP1. (D) sFRP1 concentration dependent assay in MSC/shsFRP1 P5. Cells were counted at 5 days after treatment of rhsFRP1. (E) sFRP1 concentration dependent assay in MSC/control at P5. Cells were counted at 3 days after treatment of rhsFRP1
22
Fig. 6. Down regulation of ROR accelerates proliferation of hMSCs
(A) Western blot showed that ROR was increased in the late passage of hMSCs. (B) RT-PCR was performed to verify ROR expression after transduction of lentivirus shROR. (C) After puromycin selection, growth kinetics of the transduced cells was measured by subculture. (D) Western blot result confirmed that canonical Wnt signaling was activated in
23
Fig. 7. sFRP1 could inhibit ROR translocation into nucleus
(A) ICC data confirmed that nucleus translocation of ROR was inhibited after 10ng/ml rsFRP1 treatment. (-) ; untreated sample, + sFRP1 ; rhsFRP1 10ng/ml treated sample
24
Discussion
Human bone marrow derived mesenchymal stem cells (hMSC) are adult stem cells regarded to attractive source for therapeutic use (Eom et al., 2015; Richardson et al., 2015; Squillaro et al., 2015). However, for treatment of disease, there are some limitations to expand the cells in vitro. This study confirmed that there was change in cell surface marker of CD105 (Fig. 1). bFGF has been connected tumor angiogenesis, tissue healing (Fuhrmann-Benzakein et al., 2000). Meanwhile, CD105 plays a key role in angiogenesis, cell morphology and migration so this could be associated with tumor growth, survival and metastasis of cancer cells (Li et al., 1999a; Duff et al., 2003). Summarizing these, it could be a reason that there was no basic fibroblast growth factor (bFGF) in growth condition of the cells for decrease of CD105 level. Also, proliferation of hMSCs was gradually decreased as follow passages and they lost their differentiation ability. In addition to SA--gal staining data, senescence was occurred in long-term culture in vitro (Fig. 2).
It has been well known that Wnt signaling pathway regulates embryo development, cell proliferation and differentiation (Tada and Smith, 2000; Anton et al., 2007; Cai et al., 2007; Liu et al., 2014). While a various type of cancer was caused by canonical Wnt signaling (Chen et al., 2004; Chen et al., 2013; Kim et al., 2013), it had been known that non-canonical Wnt signaling could inhibit proliferation of the cells (Toyama et al., 2010; Bi et al., 2014; Cheng et al., 2014; Liu et al., 2014; Shen et al., 2014). In hMSCs, most of Wnt signaling study was focused on differentiation. Therefore, I would like to confirm the role of Wnt signaling in hMSCs’ proliferation. Western blotting and immunocytochemistry results showed that canonical Wnt signaling was activated in early passage of hMSCs but activation of non-canonical Wnt signaling was reversed (Fig. 3, 6). These results suggested that Wnt signaling could be involved in regulation of hMSCs’ proliferation.
Secreted Frizzled Related Protein 1 (sFRP1) is an antagonist of Wnt signaling. It is well known that sFRP1 suppresses Wnt signaling by binding Fzds or Wnt protein (Bhanot et al., 1996; Povelones and Nusse, 2005; Bovolenta et al., 2008). Many studies showed that sFRP1 have anti-cancer effect on a various type of cancer cell, but it could be possible that sFRP1
25
promotes cell proliferation depending on cell context or concentration (Uren et al., 2000; Qu et al., 2013; Xavier et al., 2014; Ren et al., 2015). In this study, mRNA expression of sFRP1 was gradually decreased as passages go on (Fig. 3). To prove that sFRP1 could regulate proliferation of hMSCs, sFRP1 over-expressing (MSC/sFRP1) or knock-downed cell line (MSC/shsFRP1) was made by transduction of virus. When MSC/sFRP1s were co-cultured with normal hMSCs, the growth of the cells was promoted, but not in culture MSC/sFRP1 only. Also, MSC/shsFRP1s were hardly proliferated compared with control (Fig. 4, 5). Those results indicated that sFRP1 could be involved in the regulation of hMSCs’ proliferation. Meanwhile, it was confirmed that growth of the cells could be regulated depending on the concentration of rhsFRP1. Lower concentration of rhsFRP1 promoted the growth of the cells, but not in higher concentration (Fig. 5). Those results suggested that sFRP1 has biphasic effect on proliferation of hMSCs depending on their concentration.
It has been known that non-canonical Wnt signaling can cross-talk with canonical Wnt signaling and inhibit the proliferation of a various cell types (Lee et al., 2010; Toyama et al., 2010; Abdul-Ghani et al., 2011; Shen et al., 2014). ROR, a down-stream gene of this signaling, played a role in osteocytes differentiation of hMSCs and had been regarded to anti-cancer gene in many kinds of cancer cells (Meyer et al., 2000; Du and Xu, 2012; Xiong et al., 2012). Especially, ROR could be phosphorylated by Wnt5a/PKC signal transduction on Serine 35 residue and phosphor ROR, activated form of ROR, translocated into nucleus. Then, it bound to -catenin resulting in suppression of its transcriptional regulation (Lee et al., 2010). To confirm that ROR could be involved in control of hMSCs’ proliferation as well as differentiation, ROR knock-downed cell line (MSC/shROR) was made and growth kinetics of the cells was measured. As shown in Fig. 6, the growth of MSC/shROR was promoted compared with control and this phenomenon could be caused by activation of canonical Wnt signaling. Also, localization of ROR in nucleus was inhibited and it surrounded nucleus in rhsFRP1 treated hMSCs indicating that sFRP1 could repress nucleus translocation of ROR (Fig. 7). Those results suggested that not only sFRP1 could accelerate proliferation of the cells by inhibiting non-canonical Wnt signaling, but also it could compensate limitations of hMSCs for therapy (Alfaro et al., 2008; Dufourcq et al.,
26
2008; Alfaro et al., 2010).
qRT-PCR confirmed that Wnt11 expression, not Wnt5a, was increased gradually as follow passages (Fig. 3). Wnt11 has been known as non-canonical Wnt protein containing 354 amino acid and it plays a key role in embryo development, differentiation, cell migration and adhesion (Tada and Smith, 2000; Uysal-Onganer et al., 2010; Liu et al., 2014). Wnt11 gene has TCF/LEF binding site near promoter region, so its expression could be regulated by canonical Wnt signaling (Ueno et al., 2007; Katoh and Katoh, 2009). Particularly, Wnt11 makes intracellular Ca2+ level increase, which triggers PKC activity resulting in suppression of canonical Wnt signaling so that inhibition of cell proliferation (Toyama et al., 2010). Also, based on the results that interaction between Wnt11 and Wnt5a could activate canonical Wnt signaling (Cha et al., 2008) and Wnt11 expression pattern was reversed comparing with another Wnt family in hMSC (Fig. 3), it is possible that Wnt11 could make non-canonical Wnt signaling activate.
However, it is not in all the time that non-canonical Wnt signaling inhibits cell proliferation. For instance, Wnt5a triggered non-canonical Wnt signaling could suppress canonical Wnt signaling, resulting in enhance of hematopoietic stem cell repopulation (Nemeth et al., 2007). Also, inhibition of non-canonical Wnt signaling showed suppression of proliferation and migration of epithelial cell and retarded wound closure indicating that non-canonical Wnt signaling might have a positive potential on cell function and proliferation (Cheng et al., 2008). Meanwhile, it had been reported that when Wnt11 binds to LRP6, canonical Wnt signaling could be activated (Kofron et al., 2007). This suggested that effect of Wnt signal transduction would be dependent on what kinds of receptor interact with Wnt protein.
This study showed sFRP1, a Wnt modulating protein, could promote hMSCs’ proliferation by inhibiting non-canonical Wnt signaling, and this could be dependent on its concentration. It is worthful in terms of compensation of limitation in long term culture of hMSCs in vitro. Also, Wnt signaling modification could have biphasic effect for therapy of disease.
27
References
1. Abdul-Ghani M, Dufort D, Stiles R, De Repentigny Y, Kothary R, Megeney LA: Wnt11 promotes cardiomyocyte development by caspase-mediated suppression of canonical Wnt signals. Mol Cell Biol 31: 163-178, 2011
2. Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP: The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A 105: 18366-18371, 2008
3. Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, Young PP: sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem 285: 35645-35653, 2010
4. Anton R, Kestler HA, Kuhl M: Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett 581: 5247-5254, 2007
5. Baksh D, Boland GM, Tuan RS: Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 101: 1109-1124, 2007
6. Baksh D, Tuan RS: Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol 212: 817-826, 2007
7. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R: A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382: 225-230, 1996
8. Bi L, Liu X, Wang C, Cao Y, Mao R, Li P, Geng M: Wnt5a involved in regulation of the biological behavior of hepatocellular carcinoma. Int J Clin Exp Pathol 7: 987-995, 2014
9. Boland GM, Perkins G, Hall DJ, Tuan RS: Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93: 1210-1230, 2004
10. Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J: Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121: 737-746, 2008
11. Cai L, Ye Z, Zhou BY, Mali P, Zhou C, Cheng L: Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res 17: 62-72, 2007
12. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM: Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98: 2396-2402, 2001
13. Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J: Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135: 3719-3729, 2008
28
14. Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE:
FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-FGF-20 is implicated in cancer and development. Embo j 24: 73-84, 2005
15. Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA: Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer 101: 1345-1356, 2004
16. Chen HL, Chew LJ, Packer RJ, Gallo V: Modulation of the Wnt/beta-catenin pathway in human oligodendroglioma cells by Sox17 regulates proliferation and differentiation. Cancer Lett 335: 361-371, 2013
17. Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS: Wnt5a-mediated
non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 365: 285-290, 2008
18. Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q: Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol 229: 1908-1917, 2014
19. Cho HH, Kim YJ, Kim SJ, Kim JH, Bae YC, Ba B, Jung JS: Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng 12: 111-121, 2006
20. Daniels DL, Weis WI: Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12: 364-371, 2005
21. De Boer J, Wang HJ, Van Blitterswijk C: Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 10: 393-401, 2004 22. Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI: A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res 14: 700-709, 1999
23. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317, 2006
24. Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L: Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells 23: 1489-1501, 2005
25. Du J, Xu R: RORalpha, a potential tumor suppressor and therapeutic target of breast cancer. Int J Mol Sci 13: 15755-15766, 2012
26. Duff SE, Li C, Garland JM, Kumar S: CD105 is important for angiogenesis: evidence and potential applications. Faseb j 17: 984-992, 2003
27. Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C: Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26: 2991-3001, 2008
28. Eom YW, Shim KY, Baik SK: Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med 30: 580-589, 2015
29
29. Erices A, Conget P, Minguell JJ: Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109: 235-242, 2000
30. Etheridge SL, Spencer GJ, Heath DJ, Genever PG: Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22: 849-860, 2004
31. Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, Liu B, Han ZB, Han ZC:
Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant 14: 311-321, 2005
32. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP: Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230-247, 1968
33. Fuhrmann-Benzakein E, Ma MN, Rubbia-Brandt L, Mentha G, Ruefenacht D, Sappino AP, Pepper MS: Elevated levels of angiogenic cytokines in the plasma of cancer patients. Int J Cancer 85: 40-45, 2000
34. Galderisi U, Giordano A: The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev 34: 1100-1126, 2014
35. Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G: Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev 8: 538-553, 1994
36. Giles RH, van Es JH, Clevers H: Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1-24, 2003
37. Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F:
Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity 37: 463-474, 2012
38. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI: Characterization of cells with osteogenic potential from human marrow. Bone 13: 81-88, 1992
39. He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 281: 1509-1512, 1998
40. Hirose T, Smith RJ, Jetten AM: ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun 205: 1976-1983, 1994
41. Huelsken J, Behrens J: The Wnt signalling pathway. J Cell Sci 115: 3977-3978, 2002 42. Huelsken J, Birchmeier W: New aspects of Wnt signaling pathways in higher
vertebrates. Curr Opin Genet Dev 11: 547-553, 2001
43. Ikeya M, Takada S: Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 125: 4969-4976, 1998
44. Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23: 131-139, 2003
45. Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K: The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and
30
transcription factor TCF. Nature 399: 798-802, 1999
46. Jeoung JY, Nam HY, Kwak J, Jin HJ, Lee HJ, Lee BW, Baek JH, Eom JS, Chang EJ, Shin DM, Choi SJ, Kim SW: A decline in Wnt3a signaling is necessary for mesenchymal stem cells to proceed to replicative senescence. Stem Cells Dev 24: 973-982, 2015
47. Jetten AM, Kurebayashi S, Ueda E: The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol 69: 205-247, 2001
48. Katoh M, Katoh M: Integrative genomic analyses of WNT11: transcriptional mechanisms based on canonical WNT signals and GATA transcription factors signaling. Int J Mol Med 24: 247-251, 2009
49. Kawano Y, Kypta R: Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627-2634, 2003
50. Kim JT, Li J, Jang ER, Gulhati P, Rychahou PG, Napier DL, Wang C, Weiss HL, Lee EY, Anthony L, Townsend CM, Jr., Liu C, Evers BM: Deregulation of Wnt/beta-catenin signaling through genetic or epigenetic alterations in human neuroendocrine tumors. Carcinogenesis 34: 953-961, 2013
51. Ko CH, Takahashi JS: Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No 2: R271-277, 2006
52. Ko J, Ryu KS, Lee YH, Na DS, Kim YS, Oh YM, Kim IS, Kim JW: Human secreted
frizzled-related protein is down-regulated and induces apoptosis in human cervical cancer. Exp Cell Res 280: 280-287, 2002
53. Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, Heasman J: Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development 134: 503-513, 2007
54. Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K: Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109: 47-60, 2002
55. Kuhl M, Sheldahl LC, Malbon CC, Moon RT: Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 275: 12701-12711, 2000
56. Kutner RH, Zhang XY, Reiser J: Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 4: 495-505, 2009
57. Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG: Circulating skeletal stem cells. J Cell Biol 153: 1133-1140, 2001
58. Lee J: Role of Wnt signaling in proliferation of human bone marrow-derived mesenchymal stem cells (Thesis), Ajou University, 2013
59. Lee JM, Kim IS, Kim H, Lee JS, Kim K, Yim HY, Jeong J, Kim JH, Kim JY, Lee H, Seo SB, Kim H, Rosenfeld MG, Kim KI, Baek SH: RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol Cell 37: 183-195, 2010
60. Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP: Defective angiogenesis in mice lacking endoglin. Science 284: 1534-1537, 1999a
31
61. Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D: Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. Embo j 18: 4233-4240, 1999b
62. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak
DR, Flake AW: Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6: 1282-1286, 2000
63. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X: Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837-847, 2002
64. Liu F, Kohlmeier S, Wang CY: Wnt signaling and skeletal development. Cell Signal 20: 999-1009, 2008
65. Liu S, Zhang E, Yang M, Lu L: Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-beta. Mol Cell Biochem 390: 123-131, 2014
66. Lu W, Yamamoto V, Ortega B, Baltimore D: Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119: 97-108, 2004
67. Ma J, Cheng J, Gong Y, Tian L, Huang Q: Downregulation of Wnt signaling by sonic hedgehog activation promotes repopulation of human tumor cell lines. Dis Model Mech 8: 385-391, 2015
68. Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE: WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res 11: R32, 2009
69. Meyer T, Kneissel M, Mariani J, Fournier B: In vitro and in vivo evidence for orphan nuclear receptor RORalpha function in bone metabolism. Proc Natl Acad Sci U S A 97: 9197-9202, 2000
70. Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR: Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J 376: 607-613, 2003
71. Mosimann C, Hausmann G, Basler K: Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10: 276-286, 2009
72. Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM: Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A 104: 15436-15441, 2007
73. Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, ten Berge D, Kalani Y: Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol 73: 59-66, 2008
74. Odawara H, Iwasaki T, Horiguchi J, Rokutanda N, Hirooka K, Miyazaki W, Koibuchi Y, Shimokawa N, Iino Y, Takeyoshi I, Koibuchi N: Activation of aromatase expression by retinoic acid receptor-related orphan receptor (ROR) alpha in breast cancer cells: identification of a novel ROR response element. J Biol Chem 284: 17711-17719, 2009
75. Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y: The receptor
32
tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8: 645-654, 2003
76. Pavlaki K, Pontikoglou CG, Demetriadou A, Batsali AK, Damianaki A, Simantirakis E, Kontakis M, Galanopoulos A, Kotsianidis I, Kastrinaki MC, Papadaki HA: Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev 23: 1568-1581, 2014
77. Perry AS, O'Hurley G, Raheem OA, Brennan K, Wong S, O'Grady A, Kennedy AM,
Marignol L, Murphy TM, Sullivan L, Barrett C, Loftus B, Thornhill J, Hewitt SM, Lawler M, Kay E, Lynch T, Hollywood D: Gene expression and epigenetic discovery screen reveal methylation of SFRP2 in prostate cancer. Int J Cancer 132: 1771-1780, 2013
78. Povelones M, Nusse R: The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. Embo j 24: 3493-3503, 2005
79. Qu Y, Ray PS, Li J, Cai Q, Bagaria SP, Moran C, Sim MS, Zhang J, Turner RR, Zhu Z, Cui X, Liu B: High levels of secreted frizzled-related protein 1 correlate with poor prognosis and promote tumourigenesis in gastric cancer. Eur J Cancer 49: 3718-3728, 2013
80. Ren J, Wang R, Huang G, Song H, Chen Y, Chen L: sFRP1 inhibits epithelial-mesenchymal transition in A549 human lung adenocarcinoma cell line. Cancer Biother Radiopharm 28: 565-571, 2013
81. Ren XY, Zhou GQ, Jiang W, Sun Y, Xu YF, Li YQ, Tang XR, Wen X, He QM, Yang
XJ, Liu N, Ma J: Low SFRP1 expression correlates with poor prognosis and promotes cell invasion by activating the Wnt/beta-catenin signaling pathway in NPC. Cancer Prev Res (Phila), 2015
82. Renstrom J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A, Kroger M, Schiemann M, Busch DH, Esposito I, Lang R, Peschel C, Oostendorp RA: Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell 5: 157-167, 2009
83. Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL: A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409-414, 2003
84. Reya T, O'Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R: Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13: 15-24, 2000
85. Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini
A, Mobasheri R, Poletti FL, Hoyland JA, Mobasheri A: Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods, 2015
86. Sathi GA, Inoue M, Harada H, Rodriguez AP, Tamamura R, Tsujigiwa H, Borkosky SS, Gunduz M, Nagatsuka H: Secreted frizzled related protein (sFRP)-2 inhibits bone formation and promotes cell proliferation in ameloblastoma. Oral Oncol 45: 856-860, 2009
33
stem cell-derived Wnt5a inhibits leukemia cell progression via activation of the non-canonical Wnt signaling pathway. Oncol Lett 8: 85-90, 2014
88. Shih YL, Hsieh CB, Lai HC, Yan MD, Hsieh TY, Chao YC, Lin YW: SFRP1 suppressed hepatoma cells growth through Wnt canonical signaling pathway. Int J Cancer 121: 1028-1035, 2007
89. Slusarski DC, Yang-Snyder J, Busa WB, Moon RT: Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 182: 114-120, 1997
90. Squillaro T, Peluso G, Galderisi U: Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant, 2015
91. Tada M, Smith JC: Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127: 2227-2238, 2000
92. Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G: Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 53: 786-793, 2012
93. Toyama T, Lee HC, Koga H, Wands JR, Kim M: Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res 8: 254-265, 2010
94. Turashvili G, Bouchal J, Burkadze G, Kolar Z: Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73: 213-223, 2006
95. Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon
RT, Murry CE: Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A 104: 9685-9690, 2007
96. Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS: Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 275: 4374-4382, 2000
97. Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, Waxman
J, Kypta RM: Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer 9: 55, 2010
98. Wang J, Wang Y, Wang S, Cai J, Shi J, Sui X, Cao Y, Huang W, Chen X, Cai Z, Li H, Bardeesi AS, Zhang B, Liu M, Song W, Wang M, Xiang AP: Bone marrow-derived mesenchymal stem cell-secreted IL-8 promotes the angiogenesis and growth of colorectal cancer. Oncotarget, 2015
99. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM: Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol 121: 368-374, 2003
100. Wu Y, Li J, Sun CY, Zhou Y, Zhao YF, Zhang SJ: Epigenetic inactivation of the canonical Wnt antagonist secreted frizzled-related protein 1 in hepatocellular carcinoma cells. Neoplasma 59: 326-332, 2012
101. Xavier CP, Melikova M, Chuman Y, Uren A, Baljinnyam B, Rubin JS: Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/beta-catenin signaling. Cell Signal 26: 94-101, 2014
34
102. Xiong G, Wang C, Evers BM, Zhou BP, Xu R: RORalpha suppresses breast tumor
invasion by inducing SEMA3F expression. Cancer Res 72: 1728-1739, 2012
103. Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B: The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80: 1745-1757, 1998
104. Zhao K, Lou R, Huang F, Peng Y, Jiang Z, Huang K, Wu X, Zhang Y, Fan Z, Zhou H, Liu C, Xiao Y, Sun J, Li Y, Xiang P, Liu Q: Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21: 97-104, 2015
105. Zhu Z, Yin J, Guan J, Hu B, Niu X, Jin D, Wang Y, Zhang C: Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3beta-dependent beta-catenin/Wnt pathway activation. Febs j 281: 5371-5389, 2014 106. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP,
Hedrick MH: Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228, 2001