E
E
Ex
x
xp
p
pr
r
re
e
es
s
ss
s
si
i
i
o
o
on
n
na
a
an
n
nd
d
dF
F
Fu
u
un
n
nc
c
ct
t
ti
i
i
o
o
on
n
no
o
of
f
fT
T
To
o
ol
l
l
l
l
l
-
-
-L
L
Li
i
i
k
k
ke
e
e
R
R
Re
e
ec
c
ce
e
ep
p
pt
t
to
o
or
r
r2
2
2a
a
an
n
nd
d
d4
4
4i
i
i
n
n
nH
H
Hu
u
um
m
ma
a
an
n
nM
M
Me
e
el
l
l
a
a
an
n
no
o
oc
c
cy
y
yt
t
te
e
es
s
s
아
아
아 주
주
주 대
대
대 학
학 교
학
교
교 대
대
대 학
학
학 원
원
원
의
의
의 학
학
학 과
과
과
안
안
안 주
주
주 희
희
희
in
in
in
in Human
Human
Human
Human Melanocytes
Melanocytes
Melanocytes
Melanocytes
by
by
by
by
Joo
Joo
Joo
Joo Hee
Hee
Hee
Hee Ahn
Ahn
Ahn
Ahn
A
A
A
A Dissertation
Dissertation Submitted
Dissertation
Dissertation
Submitted
Submitted
Submitted to
to
to
to The
The
The Graduate
The
Graduate School
Graduate
Graduate
School
School of
School
of
of
of Ajou
Ajou
Ajou University
Ajou
University
University
University
in
in
in
in Partial
Partial
Partial
Partial Fulfillment
Fulfillment
Fulfillment
Fulfillment of
of
of
of the
the
the Requirements
the
Requirements for
Requirements
Requirements
for
for
for the
the
the Degree
the
Degree
Degree of
Degree
of
of
of
MASTER
MASTER
MASTER OF
MASTER
OF
OF
OF MEDICAL
MEDICAL
MEDICAL
MEDICAL SCIENCES(
SCIENCES(이학
SCIENCES(
SCIENCES(
이학
이학
이학:
:
:
: SCIENCE)
SCIENCE)
SCIENCE)
SCIENCE)
Supervised
Supervised
Supervised
Supervised by
by
by
by
Hee
Hee
Hee Young
Hee
Young
Young
Young Kang,
Kang,
Kang,
Kang, M.D.,
M.D.,
M.D.,
M.D., Ph.D.
Ph.D.
Ph.D.
Ph.D.
Department
Department
Department
Department of
of
of Medical
of
Medical
Medical
Medical Sciences
Sciences
Sciences
Sciences
The
The
The
The Graduate
Graduate
Graduate
Graduate School,
School,
School,
School, Ajou
Ajou
Ajou
Ajou University
University
University
University
August,
August,
August,
안
안
안주
주
주희
희
희의
의
의 의
의
의학
학
학 석
석
석사
사
사학
학
학위
위
위 논
논
논문
문
문을
을
을 인
인
인준
준
준함
함
함.
.
.
심
심
심사
사
사위
위
위원
원
원장
장
장
강
강
강 희
희
희 영
영
영
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
이
이
이 은
은
은 소
소
소
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
임
임
임 인
인
인 경
경
경
인
인
인
아
아
아 주
주
주 대
대
대 학
학 교
학
교
교 대
대
대 학
학
학 원
원
원
2
2
20
0
00
0
06
6
6년
년
년 6
6
6월
월
월 2
2
22
2
2일
일
일
-ABSTRACT-
Expression and Function of Toll-Like Receptor 2 and 4 in
Human Melanocytes
Toll-like receptors (TLRs) are crucial players in the innate immune response to microbial invaders. There have been reported that keratinocyte TLRs represent the first barrier against exogenous pathogens in human skin. Recently, it was reported that melanocytes not only function melanin synthesis but also act as immunocompetent cell. Melanosome and melanin in melanoyctes have antimicrobial function. In this study, we focused that microbial induced pigmentation may be related to TLRs in human skin. Therefore we investigated whether TLRs are expressed in melanocytes and played a role in microbial-induced melanogenesis. First, the mRNA expression of TLR2, TLR4 and their adaptor proteins, CD14 and myeloid differentiation factor-88 (MyD88) was shown by RT-PCR. Protein expression and localization of TLR2, TLR4 and CD14 were detected by western blot, flow cytometry and immunocytochemistry. Lipopolysaccharide (LPS) was used as microbial stimulator on melanocytes. The expression of TLR2, TLR4 and MyD88 at mRNA and protein level were increased after LPS stimulation. LPS affected cell proliferation and melanin synthesis. TLR2 expression was decreased in vitiligo lesion compared with normal skin. These findings suggested that TLR2 and TLR4 may play a role in the microbial-induced melanogenesis.
Key words: Toll-like receptor 2, 4, Lipopolysaccharide, melanocyte, melanin, pigmentation
TABLE OF CONTENTS
ABSTRACT ··· i TABLE OF CONTENTS ··· ii LIST OF FIGURES ··· iv ABBREVIATION··· v I. INTRODUCTION ··· 1Ⅱ. MATERIALS AND METHODS ··· 4
A. MATERIALS ··· 4
B. METHODS ··· 4
1. Cell Culture ··· 4
2. Reverse Transcription Polymerase Chain Reaction (RT-PCR) ··· 5
3. Flow Cytometry ··· 6
4. Western Blotting ··· 7
5. Immunocytochemistry ··· 7
6. Melanocyte growth assay ··· 8
7. Melanin content determination ··· 8
8. Immunohistochemistry ··· 8
9. Statistical analysis ··· 9
. RESUL Ⅲ TS ··· 10
A. Human melanocytes express TLR2, TLR4, CD14 and MyD88 mRNA ··· 10
B. Human melanocytes express cell surface TLR2, TLR4 and CD14 ··· 10
C. Human melanocytes express TLR2, TLR4 and CD14 protein ··· 12
D. LPS regulates the expression of TLR2, TLR4 and MyD88 in human melanocytes ···13
E. LPS induces melanogenesis in human melanocytes ··· 15
F. Expression of TLR2 in vitiligo ··· 16
Ⅴ. CONCLUSION ··· 20
REFERENCES ··· 21
LIST OF FIGURES
Fig. 1. Human melanocytes express TLR2, TLR4, CD14 and MyD88 mRNA ··· 10
Fig. 2. TLR2, TLR4 and CD14 were expressed in surface human melanocytes ··· 11
Fig. 3. TLR2, TLR4 and CD14 protein expression in human melanocytes ··· 12
Fig. 4. LPS increases TLR2, TLR4, and MyD88 mRNA in human melanocytes ··· 13
Fig. 5. LPS induces TLR4 protein in human melanocytes ··· 14
Fig. 6. LPS stimulation increased melanin synthesis and decreased cell proliferation in human melanocytes ··· 15
ABBREVIATION
TLR, Toll-like receptor; MyD88, myeloid differentiation factor-88; PAMP, pathogen-associated molecular pattern; LPS, lipopolysaccharide, PGN, peptidoglycan, NF-κB, nuclear factor-κB; IL-1α(β) interleukin-1α(β); ICAM-1, intercellular adhesion molecule-1
I. INTRODUCTION
Toll gene was first discovered as encoding for a receptor responsible for the dorsal ventral pattern in Drosophila embryos (Lien and Ingalls, 2002). Toll-like receptors (TLRs) are type I integral membrane glycoproteins, they are members of a larger superfamily that includes the interleukin-1 receptors (IL-1Rs). TLRs are expressed by various cells of the innate immune system, such as monocytes, macrophages, neutrophils, and dendritic cells. Eleven members of the TLR family have been identified in mammals and TLR2 and TLR4 have been studied most extensively. TLRs recognize various but specific microbial ligands, which have been given the general name of pathogen-associated molecular patterns (PAMPs), act via TLRs (O’Neill, 2003). TLR2 plays an important role in the innate recognition of ligands associated with gram-positive bacteria. Ligands recognized by TLR2 are peptidoglycan (PGN) and lipoteichoic acid (LTA). TLR4, together with CD14, recognizes lipopolysaccharides (LPS) on gram-negative bacteria (Baker et al, 2003). Recently, it has also been shown that TLR4 is a possible receptor for endogenous factors released during tissue injury and inflammation, such as heat shock protein (HSP) 60 (Ohashi et al, 2000) and fibronectin fragments (Okamura et al, 2001). When TLRs are activated by ligand exposure, they form dimer and activate nuclear factor-κB (NF-κB) pathway and mitogen-activated protein kinase (MAPK) pathway. Activated NF-κB is translocated in the nucleus and regulates transcription of inflammatory cytokine-related genes. Roles for TLRs are emerging in conditions such as systemic lupus erythromatosis, artherosclerosis, rheumatoid arthritis, asthma and cardiovascular diseases (O'Neill, 2003; de Kleijin and Pasterkamp, 2003; Edfeldt et al, 2002). These reports suggest that TLRs may be a potential therapeutic target in inflammatory diseases as well as in infectious diseases.
Skin is a complex organ that serves as the interfaced between the human host and the environment. It functions as apart of the immune system by playing a role in both non-specific and non-specific immune responses. The epidermis, the outermost skin layer, provides
the first line of defense against the external environment and keratinocyte is the main cell type in epidermis. The epidermal keratinocytes contribute to the protectivebarrier of the epithelia and participate in the host defenseby killing invading microorganisms. In skin, TLRs are instruments in both launching innate immune responses and influencing adaptive immunity. Keratinocytes express TLR2 and TLR4, although they have a little different results (Kawai et al, 2002; Song et al., 2002; Pivarcsi et al, 2003; Mempel et al, 2003; Köllisch et al, 2005). Propionibacterium acnes induces IL-12 and IL-8 release from human monocytes via activation of TLR2 (Kim et al, 2002). TLR2 expression was also demonstrated in biopsied acne lesions, particularly in perifollicular regions, and TLR2 positive cells were increased with the increasing age of the lesion. In leprosy, the activation and regulation of TLR2 and TLR1 at the site of disease may contribute to the host’s defense against Mycobacterium leprae (Krutzik et al, 2005). In lesional epidermis from patients with psoriasis, TLR2 was more highly expressed on the keratinocytes of the upper epidermis than the basal layer, while TLR5 was downregulated in basal keratinocytes compared with corresponding nonlesional psoriatic epidermis (Baker et al, 2003). Curry et al (2003) found that the basal keratinocytes of psoriatic skin demonstrated a strong and diffuse expression of TLR1. In lyme disease, macrophages and monocytoid and plasmacytoid DCs all exhibited increased expression of TLR1, 2 and 4 (Salazar et al, 2003)
Recently, it was reported that melanocytes, melanosomes and melanin function to inhibit the proliferation of bacterial, fungal and other parasitic infections of the dermis and epidermis. Melanocytes are also morphologically highly dendritic, and their central localization at the basal layer of the epidermis raises the possibility that they are immunologically important (Lu et al, 2002). Lu et al (2002) have found that cultured melanocytes express low levels of immunologic surface markers such as intercellular adhesion molecule-1 (ICAM-1) and CD40. Radicals and other compounds produced during melanogenesis are believed to exert strong antimicrobial activity (Mackintosh, 2001). It was suggested that melanosomes are lysosomal structures (Schraermeyer, 1995; Kim & Choi,
1998; Le Poole et al, 1993). Functional similarity between melanosomes and lysosomes was further confirmed by Schraermeyer et al (1999). Le Poole et al (1993) demonstrated that normal melanocytes processed and presented the mycobacterial protein HSP65 and whole cell sonicate of Mycobacterium leprae (the causative agent of leprosy) to CD4+ T cells in an antigen-specific MHC Ⅱ restricted manner. They suggested melanocytes function as antigen presenting cells. Taken together, melanocytes are not only professional melanin producing cells but are also immunocompetent cells.
Bacteia inducible melanization of fish skin was demonstrated in aquarium-reared cichlids (Oreochromis mossabicus) naturally infected with the bacterium Mycobacterium marinum. Noga et al (1990) has noticed that cutaneous injuries on frogs become deeply melanized in response to injury, as is characteristic of many invertebrates. These reports suggest that melanis synthesis is related to infection and inflammation in skin. In human, a genetic link between immunity and melanization is demonstrated by a number of clinical conditions that result in albism and impared immunity (Baumeister et al, 2000; Introne et al, 1999). In addition to, Knox et al (1979) found that dark-skinned men and women were significantly less likely than those of light skin to be infected with scabies.
Therefore we hypothesized that microbial induced pigmentation may be involed in TLRs activity in human melanocytes. First, we have investigated whether TLR2 and TLR4 are expressed in melanocytes. Also, we investigated possible role of TLR2 and TLR4 during microbial infection. Finally, we studied the expression of TLR2 in vitiligo to investigate the possible role of TLR in inflammation-induced pigmentary disorders.
. M
Ⅱ
Ⅱ
Ⅱ
Ⅱ
ATERIALS AND METHODS
A. Meterials
F12, fetal bovine serum (FBS), antibiotic/antimycotic solution and 0.25% trypsin- ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco-BRL (Grand Island, NY). MCDB153, phosphate buffered saline (PBS), 3-isobutyl-1-methylxanthine (IBMX), basic fibroblast growth factor (bFGF), 12-O-tetradecanoyl phorbor 13-acetate (TPA), Vitamin E, transferrin, insulin and cholera toxin were purchased from Sigma Chemical Co. (St. Louis, MO). Specific primers for GAPDH, TLR2, TLR4, CD14, and MyD88 were purchased from Bioneer (Alameda, CA). Rabbit polyclonal anti-TLR2, TLR4 and mouse monoclonal CD14 were purchased from Santa Cruz. Biotechnology, Inc. (Santa Cruz, CA), Torrey Pines Biolabs, Inc. (Houston, TX) and Biomeda (Foster City, CA), respectively. All secondary antibodies were purchased from Zymed Laboratories (San Francisco, CA). Lipopolysaccharide (LPS) purified from E.coli (026:B6) was also purchased from Sigma.
B. Methods
1. Cell culture
Normal human melanocytes were obtained from the foreskins of individuals undergoing circumcision. After removal of the subcutaneous tissue and much of the reticular dermis, the tissue samples were cut into small strips and incubated in 0.25 % trypsin solution at 37℃ for 4 hours. The epidermis was peeled off the dermis and separated to single cells by vortex. Then melanocytes were plated in 100 mm dishes. On the following day, the dishes were changed fresh medium. Melanocytes were maintained in F12 supplemented with 10% heat-inactivated FBS, 1% antibiotic/antimycotic solution, 24 µg/ml IBMX, 80 nM TPA, 1.2 ng/ml bFGF and 0.1 µg/ml cholera toxin. The cells were maintained at 37℃ in a humidified atmosphere containing 5% CO2. The medium was changed twice a week. Melanocytes were
subcultured when confluent and used for experiments at passage 2~3. Cells were seeded in 60 mm culture dishes at 1.5 × 105 cells and grown to confluency. Before 24h of stimulation, the medium was changed to MCDB153 containing 4% heat-inactivated FBS, 1% antibiotic/antimycotic solution, 0.6 ng/ml bFGF, 5 µg/ml insulin, 1 µg/ml Vitamin E, 1 µg/ml transferrin.
2. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
RT-PCR analysis was carried out to examine whether TLR2, TLR4, CD14 and MyD88 mRNA is expressed in human melanocytes and their expression was increased in LPS treated melanocytes at different time (6, 12, 24h) compared with control (0h). Total cellular RNA was extracted from cultured melanocytes using RNeasy Mini Kit (Qiagen Inc., Valencia, CA) and was quantified by measuring the optical density at 260 nm. First strand cDNA was synthesized from 1 µg total RNA in a 13 µl volume by using dNTP and OligodT primer (iNtRON, Korea). The samples were incubated at 65℃ for 5 min. The cDNA was then amplified in a 20 µl final volume using SuperScriptTM Ⅲ (Invitrogen, USA) following the recommendations of the manufacturer. SuperScriptTM Ⅲ contains 5ⅹ First-Strand Buffer, 0.1 M DTT, RNase OUTTM Recombinant RNase Inhibitor, and SuperScriptTM Ⅲ RT (200 units/µl). The reaction was incubated at 55℃ for 1 hour, and terminated by 72℃ for 15 min. RT-PCR analyses were carried out in a reaction mixture (Bioneer, Alameda, CA) containing, in a final volume of 20 µl, 1U Taq polymerase, 250 µM dNTP, 10 mM Tris-HCl (pH 9.0), 40 mM KCl, 1.5 mM MgCl2 and 10 pmol primers for TLR2, TLR4, CD14 and MyD88
(Bioneer). For the co-amplification primers for glyceraldehydes-3-phosphate (GAPDH) were used. The reaction was carried out in a DNA Thermal Cycler (model # : RTC-200, MJ Research, MA) using the following primer sets, these primers are based on the published sequences : The TLR2 primer sequences used were 5’-GCCAAAGTCTTGATTGATTGG-3’ for the sense primer and 5’-TTGAAGTTCTCCAGCTCCTG-3’ for the antisense primer (Zhang et al, 1999). The TLR4 primer sequences used were
5’-GCTTACTTTCACTTCCA-ACAA-3’ for the sense primer and 5’-CAATCACCTTTCGGCTTTTAT-3’ for the antisense primer (Song et al, 2002). The CD14 primer sequences used were 5’-CGTGGGCGACAGG-GCGTTCT-3’ for the sense primer and 5’-TAAAGGTGGGGCAAAGGGTT-3’ for the antisense primer (Song et al, 2001). The MyD88 primer sequences used were 5’- TAAGAAGGACCAGCAGAGCC-3’ for the sense primer and 5’-CATGTAGTCCAGCA-ACAGCC-3’ for the antisense primer (Baroni et al, 2005). The GAPDH primer sequences used were 5’-GAAGGTGAAGGTCGGAGTCAACG-3’ for the sense primer and 5’- AGTCCTTCCACGATACCAAAGTTG-3’ for the antisense primer. The reaction was performed following condition : TLR2, TLR4 and CD14 : 30 cycles at 94℃ for 30s, 54℃ for 30s and 72℃ for 30s. MyD88 : 34 cycles at 94℃ for 30s, 63℃ for 40s and 72℃ for 2 min. GAPDH : 28 cycles at 94℃ for 30s, 56℃ for 30s and 72℃ for 30s. The PCR products were analysed by electrophoresis on 1% agarose gel in Tris-Acetate-EDTA (TAE) buffer. The identity of the amplification products was confirmed by comparing their size with the size expected from the known gene sequence. To quantify the expression of the transcripts, the intensities of the PCR bands were measured by densitometry using Image-Pro Plus Version 4.5 (Media Cybertics Co, MD) and are expressed as intensities relative to GAPDH.
3. Flow cytometry
Human melanocytes were stained with primary monoclonal antibodies specific for TLR2, TLR4, and CD14 conjugated with FITC for 30 min at room temperature. After washing, cells were suspended in PBS and immediately analyzed on a FACScan flow cytometer (Becton-Dickinson, San Jose, CA).
4. Western blotting
Western blotting analysis was carried out to examine whether the expression of TLR2, TLR4 protein was increased in LPS (10 µg/ml) treated melanocytes at different time (12, 24, 36h) compared with control (0h). Human melanocytes were lysed in RIPA buffer (1% NP-40, 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA) with 10 µg/ml aprotinin, 1 mM sodium orthovanadate and 100 µg/ml Phenylmethylsulphonylfluoride (PMSF) and separated with a 10% SDS-PAGE gel. The protein was then transferred onto a polyxinylidene difluoride (PVDF) membrane, and then the membrane was probed with anti-TLR2, TLR4, and CD14 antibodies. After incubation with horseradish peroxidase-conjugated goat anti-rabbit (TLR2 and TLR4) and goat anti-mouse (CD14) antibody, the membrane was developed with an enhanced chemiluminescence (ECL) detection kit.
5. Immunocytochemistry
Melanocytes grown on Laboratory-Tek chambers (Nalge Nunc International, Naperville, IL) were fixed in 4% paraformaldehyde for 30 min at room temperature, and permeated in methanol followed by 0.1% Triton X-100 to achieve a nuclear permeance. Slides were placed in methanol containing 0.3% hydrogen peroxide for 10 min and the nonspecific activity was blocked by normal goat serum for 10 min. Then they were incubated with rabbit polyclonal anti-TLR2, TLR4 and mouse monoclonal anti-CD14 antibodies overnight at 4℃ at 1:50 dilution. Biotinylated antibody against both mouse and rabbit (Dako, Carpinteria, CA) was incubated for 20 min at room temperature. The substrate chromogen 3-amino-9-ethyl-carbazol (Biomeda Corp., Foster City, CA) was applied for 20 min. Negative controls were made by applying PBS instead of the primary antibody. They consistently yielded negative results.
6. Melanocyte growth assay
Melanocytes were plated at a density of 1.5×105 cells in 60 mm culture dishes. Before 24h of stimulation, the medium was changed to MCDB153 containing 4% FBS and 8 nM TPA with other supplements like above. Melanocytes were stimulated with 5, 10 µg/ml LPS. After 5 days of stimulation, melanocytes were collected using 0.25% trypsin-EDTA. After harvesting, the cell numbers were counted with Coulter counter (ZM Coulter Co., England).
7. Melanin content determination
After cell counting, 1×105 cells were spun down and supernatant was discarded. The pellet was solubilized in 1 N NaOH and incubated in 37℃ for 90 min. The optical densities were measured at 490 nm using an enzyme-linked immunosorbent assay (ELISA) reader, Model 680 (Bio-Rad, USA). The absorbance was compared with a standard curve.
8. Immunohistochemistry
Normal and lesion skin were appreciatively provided vitiligo patients who attended the Department of Dermatology, Ajou University Hospital, Suwon, Korea. Two millimeter punch biopsies from lesional and normal appearing skin was done. Tissues were prepared for light microscopic study by 10% formalin fixation. 4 µm paraffin-embeded sections of both lesional and normal skin were mounted on Polysine microscope slide (Menzel-Glaser, Germany) coated with 0.1% poly p-lysine. Tissues were deparaffinized and rehydrated by sequential immersion in xylene, graded concentrations of ethanol, and distilled water. They were incubated for 30 min at room temperature in a solution of 0.5% hydrogen peroxidase in methanol to quench endogenous peroxides activity, followed by washing three times in Tris-buffered saline (TBS, 0.1 M, pH 7.4, Dako, Carpinteria, CA). After washing three times in TBS, they were flooded with a protein-blocking agent (PBA, Immunon, Pittsburgh, PA) for 10 min at room temperature. Excess PBA was drained and the TLR2 primary antibodies
were applied to the tissue sections (1:200). The slides were then incubated at 4℃, over night. Following three times washing in TBS, sections were incubated for 30 min at room temperature while being flooded with a anti-rabbit biotinylated universal secondary antibody reagent (Immunon). The slides were then washed in TBS, followed by incubation in streptavidin alkaline phosphatase reagent (Immunon) for 30 min. After washing in TBS, sections were incubated in fast red chromogen (Immunon) for 10 min. The sections were counterstained with haematoxylin modified solution (Merck, Darmstadt, Germany) and mounted in an aqueous mounting medium (Biomeda, Forster City, CA). The image analysis was evaluated using Image Pro Plus Version 4.5 (Media Cybertics Co., MD). The stained area per epidermal area (SA/EA) was measured in normal appearing and hypopigmented skin.
9. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical significance was tested
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS
A. Human melanocytes express TLR2, TLR4, CD14 and MyD88 mRNA
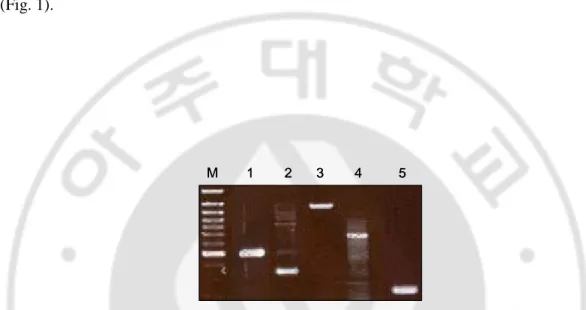
To investigate whether TLR2, TLR4 and their adapter molecules, CD14 and MyD88 are constitutively expressed in cultured human melanocytes, we performed RT-PCR analysis. The result showed that human melanocytes express TLR2, TLR4, CD14 and MyD88 mRNA (Fig. 1).
M 1 2 3 4 5
M 1 2 3 4 5
Fig. 1. Human melanocytes express TLR2, TLR4, CD14 and MyD88 mRNA. Human
melanocytes were cultured by approximately 80% confluency and RNA was obtained. RT-PCR analysis showed TLR2 (lane 2 : 394 bp), TLR4 (lane 3 : 1139 bp) and their adapter molecules, CD14 (lane 4 : 777 bp) and MyD88 (lane 5 : 200 bp) mRNA was expressed in human melanocytes. Lane 1 : GAPDH (516 bp), M : molecular weight marker.
B. Human melanocytes express cell surface TLR2, TLR4 and CD14
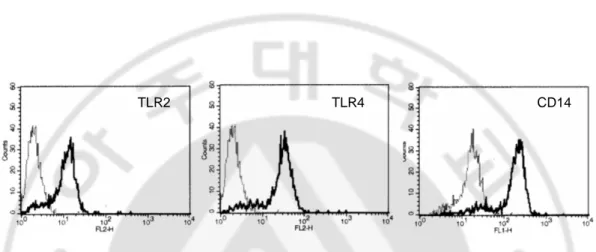
We then examined the surface expression of TLR2, TLR4 and CD14 in human melanocytes using flow cytometric analysis. Figure 2 showed that TLR2, TLR4 and CD14 were expressed in cell surface of melanocytes indicating human melanocytes not only expressed TLR2, TLR4 and CD14 mRNA but also expressed cell surface TLR2, TLR4 and CD14.
TLR2 TLR4 CD14
TLR2 TLR4 CD14
Fig. 2. TLR2, TLR4 and CD14 were expressed in surface human melanocytes. Surface
expression of TLR2, TLR4 and CD14 on human melanocytes was assessed by flow cytometry. These proteins expression on the surface of human melanocytes is shown in each shaded histogram using TLR2, TLR4 and CD14 specific antibodies.
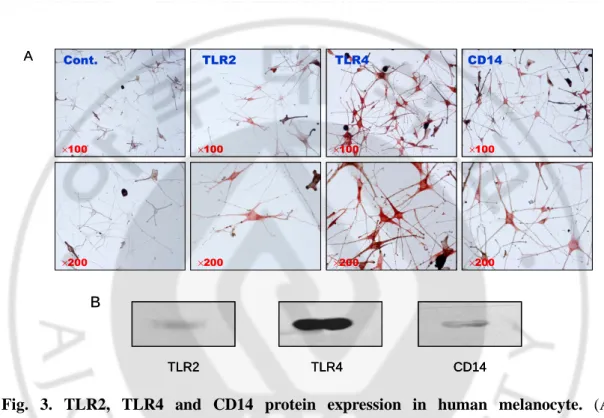
We then examined whether TLR2, TLR4 and CD14 protein could be detected in cultured melanocytes. Immunocytochemical staining showed immunoreactivity for anti-TLR2, TLR4 and CD14 proteins in human melanocytes (Fig. 3A). The expression of TLR2, TLR4 and CD14 protein was further confirmed by western blot analysis (Fig. 3B).
Cont. TLR2 TLR4 CD14 ×100 ×100 ×100 ×100 ×200 ×200 ×200 ×200 A Cont. TLR2 TLR4 CD14 ×100 ×100 ×100 ×100 ×200 ×200 ×200 ×200 A TLR2 TLR4 CD14 B TLR2 TLR4 CD14 TLR2 TLR4 CD14 B
Fig. 3. TLR2, TLR4 and CD14 protein expression in human melanocyte. (A)
Immunocytochemistry was carried out with melanocyte TLR2, TLR4 and CD14 specific antibodies. These proteins were stained positively on cell surface. In control study human melanocytes were treated with only a secondary biotin-conjugated anti-rabbit IgG. (B) Melanocyte lysates were separated by SDS-PAGE and probed with anti-TLR2, TLR4 and CD14 antibodies. Western blot analysis showed that TLR2, TLR4 and CD14 proteins are present in human melanocytes.
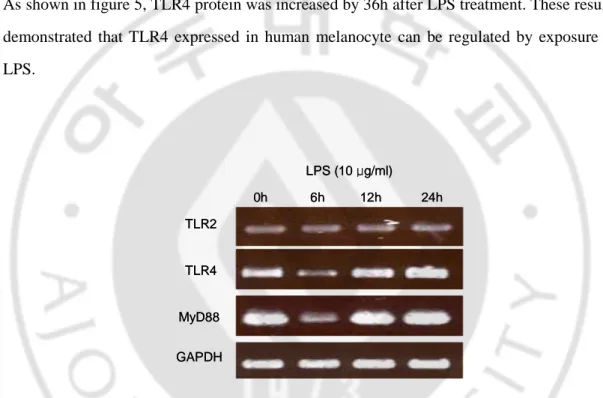
To determine whether the TLR2 and TLR4 expression was regulated by gram-negative bacterial derived LPS, the relative levels of TLR4 mRNA expression in response to LPS were analyzed by semiquantitative RT-PCR. Since MyD88 is implicated in the signaling pathway of many TLRs, the expression of MyD88 mRNA was also examined. Figure 4 showed LPS (10 µg/ml) treatment induced an early upregulation of TLR2, TLR4 and MyD88 mRNA in human melanocytes. This increment increased by 24h after the addition of the LPS. The protein expression of TLR4 was also regulated by LPS treatment (10 µg/ml). As shown in figure 5, TLR4 protein was increased by 36h after LPS treatment. These results demonstrated that TLR4 expressed in human melanocyte can be regulated by exposure to LPS. GAPDH TLR2 TLR4 MyD88 0h 6h 12h LPS (10 μg/ml) 24h GAPDH TLR2 TLR4 MyD88 0h 6h 12h LPS (10 μg/ml) 24h
Fig. 4. LPS increases TLR2, TLR4, and MyD88 mRNA in human melanocytes. TLR2,
TLR4 and MyD88 mRNA expression in human melanocytes was examined at 0, 6, 12, 24h after the addition of 10 µg/ml LPS by semiquantitative RT-PCR analysis.
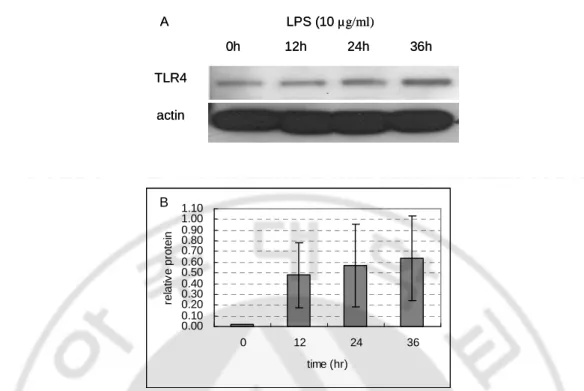
0h 12h 24h 36h LPS (10 µg/ml) TLR4 actin A 0h 12h 24h 36h LPS (10 µg/ml) TLR4 actin A 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 1.10 0 12 24 36 time (hr) re la tiv e p ro te in B 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 1.10 0 12 24 36 time (hr) re la tiv e p ro te in B
Fig. 5. LPS induces TLR4 protein in human melanocytes. Regulation of TLR4 protein
expression was investigated by western blot analysis. Melanocytes stimulated with LPS (10 µg/ml) for 0, 12, 24, 36h were lysed and probed polyclonal anti-TLR4. Anti-actin was used as an internal control. (A) Fluorogram. (B) Densitometric analysis. The data shown is representative of triplicate experiments. The values indicate TLR4/actin relative values. They are expressed as mean ± SD. Protein was gradually increased by 36h after LPS treatment.
E. LPS induces melanogenesis in human melanocytes
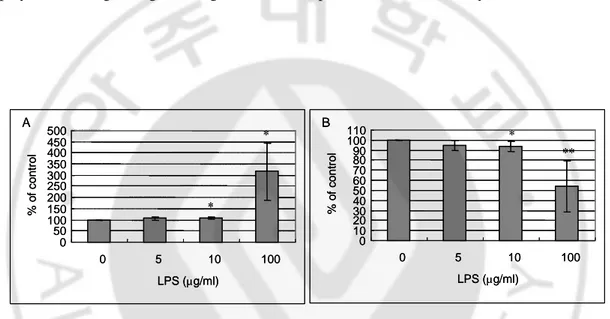
To investigate the functional role of TLR2 and TLR4 regulating melanogenesis, melanocytes were treated with LPS (1-100 µg/ml) for 5 days. The melanin content was induced by 106 ± 7 % in cells treated with 10 µg/ml LPS compared with control (100%) (Fig. 6A). LPS inhibited melanocyte proliferation in a concentration-dependent fashion (Fig. 6B) (mean ± SD, n=5, *p<0.05, **p<0.001). Treating 100 µg/ml LPS, melanin content was significantly increased but it would be cytotoxicity. These results indicate that TLR4 may play a role in regulating melanogenesis and cell proliferation in melanocytes.
A B 0 50 100 150 200 250 300 350 400 450 500 0 5 10 100 LPS (µg/ml) % o f c o n tr o l 0 10 20 30 40 50 60 70 80 90 100 110 0 5 10 100 LPS (µg/ml) % o f c o n tr o l * ** * * A B 0 50 100 150 200 250 300 350 400 450 500 0 5 10 100 LPS (µg/ml) % o f c o n tr o l 0 10 20 30 40 50 60 70 80 90 100 110 0 5 10 100 LPS (µg/ml) % o f c o n tr o l * ** * *
Fig. 6. LPS stimulation increased melanin synthesis and decreased cell proliferation in
human melanocytes. 1.5 × 105 melanocytes were grown to confluency and treated with 0, 5, 10, 100 µg/ml LPS for 5 days. After harvesting, melanin assay and cell counting were performed. The values indicate percentage of control. (A) Melanin assay. 1 × 105 cells were solubilized in 1N NaOH and measured at 490 nm. 10 µg/ml LPS stimulation significantly increased melanin content. (B) Cell counting. 10 µg/ml LPS stimulation significantly decreased cell proliferation. Columns show the mean ± SD (n=5, *p< 0.05, **p<0.001, ).
F. Expression of TLR2 in vitiligo
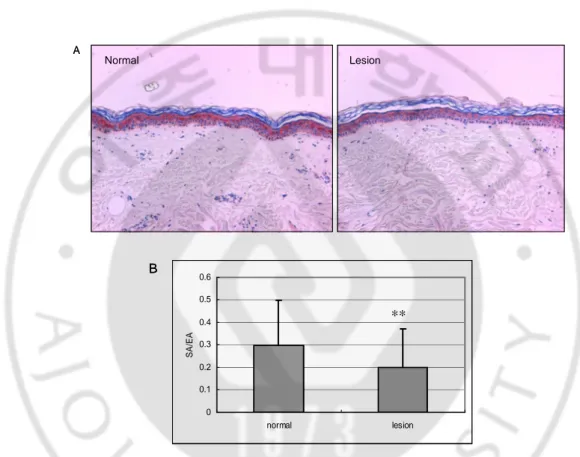
To investigate the role of TLR in pigmentary disorder, we compared the expression of TLR2 in vitiligo lesion and perilesional normal skin (n=50) by immunohistochemistry (Fig. 7A). Image analysis showed a significant decrease of SA/EA in lesion (0.20±0.17) as compared with that in normal skin (0.30±0.20) (**p<0.001) (Fig. 7B).
Normal A Lesion Normal A Lesion 0 0.1 0.2 0.3 0.4 0.5 0.6 normal lesion S A /E A ∗∗ B 0 0.1 0.2 0.3 0.4 0.5 0.6 normal lesion S A /E A ∗∗ B
Fig. 7. Immunohistochemical analysis of TLR2 expression in vitiligo. (A) The expression
of TLR2 in epidermis of vitiligo lesion was decreased compared with perilesional normal skin. Magnification × 200. (B) SA/EA value of TLR2 immunostaining. Data are expressed as mean ± SD. (n=50, ∗∗P<0.001.)
Ⅳ
Ⅳ
Ⅳ
Ⅳ. DISCUSSION
In this study, we have demonstrated that the TLR2 and TLR4 are expressed in human melanocytes both at the mRNA and protein levels. We also showed that LPS induced TLR2, TLR4 expression in melanocytes, suggesting that the effect of LPS on melanocytes is mediated by TLR4. CD14, which is known to associate with TLR4, could also be involved in the mediation of LPS effects in melanocytes. We demonstrated that melanocytes constitutively express CD14. CD14 has a role as adapter molecule to increase the signaling sensitivity for LPS by the TLR4. CD14 is a 55-kDa glycosyl phosphatidylinositol (GPI)-anchored glycoprotein identified on the surface of monocytes, macrophages, and polymorphonuclear leukocytes (PMNs) (Song et al, 2001). TLR4, together with CD14, recognizes LPS of gram-negative bacteria. MyD88 is required for the intracellular signaling of TLRs. The signaling pathway of the TLR family leads to the activation of NF-κB and MAPK (p38 and JNK) through the adaptor proteins MyD88 and IRAK (Zhang and Ghosh, 2002; Akira and Takeda, 2004). We have shown here for the first time that MyD88 mRNA is also expressed and regulated by LPS treatment in melanocytes.
It is now well established that TLR4 mediated signaling events upon LPS stimulation (Molteni et al, 2006; Poltorak et al, 1998). LPS, a major component of the outer membrane of gram-negative bacteria. LPS and other bacterial or viral by-products bind to a family of specific receptors, the TLRs, which regulate both innate and adaptive immunity. Exposure to bacterial compounds such as LPS increased the expression of TLR4 in several cells including human keratinocytes and monocytes (Song et al, 2002, Guha and Mackman, 2001). Some studies showed that LPS increased TLR2 expression and LPS stimulation of TLR2 initiated an interleukin 1 receptor-like NF-κB signaling cascade. These demonstrated that TLR2 also participated in sensing LPS (Yang et al, 1998; Kirschning et al, 1998). We
increased after stimulation with LPS. LPS also increased the expression of MyD88. We have also demonstrated that incubation of cultured melanocytes with LPS increased melanin, suggesting gram-negative microbial infection induces melanogenesis. It was suggested that human melanocytes produce nitric oxide in response to LPS (Tsatmali et al, 2000). Tsatmali et al. further found that melanin synthesis was increased in cultured human melanocytes in response to increased levels of nitric oxide, suggesting LPS up regulated both melanogenesis and nitric oxide production for antimicrobial role. These findings including ours are in keeping with an antimicrobial role for melanin. The expression of TLR4 mRNA in melanoma cells was shown recently by Molteni et al. The authors showed that LPS significantly up-regulated the production of IL-8 of the cells and suggested direct link between LPS and tumor cell metastasis. Because IL-8 is an important chemotactic factor, we presume that the induction of IL-8 in melanoma cells by pathogens could lead to the recruitment of inflammatory cells to the site of infection (Molteni et al, 2006). Indeed, melanocyte TLR4 may recognize pathogens leads to the production of melanin, which might be responsible for the defense mechanism. Our findings stress the importance of the melanocytes as a component of the innate immune response.
Melanocytes are not simply pigment-producing cells, but produce substances with a range of biological functions, including structural strengthening by cross-linking proteins, antimicrobial defense, photon shielding, and chemoprotection (Burkhart CG and Burkhart CN, 2005). There is evidence that a major function of melanocytes, melanosomes, and melanin in human skin is to inhibit the proliferation of bacterial, fungal, and parastitic infections in the epidermis and dermis (Mackintosh, 2001).
We are planning to study about role of TLRs during inflammatory stimulation, such as TNF-α or UVB irradiation. Because the inflammatory signaling including TNF-α may activate TLR, TLRs may play a role in inflammation induced pigmentary disorders. We observed expression of TLR2 was significantly decreased in vitiligo compared with in
melanocytes from the cutaneous epidermis. Although the exact etiology of vitiligo has not yet been established, the abnormal immune responses frequently observed in vitiligo patients have led to the suggestion that, in some cases, the condition has an autoimmune component (Kemp et al, 2001). Because vitiligo skin usually has no melanocytes, this decreased TLR2 expression may be on keratinocytes. It was reported that vitiliginous keratinocytes as well as melanocytes are more vulnerable to assaults from extracellular factors. Vitiligo skin having decreased TLR may not respond well against inflammatory insults compared with normal skin. Further study needs to delineate the role for TLR2 in vitiligo skin. Also, the role of TLRs in inflammation-induced pigmentary disorders required further investigation. Release of inflammatory mediators and cytokines from inflammatory cells, as well as epidermal cells and melanocytes play a role in inflammatory induced pigmentation. A study performed by Halder et al concerning acne in darker skin showed a high degree of histologic inflammation in lesions that did not show significant clinical inflammation.
Our finding suggested that microbial infection stimulates melanin production in human melanocytes. This increased melanogenesis may play a role in antimicrobial immunity. In summary, here we demonstrated that TLR2 and 4 are expressed in human melanocytes and TLR4 are involved in LPS-induced melanogeneis. TLRs would be important therapeutic target on microbial or inflammatory induced cutaneous pigmentation.
In this study, we investigated that TLR2, TLR4, and CD14 were expressed in cultured human melanocytes at mRNA and protein levels. mRNA expression of MyD88 which is TLRs adapter molecule, was also demonstrated in melanocytes. And we studied LPS increased the expression of TLR2, TLR4 and MyD88. This LPS induced pigmentation, suggesting that TLR4 may play a role in microbial-induced melanogenesis. TLR2 expression was decreased in vitiligo in vivo, suggesting that TLRs may play role in the inflammation-induced pigmentary disorders.
1. Akira S, Takeda K: Toll-like receptor signalling. Nat Rev Immunol 4:499-511, 2004
2. Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L: Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: Modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 148:670-679, 2003
3. Baroni A, Orlando M, Donnarumma G, Farro P, Iovene MR, Turano MA, Buommino E: Toll-like receptor 2 (TLR2) mediates intracellular signaling in human keratinocytes in response to Malassezia furfur. Arch Dermatol Res 297:280-288, 2006
4. Baumeister FA, Stachel D, Schuster F, Schmid I, Schaller M, Wolff H, Weiss M, Belohradsky BH: Accelerated phase in partial albinism with immunodeficiency (Griscelli syndrome): genetics and stem cell transplantation in a 2-month-old-girl.
Eur J Pediatr 159:74-78, 2000
5. Burkhart CG, Burkhart CN: The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection).
Int J Dermatol 44:340-342, 2005
6. Curry JL, Qin JZ, Bonish B, Carrick R, Bacon P, Panella J, Robinson J, Nickoloff
BJ: Innate immune-related receptors in normal and psoriatic skin. Arch Pathol Lab
Med 127:178-186, 2003
8. Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ: Expression of Toll-like receptors in human atherosclerotic lesions. Circulation 105:1158-1161, 2002
9. Guha M, Mackman: LPS induction of gene expression in human monocytes. Cell
Signal 13:85-94, 2001
10. Halder RM, Brooks HL, Callender VD: Acne in ethnic skin. Dermatol Clin 21(4):609-615, 2003
11. Introne W, Boissy RE, Gahl WA: Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab 68:283-303, 1999
12. Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M: Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci 30:185-194, 2002
13. Kemp EH, Waterman EA, Weetman AP: Immunological pathomechanisms in vitiligo. Expert Rev Mol Med 23;1-22, 2001
14. Kim IT, Choi JB: Melanosomes of retinal pigment epithelium--distribution, shape, and acid phosphatase activity. Korean J Ophthalmol 12(2):85-91, 1998
15. Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, Brightbill HD, Holland D, Cunliffe WJ, Akira S, Sieling PA, Godowski PJ, Modlin RL: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol
16. Kirschning CJ, Wesche H, Ayres TM, Rothe M: Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 188(1):2091-2097, 1998
17. Knox DW, Yoder F, Kramer M: Incidence of scabies in white and black populations.
Arch Dermatol 115:1286, 1979
18. Köllisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M: Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 114:531-541, 2005
19. Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL: Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med 9:525-532, 2003
20. Le Poole IC, van den Wijngaard RM, Westerhof W, Verkruisen RP, Dutrieux RP, Dingemans KP, Das PK: Phagocytosis by normal human melanocytes in vitro. Exp
Cell Res 205:388-395, 1993
21. Lien E, Ingalls RR: Toll like receptors. Crit Care Med 30(suppl):S1-S11, 2002
22. Lu Y, Zhu WY, Tan C, Yu GH, Gu JX: Melanocytes are potential immunocompetent cells: Evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment Cell Res 15:454-460, 2002
melanin and the evolution of black skin. J Theor Biol 211:101-113, 2001
24. Mempel M, Voelcker V, Köllisch G, Plank C, Rad R, Gerhard M, Schnopp C, Frnaunberger P, Walli AK, Ring J, Abeck D, Ollert M: Toll-like receptor expression in human keratinocytes: Nuclear factor κB controlled gene activation by
Staphylococcus aureus is Toll-like receptor 2 but not Toll-like receptor 4 or platelet
activating factor receptor dependent. J Invest Dermatol 121:1389-1396, 2003
25. Molteni M, Marabella D, Orlandi C, Rossetti C: Melanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4. Cancer Lett 235(1):75-83, 2005
26. Noga EJ, Wright JF, Pasarell L: Some unusual features of mycobacteriosis in the cichlid fish Oreochromis mossambicus. J Comp Pathol 102:335-344, 1990
27. Ohashi K, Burkart V, Flohe S, Kolb H: Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol 164:558-561, 2000
28. Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF Ⅲ: The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem
276:10229-10233, 2001
29. O’Neill AJ: Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol 3:396-403, 2003
function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol 15(6):721-730, 2003
31. Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282:2085-2088, 1998
32. Salazar JC, Pope CD, Sellati TJ, Feder HM Jr, Kiely TG, Dardick KR, Buckman RL, Moore MW, Caimano MJ, Pope JG, Krause PJ, Radolf JD: Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol 171:2660-2670, 2003
33. Schraermeyer U: Transport of endocytosed material into melanin granules in cultured choroidal melanocytes of cattle--new insights into the relationship of melanosomes with lysosomes. Pigment Cell Res 8:209-214, 1995
34. Schraermeyer U, Peter S, Thumann G, Kociok N, Heimann K: Melanin granules of retinal pigment epithelium are connected with the lysosomal degradation pathway.
Exp Eye Res 68(2):237-245, 1999
35. Song PI, Abraham TA, Park Y, Zivony AS, Harten B, Edelhauser HF, Ward SL, Armstrong CA, Ansel JC: The expression of functional LPS receptor proteins CD14 and Toll-like receptor 4 in human corneal cells. Invest Ophth Vis Sci 42:2867-2877, 2001
Ansel JC: Human keratinocytes express functional CD14 and Toll-like receptor 4. J
Invest Dermatol 119:424-432, 2002
37. Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, McNeil CJ, Graham AM, Thody AJ: α-Melanocyte-stimulating hormone modulates nitric oxide production in melanocytes. J Invest Dermatol 114:520-526, 2000
38. Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ: Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288, 1998
39. Zhang G, Ghosh S: Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem 277(9):7059-7065, 2002
인간
인간
인간
인간 멜라닌
멜라닌
멜라닌
멜라닌 세포에서
세포에서
세포에서
세포에서 Toll
Toll
Toll-
Toll
-Like Receptor 2
-
-
Like Receptor 2
Like Receptor 2
Like Receptor 2와
와
와
와 4
4
4의
4
의
의
의
발현과
발현과
발현과
발현과 기능
기능
기능
기능
아주대학교 대학원의학과 안 주 희 (지도교수: 강 희 영) Toll-like receptors (TLRs)는 선천성 면역 체계에서 중요한 역할을 하는 세포막에 존재하는 수용체이다. 피부에서는 미생물과 같은 외부 병원체에 의한 세균성 감염질환 외에 건선이나 여드름 등의 만성 염증성 질환의 병인에 TLR 이 중요한 역할을 할 것으로 생각되고 있으며 외부 병원체에 대한 1 차 방어벽인 각질형성세포에서 TLR 의 역할이 밝혀지고 있다. 최근 멜라닌 세포가 멜라닌 형성뿐 아니라 자가면역에 관여한다는 사실이 보고되었고, 멜라닌 색소의 자외선 방어의 역할 뿐만 아니라 외부 미생물에 저항하는 기능이 보고되었다. 따라서 본 연구에서는 멜라닌 세포에서 세균감염방어에 있어서의 TLR 의 역할을 알아보고자 하였다. 먼저 인간 멜라닌 세포에서 TLR2, TLR4 와 adapter molecule 인 CD14 과 MyD88 이 발현됨을 확인하였다. 멜라닌 세포에 병원체 산물인 Lipopolysaccharide (LPS)를 처리한 결과 TLR2, TLR4 의 발현이 증가하였다.결과는 멜라닌 세포에 존재하는 TLR2 또는 TLR4 가 병원체 감염에 의한
멜라닌 합성증가에 관여할 것을 시사하였다. 또한 TLR2 의 발현이 백반증
환자의 병변 표피 조직에서 인접 정상 표피보다 현저히 감소되어 있었는데, 이
결과는 염증성 색소질환의 병인에 TLR 의 역할을 시사하였다.
핵심어 : Toll-like receptor 2, 4, Lipopolysaccharide, 멜라닌 세포, 멜라닌