저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Characteristic analysis of mobility inducers for
neural stem cells
by
Jung Mi Kim
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Characteristic analysis of mobility inducers for
neural stem cells
by
Jung Mi Kim
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for
the Degree of Master in NeuroScience
Supervised by
Myung Ae Lee, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Jung Mi Kim is approved.
SUPERVISORY COMMITTEE
( Sign)
Myung Ae Lee
(Sign)
Hye Sun Kim
(Sign)
Joon Kyu Lee
The Graduate School, Ajou University
December, 14th, 2012
ACKNOWLEDGEMENTS
먼저 타지에서 올라와 많은 것이 부족했던 저에게 지속적으로 진심 어린 조언과 아낌없는 지도를 해주고 제가 이 자리에 올 수 있도록 이끌어 주신 이명애 교수님께 진심으로 존경과 감사의 말씀을 전하고 싶습니다. 또한, 이 논문에 대하여 자상한 조언과 심사를 해주신 김혜선 교수님과 이준규 교수님께도 감사의 말씀을 전하고 싶습니다. 처음 올라와 아무것도 모르는 저에게 실험뿐 아니라 실험실 생활에 대한 전반적인 조언을 아낌없이 해주신 정용오빠, 실험적인 것뿐 아니라 모든 것을 친절하게 가르쳐 주신 친언니 같았던 현숙언니, 묵묵하게 열심히 실험하시는 듬직한 민웅오빠, 꼬맹이라고 예뻐해 주시고 실험실 생활을 재미있고 활기차게 지낼 수 있도록 도와주신 정아언니, 실험적 조언을 아끼지 않으셨던 황동훈 박사님, 최준영선생님, 언제나 멋진 민정언니, 재미있는 두현오빠, 같은 나이지만 언니 같은 혜영이, 항상 긍정적인 이쁜 은아, 서로 의지하고 큰 힘이 되었던 영원한 동기 진하, 나이. 힘이 들 때면 위로해주고, 기쁜 일은 같이 기뻐해주며 나에게 힘이 되어주었던 모두에게 감사하다는 말씀을 전하고 싶습니다. 또 멀리 부산에서, 대전에서 같은 석사생활 하며 많은 대화를 나눴던 경빈이 그리고 송이와 나를 응원해주고 웃게 해준 모든 친구들에게 항상 더 좋은 일만 생기길 바랍니다. 그리고 항상 옆에서 큰 힘이 되어주고 의지가 되어준 언석오빠에게도 고맙다는 말을 전하고 싶습니다. 마지막으로 대학원에 들어 올 수 있게 지원해주시고 저를 믿어주신 우리 가족들 아빠, 엄마, 오빠 사랑합니다. 진심으로 감사 드립니다.i
- ABSTRACT -
Characteristic analysis of mobility inducers for neural stem cells
Recently neural stem cells (NSCs) were reported to migrate to damaged brain areas, such as brain tumors or ischemic regions. Systemic strategy can be effective to cancer therapeutic effect through cell-based gene therapy. From our previous studies, we identified chemoattractant molecules-1(CM-1) and CM-2 as novel chemoattractants that induced tumor-tropism of neural stem cells, and showed that CM-1 accelerated tumor tropism of human NSCs encoding therapeutic gene and increased targeting in vivo model of rats. Previous our results showed that in rats transplanted with NIH 3T3 cells overexpressing CM-1 and glioma cells, the edge of tumor mass did not infiltrate and invasive tumor cells were eliminated from surrounding region compared with only glioma cells. So, we examined that CM-1 bound to the integrin αvβ5, known as candidate CM-1 receptor in NSCs and resulted in promoting activation of signal pathway for mobility. In migration assay using functional blocking antibody, we identified that integrin αvβ5 interacted with CM-1 and induced functional cell migration in human glioblastoma primary cultured cells and rat glioma cells as well as human NSCs. In the presence of CM-1, NSC migrated toward opposite area significantly compare with control group. According to that, CM-1 made brain cancer stem cells to assemble toward tumor mass. To investigate whether CM-1-induced NSC migration can have in vivo therapeutic efficacy, we determined survival time of brain tumor animal model transplanted with NSC harboring suicidal gene and CM-1. The result of the in vivo
ii
experiment for CM-1 showed that human NSCs encoding cytosine deaminase (CD) migrated towards co-transplantation sites of NIH3T3 cells overexpressing CM-1 and glioma cells compared to only glioma cells. In addition, bystander effect by repetitive infusion of prodrug, 5-fluorocytosine (5-FC), reduced tumor mass with increasing number of CM-2-expressing cells. Transplanted group of CM-1-producing NIH 3T3 cells significantly extended survival time over 105 days than transplantation of glioma cells. PET imaging data showed that increase of survival correlated with reduction of tumor mass volume.
Our previous study showed that, the other Chemoattractants molecule, CM-2 induced NSCs migration by interacting with CD63 and CM-2-CD63complex bound to integrin β1. To investigate the detailed molecular mechanism for NSCs migration by CM-2, in migration assay, we confirmed that integrin β1 and PI3K signaling pathways were implicated in the induction of NSCs migration via CM-2. We investigated phosphorylation of tyrosine 397 site of FAK by using CD63-downregulated HB1.F3 cells with shRNA strategy and pREP4 F3 cells. Our results showed that human NSCs activated phosphorylation of FAK by CM-2 for migration with the exception of shCD63 knockout NSCs.
These findings provide us an important step towards the development of new NSCs based gene therapies to cure brain tumor and hold promise for use as a therapeutic approach to treat aggressively invasive gliomas such as glioblastoma multiforme.
Key words: Neural stem cell, Tumor tropism, Stem cell therapy, Glioblastoma multiforme (GBM), Mobility inducers for neural stem cells.
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FITURES ··· Ⅴ Ⅰ. INTRODUCTION ··· 1Ⅱ. MATERIAL AND METHODS ··· 6
A. Cells and cell culture ··· 6
B. Brain tumor animal model ··· 6
C. 5-FC treatment ··· 7
D. PET scan and tumor volume evaluation ··· 7
E. Blocking antibody migration assay ··· 8
F. Boydenchamber assay ··· 9
G. Western blot analysis ··· 9
Ⅲ. RESULTS ··· 11
A. Cancer stem cells induced migration by 1 via integrin αvβ5 as receptor of CM-1 as well as NSCs ··· CM-1CM-1
B. Systemic strategy using CM-1 as chemoattractants and CD-5-FC was effective to prolong survival time ··· 15
C. CM-2 was crucial interaction with integrin β1 in induction of NSCs migration · 21 D. FAK and PI3K signal pathway were involved in the NSCs migration ··· 23
iv
Ⅴ. CONCLUSION ··· 29 REFERENCES ··· 30 국문요약 ··· 35
v
LIST OF FIGURES
Fig.1. Role of αvβ5-integrin receptors in CM-1-induced human neural stem cell, glioma
cell lines migration ··· 13
Fig.2. In vivo Experiment schedule for extensive survival period by using CM-1 treatment ··· 17
Fig.3. CM-1-induced migration of F3.CD was significantly effective to therapy of tumor in PET analysis ··· 18
Fig.4. Bystander effect of CM-1-induced migration of F3.CD was significantly decreased to tumor volume by tumor volume analysis ··· 19
Fig.5. In vivo analysis of extended survival period by therapeutic effect··· 20
Fig.6 Integrin β1 mediated functional cell migration by induction of CM-2 ··· 22
Fig.7 PI3K signal pathway controlled NSC-induced migration by CM-2 ··· 24
1
Ⅰ. INTRODUCTION
Malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) occur more frequently and lethally than other types of primary CNS tumors, having a combined incidence of 5–8/ 100,000population. (Henry et al, 2000; Louis et al, 2007) Among them, glioblastoma multiformes (GBMs) are the most frequent and malignant form of brain tumors. (Lim et al, 2011; Munshi et al, 2009) The tumor microenvironment exhibits expression of pro-inflammatory molecules that promote migration and invasion of tumor cells. (Tafani et al, 2011; Goldbrunner et al, 1998) So, glioma can infiltrate aggressively and invasively into surrounding regions. Despite combined treatment of chemotherapy and radiotherapy after surgical excision, the median survival time after diagnosis is still in the range of just 14 months. (Smith et al, 2000; Ohgaki et al., 2004; Yamanaka et al, 2009) Moreover, although advanced in medical technology and discovery of new anticancer drugs, malignant glioma is still a serious health problem and conventional therapeutic effects on it still show poor prognosis and incurable in large proportion of patients. So, new and efficient therapy is required for malignant gliomas. In this regard, neural stem cell-based gene therapy can be regarded as a promising alternative. (SHI et al, 2009; Donghong et al, 2008; Seung U Kim, 2011)
Recent research indicates that the discovery of the inherent tumor-tropic properties of neural stem cells (NSC) can specifically target invasive solid tumors, including glioma, and thus provide a novel platform for targeted delivery of therapeutic agents to tumors and
2
overcoming the primary challenge in developing chemotherapy regimens, resulting in significant antitumor effect. (Schmidt et al, 2005; Donghong et al, 2008)
To apply this strategy to the treatment of brain tumor, the tumor-tropic properties of neural stem cells (NSCs) led to the development of a novel strategy for delivering therapeutic genes to tumors in the brain. Human neural stem cells (hNSCs) possess an inherent tumor tropism that supports their use as a reliable delivery vehicle to target therapeutic gene products to primary and secondary invasive glioma cells throughout the brain, as well as to other types of solid tumors, including melanoma brain metastases, medulloblastoma, and neuroblastoma .(Kendall et al, 2008; Heese et al, 2005; Seung U Kim, 2011) Brain tumors including malignant gliomas are known to release numerous cytokines, chemokines and growth factors that are capable of stimulating the directed migration of NSCs into the tumor environment. Immortalized hNSC lines, such as HB1.F3, are particularly well suited for the expansion of clones that stably express therapeutic genes designed to treat invasive gliomas. (Kendall et al, 2008) The novel and significant feature of neural stem cell-based gene therapy for brain tumor is that it may be viable to exploit the tumor-targeting therapy for invasive and metastatic tumors in the brain, for which no curative treatments are currently available.
A recent studies showed that NSCs have the ability to target cytosine deaminase (CD) to tumor cells, where CD can convert the systemically administrated prodrug 5-fluorocytosine (5-FC) into the toxic anticancer agent 5-fluorouracil (5-FU), which kills tumor cells in vivo by triggering apoptosis. (SHI et al, 2009; Aboody et al, 2006; Egeblad et al, 2002) Like a suicide gene therapy has been found to be effective on malignant tumors, although its
3
application is very limited. (Springer et al, 2000) In here, availability of tumor targeting can elevate suicide gene therapy by CD and 5-FC.
Tumors secrete multiple growth factors that drive activation, migration and proliferation of vascular endothelial cells, including TGF-α, bFGF and VEGF. (Folkman et al, 2003) CM-1, also named osteoblast-specific factor 2, is a kind of bone adhesion molecule that regulates osteoblast adhesion and differentiation and is classified among the extracellular matrix (ECM) proteins. (Wang et al, 2012) The sequence of CM-1 contains a typical signal sequence, a cysteine-rich domain, a fourfold fasciclin 1- like (FAS-1) domain and a C-terminal domain. (Horiuchi et al, 1999; Takeshita et al, 1993) Recently, the overexpression of CM-1 has been found in various human cancers including non-small cell lung cancer, ovarian cancer, breast cancer, colon cancer, pancreatic cancer, liver cancer, oral cancer, head and neck cancer and neuroblastoma. (Shao et al, 2004; Zhu et al, 2010; Bao et al, 2004; Kudo et al, 2006; Baril et al, 2007; Riener et al, 2010) CM-1 is reported to be involved in tumor EMT, extracellular matrix degradation, tumor invasion, and distant metastasis, but the mechanism by which it operates is still unclear. (Wang et al, 2011; Morra et al, 2011)
CM-2 is multifunctional proteins that can inhibit the catalytic activity of MMPs, thus maintaining ECM homeostasis. Besides acting as proteinase inhibitors, TIMPs also modulate cell growth, apoptosis, and angiogenesis. (Lambert et al, 2004) Recent transgenic studies also showed that endogenous overexpression or suppression of CM-2 inhibited or enhanced the tumor growth in several cell types, respectively. (Martin et al, 1996; Kruger et al, 1998 and Martin et al, 1999) The CM-2 field was complicated in recent years by the finding that CM-2 is often overexpressed in many malignancies, including lung, ovarian and breast
4
cancer. (Egeblad et al, 2002) In addition, CM-2 has been shown to exert an antiangiogenic activity both in vitro and in vivo. (Johnson et al, 1994; Roberts et al, 1993)
Through microarray analysis, previous results showed that CM-1 and CM-2 were commonly over-expressed in tumor tissues of glioblastoma patients. First, CM-1 was proved to induce migration of NSCs in vitro/ in vivo and activated migration signaling pathway such as FAK and CDK5. In vivo, CM-1 by CM-1-producing NIH3T3 cells (p-NIH3T3) significantly induced migration of NSCs and NSC encoding cytosine deaminase (CD), suicide gene delivered bystander effect targeting to tumor more efficiently. Furthermore in vivo result showed obvious edges of tumor mass. So, we hypothesized that CM-1 was effective to assemble cancer stem cells and neural stem cells by integrin αvβ5 as well as prolonged survival time using systemic strategy.
The other chemoattractants CM-2, as prior studies, specifically led to migration of NSCs by interacting with CD63 as well as by implicating CDK5 and ERK signal pathway. Here, we determined more detail signaling mechanism whether integrin β1 and phosphoinositol-3-kinase (PI3K) signal pathways control induction of NSCs migration via CM-2. Moreover, phosphorylation of focal adhesion kinase (FAK) is regulated by CM-2 induced migration but not in CD63 knockouted NSCs.
In this study, we determined that CM-1 induced tumor-tropism of neural stem cells for brain tumor therapy along with NSCs encoding cytosine deaminase and prodrug 5-FC that resulted in effective increased survival in brain tumor models. Furthermore, we investigated functional mechanism involved in CM-2 induced in vitro mobility of neural stem cells. In the present study, we provide the evidence that CM-1 and CM-2 could serve as key molecules in
5
NSCs tropism and combination with genetically modified NSCs as well as CM-1 and CM-2 strongly block the infiltration of tumor cells.
6
Ⅱ. MATERIAL AND METHODS
A. Cells and cell culture
C6 (Rat glioma cells), F3 (immortalized human neural stem cells, NCSs), NIH3T3 (mouse fibroblast cells) were maintained in Dulbecco's modified Eagle's medium (DMEM, sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Hyclone), 10ug/ml penicillin-streptomycin (Gibco) and incubated at 37℃ in an incubator of 5% CO2/95%air.
F3.pREP4 and F3.shCD63 cell lines were maintained with hygromycin (invitrogen) 100ug/ml in Dulbecco's modified Eagle's medium (DMEM, sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Hyclone), 10ug/ml penicillin-streptomycin (Gibco).
B. Brain tumor animal model
The C6 glioma cell is a model for studying a form of malignant brain tumor glioblastoma multiform. Eight weeks old female SDS rat (orient) were anesthetized by 4% chloral hydrate (1ml/kg) and were stereotaxically injected into the rat right striatum; [anterior/posterior (AP) +0.4 mm, medial/lateral (ML) −3.1 mm, dorsal/ventral (DV) −5.0 mm] by 1 x 106 C6 rat glioma cells in 3 µl HBSS (groups 1 and 2 and 3, n = 8 for group1 and 2, n = 7 for group3) or 1 × 106 C6 rat glioma cells with 5 x 105 CM-1-overexpressing NIH3T3 cells (0.5x) in 3μl HBSS (group 4, n = 10) or 1 × 106 C6 rat glioma cells with 2 x 106 CM-1-overexpressing NIH3T3 cells (2x) in 3μl HBSS (group 5, n = 9) at a rate of 0.2μl/min using 26-gauge Hamilton syringe (Hamilton, Nevada, USA) attached to an automated microinjector (KD
7
scientific INC, MA, USA). Since then five days later, each group was injected with 3ul HBSS (group1, n = 8) or 1 x 106 F3.CD human NSCs in 3µl HBSS (groups 2 to 5, n = 8 for group 2, n = 7 for group 3, n = 10 for group 4, n = 9 for group) into left hemisphere cortex. Upon treatment of 5-FC and PET scan, a number of rats were calculated for survival period.
C. 5-FC treatment
Ten days after transplantation of F3.CD cells, 5-fluorocytosine (5-FC, Sigma) was intraperitoneal injected for period of three weeks. For first five days of a week, each group was injected into 0.9% normal saline (group 1 and 2) or 5-FC (500mg/kg, 15mg/ml in 0.9% normal saline, group 3 to 5) throughout two times a day and break for last two days of week.
D. PET scan and tumor volume evaluation
Imaging studies with 18F-FDG were conducted 15, 22, 29, 36 and 43 days for each rat. Each rat (which had been kept fasting for at least 4 h) were anesthetized with isoflurane (15 mg/kg), and 63 MBq of 18F-FDG were injected intravenously. After 60 min of distribution, the brain image of rat was acquired in a high-resolution explore Vista PET scanner (GE Healthcare, USA). Each rat was scanned for 30 min in a prone position with its brain centered in the axial and transaxial fields of view. PET images were reconstructed with a 3D ordered subsets expectation maximization algorithm with corrections for decay, detector deadtime, scatter and random coincidences. The final image resolution in the central FOV was less than 1 mm at full width half maximum. For semiquantitative evaluation, ring-shaped regions of interest were drawn onto coronal slices with the most obvious lesion and
8
contralateral side. Changes in uptake in the serial PET scans were analyzed by comparing the ratios to the contralateral side (ratio = uptake of a region of interest/uptake of the mirror region of interest) of the matched images. For tumor volume evaluation, reconstructed three-dimensional PET data were evaluated by a region of interest (ROI) analysis using the dedicated biomedical image quantification software PMOD (Pmod Technologies Ltd, Adliswil, Switzerland). ROIs were drawn manually in all coronal slices comprising the tumor, thus providing volume of the entire tumor.
E. Blocking antibody migration assay
The suspended F3 human neural stem cells, human primary cultured brain tumor cells #18, #24, C6 rat glioma cells (each 5 x 104cells) were incubated with mAb against integrin αvβ5 (P1F6 Chemicon MAB1961, 10ug/ml), integrin β1 (P5D2 Chemicon, MAB1959, 10μg/ml) or control IgG (Zymed gout anti-mouse IgG, 10μg/ml) or without antibody for 30min at 37℃ in 100μl medium (DMEM + 10% FBS + P/S). Pre-incubated cells were seeded into the upper chamber, and simultaneously 5μg/ml of Recombinant human CM-1 /OSF-2 (R&D systems, 3548-F2) or 100ng/ml of CM-2 was added to lower chamber (Corning costar, USA). Cells were incubated at 37℃. After 24hrs, the insert was removed from transwell, non-adhering cells in its upper surface were wiped off with a cotton wool. The membranes were washed with PBS for two times. The migrated cells under the membrane were fixed by using 4% paraformaldehyde during 10mins and stained with hematoxylin (Sigma) for 15min. The remained cells in all of the membrane were counted under the Image Analysis System (Olympus BX51, KY-LINK), and expressed as fold
9
compare with control.
F. Boydenchamber assay
HB1.F3 cells (5×104) were plated onto tissue culture plate inserts (pore size, 8 μm) of a 24-transwell system (Corning Costar, USA) and containing 10% FBS overnight. The cells were then treated with Recombinant human CM-2 (R&D systems) and 1mM LY294002 (10μM/ml) for 12h. In upper chamber, cells were scraped off with cotton wool. Cells that migrated to the bottom well were fixed in 4% paraformaldehyde and stained with hematoxylin (Sigma). The remaining cells in all of the membrane were counted under the Image Analysis System (Olympus BX51, KY-LINK), and expressed as fold compare with control.
G. Western blot analysis
HB1.F3, F3.pREP4, F3.shCD63 cells were seeded at density of 1 x 106 per 60mm dish. After 24hr, cells were washed one time using new medium and incubated with medium containing CM-2 100ng/ml or without CM-2 (control) for 30min. Whole cell lysates were prepared by incubating cells for 30 min in RIPA Buffer (1% sodium deoxycholate, 1% triton X-100, 0.1% SDS, 50mM Tris HCl, 50mM NaCl in distilled water, pH 7.5) containing complete protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Thermo science). Protein concentrations were determined by the Bio-Rad DC Protein Assay. 20μg of cell lysates were loaded into each lane of 8% SDS-PAGE. After electrophoresis, proteins were transferred to PVDF membrane (Millipore). The membranes were blotted with
10
antibodies to p-FAK (rabbit mAb Y397, Invitrogen), total FAK (mouse mAb, BD) followed by HRP-conjugated secondary antibodies. Bound antibodies were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce). The same membranes were then blotted with a mouse mAb to β-actin (sigma) as a loading control.
11
Ⅲ. RESULTS
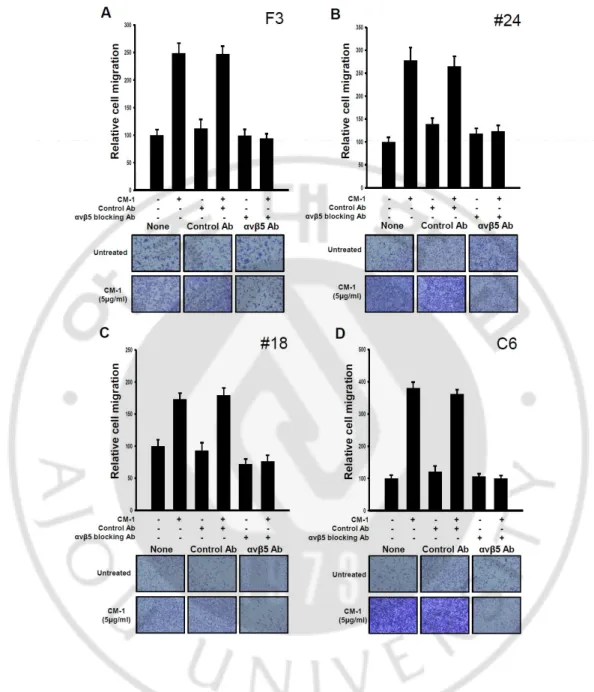
A. Cancer stem cells induced migration by CM-1 via integrin αvβ5 as receptor of CM-1 as well as NSCs.
Tumor stroma is composed of a variety of cells including cancer stem cells (CSCs). Cancer stem cells have aggressive and invasive properties and are known to play the important role for cancer metastasis. (Soltysova et al, 2005; Altaner C, 2008) In general pattern, tumor metastasis promotes the spread of tumors to local and distant sites away from primary tumors. (H Jin et al, 2004)
Previous in vivo data showed that tumor cells in 200% NIH 3T3 cells overexpressing 1 (p-NIH 3T3) implanted condition were promoted the gathering of tumors toward CM-1-secreted sites compared with 50% p-NIH 3T3 cells implanted condition. As well as, edge of tumor mass was seemed like clearly assemblage. So, we investigated whether CM-1 induced migration not only NSCs, but also in cancer stem cells. In order to determine the presence of integrin αvβ5 known as receptor of CM-1 in cell membrane, we performed migration assay using functional blocking antibody for human NSCs HB1.F3 and human glioblastoma primary cultured cells, rat glioma cells. (fig. 1) In this result, C6 and human glioblastoma primary cultured cells #18, #24 significantly increased induction of cell migration about 1.7-3.8 fold into pore of membrane via CM-1 as well as F3 and decreased migration by integrin αvβ5 functional blocking antibody though in the presence of CM-1. This data suggests that CM-1 induces migration not only in cancer stem cells, but also in
12
13
Fig. 1. Role of alpha v beta 5-integrin receptors in CM-1-induced human neural stem cell, glioma cell lines migration. (A-D) Blocking experiment with integrin αvβ5 antibodies
showed the interactions of CM-1-integrin αvβ5. (A) HB1.F3 Human neural stem cells (5x104 cells / well) were pretreated with anti-integrin αvβ5 antibody (10μg/ml) or control antibody (10μg/ml ) for 30min and transferred onto transwells in the presence or absence of
14
CM-1 (5μg/ml) for 24 hr. Migrated cells were fixed and stained using hematoxylin, followed by quantitative analysis. Human glioblastoma primary cultured #24 cells (B), human glioblastoma primary cultured #18 cells (C), C6 rat glioma cells (D) were assessed by same method. Bottom representative figures of migrated cells under membrane of transwell after 24 hr using bright microscope. Error bars indicate SD. Magnification, X100.
15
B. Systemic strategy using CM-1 as chemoattractants and CD-5-FC is effective to prolong survival time.
Previous in vivo experiments employed immortalized HB1.F3.CD cells encoding cytosine deaminase (CD) gene to investigate the bystander effect by migrating to tumor via CM-1. As prior result, F3.CD cells were affected to neighborhood cell as induced cell death fairly. So, we determined whether CM-1 was effective to extend survival time in animal model of brain tumor, following described experimental plan via divided 5 groups. (fig. 2) We confirmed the in vivo prolonged survival time of CM-1 in brain tumor animal model, which was made by injection of C6 rat glioma cells into the right striatum of 8-week-old SDS.rats. F3.CD cells were transplanted into left brain hemisphere stereotactically and 5-FC was administrated systemically.
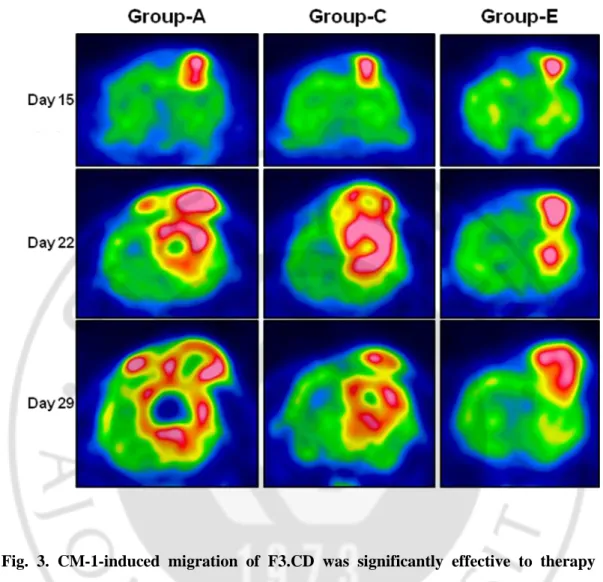
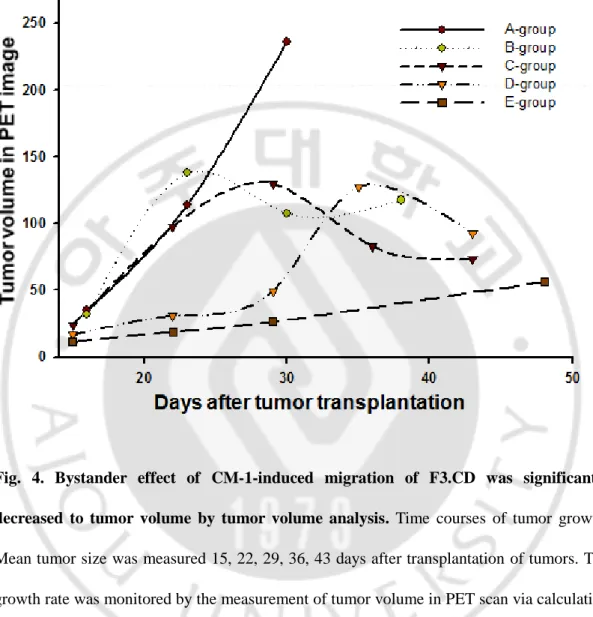
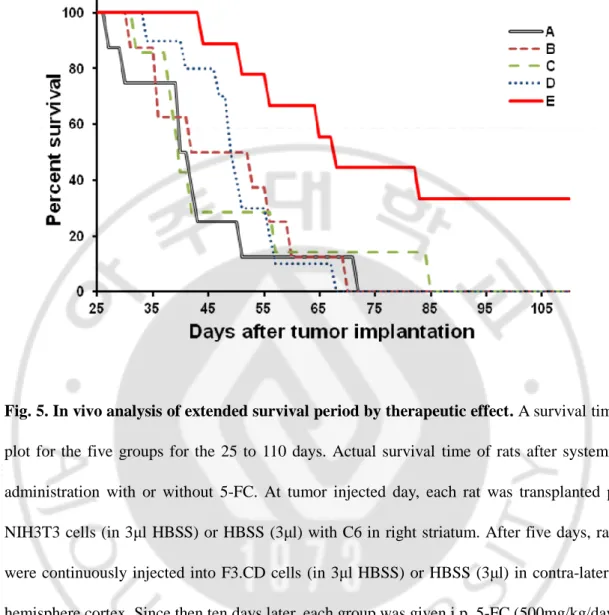
In PET images, tumor mass of group E [C6 + p-NIH 3T3 (2x) + F3.CD + 5-FC] was significantly reduced compared with group A (C6 + HBSS) as a negative control and group C (C6 + F3.CD + 5-FC). (fig. 3) This result showed that F3.CD cells with enhanced mobility via CM-1 made to slow the tumor growth more effectively. To calculate that numerical value of tumor volume, we utilized AMIDE on the basis of PET images. (fig. 4) Tumor volumes of rats implanted with p-NIH 3T3 cells were reduced significantly [group E, an average of tumor volumes = 56.2422 ± 52.8143mm3 (mean ± SD)], compared with those of control group [group A, an average of tumor volumes = 236.233 ± 119.2748mm3 (mean ± SD)]. It means that CM-1 help the targeting of F3.CD cells to tumor region and F3.CD cells hinder to increase tumor volume in established condition of tumor model. To identify whether how much survival time is extended via CM-1-overexpressed condition, we checked dead or alive
16
rats in everyday. As survival graph, until 85 days, all rats of group A-D died. But, 33.2 % rats of group E were alive without dying continuously over 110 days. There was significantly difference in survival [final survival after tumor cell implantation(minimum period – maximum period); group A (HBSS injection + saline) = 71 days (27-71)days; group B (F3.CD injection + saline) = 69 days (31–69 days); group C (F3.CD injection + 5-FC) = 84 days (32–84 days); group D (p-NIH 3T3 0.5x + F3.CD injection + 5-FC) = 67 days (34–67 days) group E (p-NIH 3T3 2x + F3.CD injection + 5-FC) = alive 3 rats (44- days)]. (fig.5) Interestingly, early tumor volume rate of implanted group D of 0.5 fold p-NIH 3T3 cells was similar with group E. But about 30 days later, rate of tumor volume was rapidly increased and survival time was not different with control group.
These results suggest that CM-1 of the more constant concentration in tumor area can improve the therapeutic effect of gene therapy using NSCs and prolong survival period.
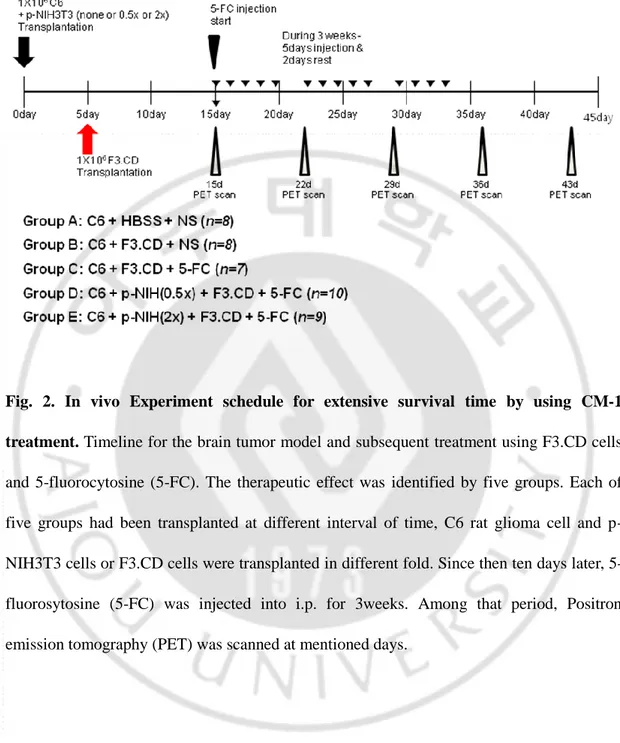
17
Fig. 2. In vivo Experiment schedule for extensive survival time by using CM-1 treatment. Timeline for the brain tumor model and subsequent treatment using F3.CD cells
and 5-fluorocytosine (5-FC). The therapeutic effect was identified by five groups. Each of five groups had been transplanted at different interval of time, C6 rat glioma cell and p-NIH3T3 cells or F3.CD cells were transplanted in different fold. Since then ten days later, 5-fluorosytosine (5-FC) was injected into i.p. for 3weeks. Among that period, Positron emission tomography (PET) was scanned at mentioned days.
18
Fig. 3. CM-1-induced migration of F3.CD was significantly effective to therapy of tumor in PET analysis. Representative PET (coronal images) data. The rats with C6 tumors
(group A : C6 + HBSS + NS) as controls were not treated with 5-FC after tumor inoculation. Group C (C6 + F3.CD + 5-FC) and E [C6 + p-NIH3T3 (2x) + F3.CD + 5-FC] were implanted with F3.CD after 5 days of tumor inoculation, Followed by daily intraperitoneal injection of 5-FC for 3 weeks. Reduction in signal intensity could be observed after treatment, indicating reduction in uptake of F-FDG in tumor at 15, 22, 29 day.
19
Fig. 4. Bystander effect of CM-1-induced migration of F3.CD was significantly decreased to tumor volume by tumor volume analysis. Time courses of tumor growth.
Mean tumor size was measured 15, 22, 29, 36, 43 days after transplantation of tumors. The growth rate was monitored by the measurement of tumor volume in PET scan via calculating the FDG uptaken by tumor cells. Group A ; C6 + HBSS + NS (n = 8), Group B ; C6 + F3.CD + NS (n = 8), Group C ; C6 + F3.CD + 5-FC (n = 7), Group D ; C6 + p-NIH3T3 (0.5x) + F3.CD + 5-FC (n = 10), Group E ; C6 + p-NIH3T3 (2x) + F3.CD + 5-FC (n = 9)
20
Fig. 5. In vivo analysis of extended survival period by therapeutic effect. A survival time
plot for the five groups for the 25 to 110 days. Actual survival time of rats after systemic administration with or without 5-FC. At tumor injected day, each rat was transplanted p-NIH3T3 cells (in 3μl HBSS) or HBSS (3μl) with C6 in right striatum. After five days, rats were continuously injected into F3.CD cells (in 3μl HBSS) or HBSS (3μl) in contra-lateral hemisphere cortex. Since then ten days later, each group was given i.p. 5-FC (500mg/kg/day) or 0.9% normal saline for three weeks. The p-NIH3T3 (2x) and CD/5-FC group yielded significantly better survival versus all of the other groups. Rat surviving 120 days were considered cured. Group A (black) ; C6 + HBSS + NS (n = 8), Group B (pink) ; C6 + F3.CD + NS (n = 8), Group C (green) ; C6 + F3.CD + 5-FC(n = 7), Group D (blue) ; C6 + p-NIH3T3 (0.5x) + F3.CD + FC (n = 10), Group E (red) ; C6 + p-p-NIH3T3 (2x) + F3.CD + 5-FC (n = 9)
21
C. CM-2 was crucial interaction with integrin β1 in induction of NSCs migration.
At the plasma membrane, the members of the tetraspanin family including CD63 are known to interact with cell adhesion molecules such as integrins, regulating intracellular signal transduction pathways including cell adhesion, motility, and survival (Berditchevski, 2001; Hemler, 2001; Yunta and Lazo, 2003). Integrin β1 is one of the main tetraspanin-interacting integrins. Previous results reported that CM-2 induced migration of NSCs by combining CD63. As well as, it complex bound to integrin β1.
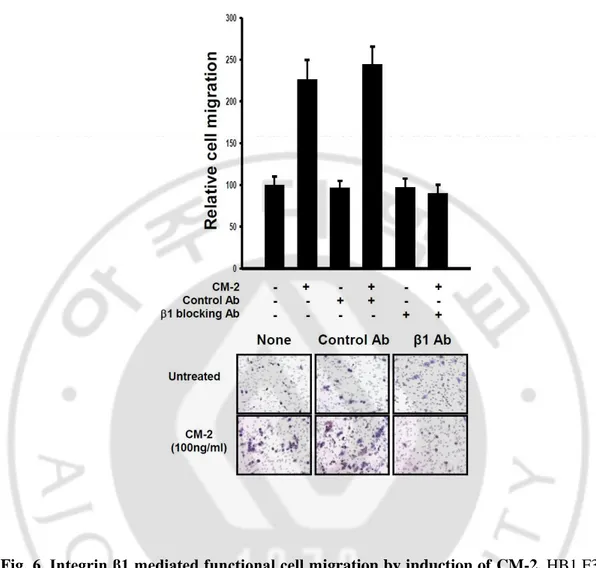
To explore the essential role of integrin β1 by which CM-2 promotes induction of NSCs migration, we used functional blocking antibody for integrin β1 in migration assay. As shown in fig. 6, migrated NSCs were 2.26-2.44 fold compare with control in treatment condition of CM-2. In the presence of functional blocking antibody for integrin β1, NSCs did not induce migration and moved cells were no almost.
Its results supported that induction of NSCs migration by CM-2 was mediated to interact with CM-2 and integrin β1 as well as CD63.
22
Fig. 6. Integrin β1 mediated functional cell migration by induction ofCM-2. HB1.F3 (5
x 104 cells/well) were preincubated with anti-integrin β1 antibody (10μg/ml) or control antibody (10μg/ml) for 30min and were cultured in a 24-well plate medium with or without CM-2 (100ng/ml) for 24hr. Migrated cells on the lower side of the membrane were fixed and stained with hematoxylin and counted under a bright microscope using a ×100 objective. Error bars indicated SD.
23
D. FAK and PI3K signal pathway were included in the NSCs migration.
The binding of ECM proteins to the extracellular domains of integrins leads to integrin activation, which recruits and activates signaling molecules such as focal adhesion kinase (FAK). FAK signaling pathway is known as cell motility related signal pathway. FAK signal pathway connected with many kinases in its downsteam and convey signal. In cell migration, phosphoinositol-3-kinase (PI3K) signal pathway was mainly used that located in FAK downstream. (Wozniak et al., 2004; Zhao et al, 2009)
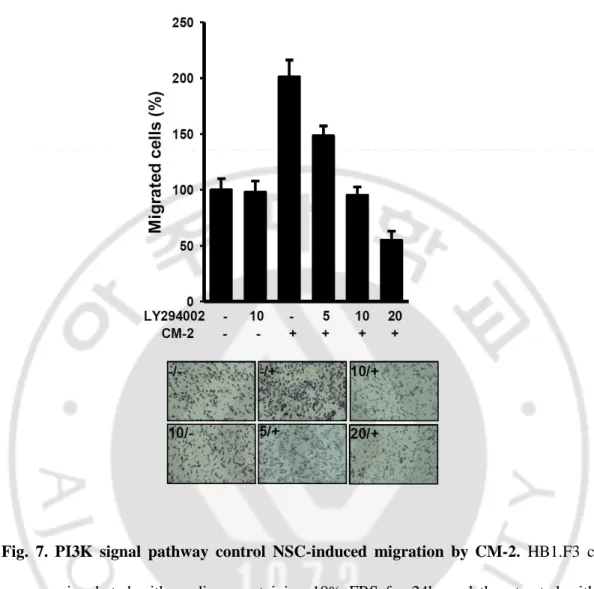
To investigate PI3K signal pathway is continuous with induction of migration by CM-2, we performed boydenchamber assay using PI3K inhibitor, LY294002 (10μM/ml). We observed that activation of PI3K signaling by CM-2 was interrupted by PI3K inhibitor, LY294002 (10μM/ml) and moving ratio was reduced in dose-dependent manner (70% in the migrated cells). (fig. 7)
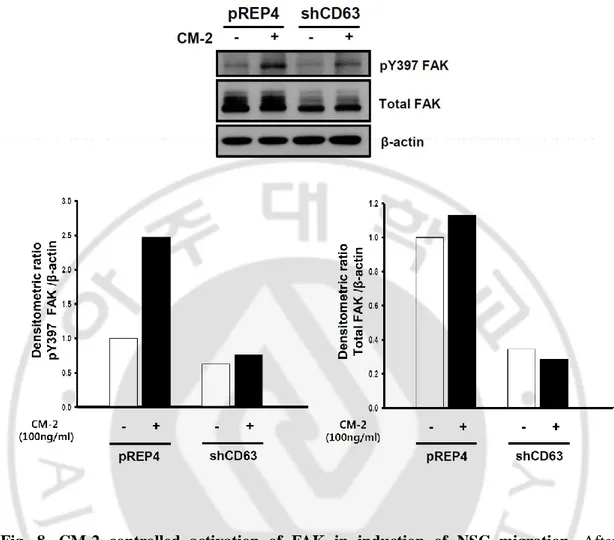
Also, we identified that the present or absent condition of CM-2, the tyrosine 397 phosphorylated level of FAK in CD63-knockdown F3 cells and pREP4 vector F3 cells (pREP4.F3 cells). CD63-knockdown F3 cells (shCD63. F3 cells) was established by using a vector-based small hairpin RNA (shRNA) strategy. As shown western blot results, phosphorylated FAK levels of CD63-knockdown F3 cells were decreased compare with pREP4 vector F3 cells in the condition of treatment CM-2. (fig. 8) To check total FAK level, same membrane with phospho-FAK detected membrane deproved and then detected again.
These results suggest that CM-2 lead to NSCs migration via tyrosine 397 phosphorylation of FAK and activation of PI3K signaling.
24
Fig. 7. PI3K signal pathway control NSC-induced migration by CM-2. HB1.F3 cells
were pre-incubated with medium containing 10% FBS for 24hr and then treated with or without LY294002. Other cell groups were treated dose-dependently PI3K inhibitor (LY294002) with CM-2. In all cases, migration was measured after 12h and was expressed relative to the migration of stimulated cells. Bottom images were representative migrated F3. Error bars indicated SD. Magnification, X100.
25
Fig. 8. CM-2 controlled activation of FAK in induction of NSC migration. After
incubation of pREP4.F3 cells and shCD63.F3 cells in the presence or absence of CM-2 for 30min, cell lysates were prepared by using RIPA buffer. Equal volumes of cell lysates were analyzed by Western blot using antibodies directed against the phosphorylated state of FAK at Tyrosine 397. The membranes were further analyzed by Western blotting using anti-FAK antibody to detect FAK expression levels. As controls, β-actin was detected. The graph of each relative activity was analyzed by densitometric quantification using Image J software.
26
Ⅳ. DISCUSSION
The present study identified CM-1 and CM-2 as novel chemoattractants molecules for mobility inducers in activating tumor tropism of NSCs. According to prior results, CM-1 and CM-2 were over-expressed in human glioblatomas tissue and possessed ability of induction of NSCs migration. Furthermore, when human NSC line HB1.F3 carrying cytosine deaminase (CD) enzyme gene (F3.CD) was transplanted intracranially at distant sites from the tumor, the NSCs migrate through normal tissue and selectively “ home in” to the glioblastoma tumor mass and upon administration of prodrug 5-FC, significant reduction in tumor volume was demonstrated in rat brain tumor model. (Kim et al, 2011; Black, 1991; Heese et al, 2005)
In previous results, tumor mass was assembled to tumor area and non-invasive signature relatively. Cancer stem cells, one of the cells that comprise the tumor, have aggressive and invasive features are related to metastasis. So, we hypothesized that cancer stem cells had integrin αvβ5 as a receptor of CM-1 and induced cell migration via CM-1. As shown in our result, human glioblastoma primary cultured cells and C6 rat glioma cells had integrin αvβ5 and led to migration toward CM-1.
As well as in this study, we identified that use of CM-1 prolonged survival time with advanced strategy using CD and 5-FC suicide gene therapy for brain tumor model. Among experimental group D of CM-1-over expressing NIH 3T3 cells 0.5x and experimental group E of CM-1-over expressing NIH 3T3 cells 2x (compare with C6 number), tumor volume rate
27
of group D was similar with group E until 30 days. But, after then, rate of tumor volume was rapidly increased and survival time was not different with control group. In contrast, the group E showed distinctly slowed the tumor growth rate as well as increased survival rate compared with other groups. It means that CM-1 of the more constant concentration was significantly effective to brain tumor treatment.
Other previous results showed that CM-2 induced migration specifically in NSCs and interacted with CD63 and integrin β1 for induction of migration. When we blocked integrin β1 using functional blocking antibody, induction of migration was significantly decreased despite the presence of CM-2. Some researchers demonstrated phosphoinositol 3-kinase (PI3K), which has been shown to be an important regulator of directed NSCs migration. (Wozniak et al, 2004; Zhao et al, 2009) These observations revealed when CM-2 induced NSCs migration, PI3K signaling pathway was important shown by migration assay. As well as, CM-2 induced phosphorylation of FAK in tyrosine 397 site but not in CD63 knockouted F3 cells. The present results support that interaction of integrin β1 and PI3K pathway and phosphorylation of FAK are related to induce NSCs migration via CM-2.
Overall, our results reveal that a novel tumor tropism signature of neural stem cells was induced by CM-1 and CM-2. As a result, these chemoattractants improve ability that the inherent tumor-tropism of NSCs to primary and invasive brain tumor foci can be exploited to deliver therapeutics to invasive brain tumor cells in humans. (Imitola et al, 2004) Thus, identification and characterization of such chemoattractants will become to use crucial for brain tumor therapy more efficiently.
28
CM-2 is also effective to induce cell migration in vivo. As well as, when CM-1 and CM-2 is introduced in the therapy of actual human brain tumor, we think about that mobility inducers can be used most effectively over a long period of time for therapeutic effect.
29
Ⅴ. CONCLUSION
This study showed that both NSCs and CSCs has receptor of CM-1 as integrin αvβ5 and induced migration by CM-1. Moreover, CM-1 enhanced migration ability and elevated tumor targeting for F3.CD. Such F3.CD cells decreased growth rate of tumor as well as prolong survival period in tumor model by converting 5-FC to cytotoxic agents, accordingly it made suicide gene therapy was more effective. Another inducer of NSCs migration, CM-2 induced migration via interaction with integrin β1 and PI3K signaling pathway. The existence of CD63 in NSCs upon interaction with CM-2 activated FAK and then, NSCs induced migration. These results demonstrated that CM-1 and CM-2 as chemoattractants led to migration of NSCs & CSCs and can improve the effect of neural stem cell-based gene therapy for brain tumor.
30
REFERENCES
1. Aboody, K, Najbauer, J, Schmidt, NO, Yang, W, Wu, JK, Zhuge, Y et al: Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol 8: 119–126, 2006
2. Altaner C. Prodrug cancer gene therapy: Cancer Lett. 8;270(2):191-201. 2008
3. Alves TR, Lima FR, Kahn SA, Lobo D, Dubois LG, Soletti R et al: Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma.. Life Sci 89: 532–539, 2011
4. Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF: Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5:329-339,2004
5. Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR: Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxiainduced cell death: Role of the beta4 integrin and the PI3K pathway. Oncogene 26:2082-2094, 2007
6. Berditchevski F: Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114: 4143–4151. 2001
7. Black, P.M: Brain tumors. Part I. N. Engl. J. Med 324, 1471–1476, 1991
8. Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161– 174, 2002
9. Folkman J: Fundamental concepts of the angiogenic process. Curr Mol Med 3(7):643-651, 2003
10. Glaser, T., Brose, C., Franceschini, I., et al: Neural cell adhesion molecule polysialylation enhances the sensitivity of embryonic stem cell-derived neural precursors to migration guidance cues. Stem Cells 25: 3016–3025, 2007
11. Goldbrunner RH, Bernstein JJ, Tonn JC: ECM-mediated glioma cell invasion. Microsc Res Tech 43: 250–257, 1998
31
12. H Jin and J Varner. Integrins: roles in cancer development and as treatment targets. British Journal of Cancer 90: 561 – 565, 2004
13. Heese O, Disko A, Zirkel D, Westphal M, Lamszus K: Neural stem cell migration toward gliomas in vitro. Neuro Oncol 7(4):476-84, 2005
14. Hemler ME: Specific tetraspanin functions. J Cell Biol 155: 1103–1107. 2001
15. Henry S. Friedman, Tracy Kerby, and Hilary Calvert: Temozolomide and Treatment of Malignant Glioma. Clin Cancer Res 6: 2585–2597, 2000
16. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A: Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239-1249, 1999
17. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ: Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 28;101(52):18117-22, 2004
18. Johnson MD, Kim HR, Chesler L, Tsao-Wu G, Bouck N, Polverini PJ: Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J Cell Physiol 160:194 – 202, 1994 19. Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, Garcia E, Kim SU, Barish
ME, Aboody KS, Glackin CA: Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells 26: 1575-86, 2008
20. Kim SU: Neural stem cell-based gene therapy for brain tumors. Stem Cell Rev. 2011; 7(1):130-38.
21. Kruger A, Sanchez-Sweatman OH, Martin DC, et al: Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene 16:2419 – 23, 1998
22. Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M, Takata T: Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res 66:6928-6935, 2006
32
23. Lambert E, Dasse E, Haye B, Petitfrere E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol 49:187 – 98, 2004
24. Lim SK, Llaguno SR, McKay RM, Parada LF: Glioblastoma multiforme: a perspective on recent findings in human cancer and mouse models. BMB Rep 44: 158–164, 2011
25. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P:
26. Martin DC, Ruther U, Sanchez-Sweatman OH, Orr FW, Khokha R: Inhibition of SV40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene 13:569 – 76, 1996
27. Martin DC, Sanchez-Sweatman OH, Ho AT, Inderdeo DS, Tsao MS, Khokha R: Transgenic TIMP-1 inhibits simian virus 40 T antigen-induced hepatocarcinogenesis by impairment of hepatocellular proliferation and tumor angiogenesis. Lab Invest 79:225 – 34, 1999
28. Morra L, Moch H: Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update.Virchows Arch 459: 465–475, 2011
29. Munshi A, Jalali R. Therapy for glioma: Indian perspective. Indian J Cancer 46: 127–131, 2009
30. Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al: Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892-9. 2004
31. Riener MO, Fritzsche F R, Sol l C, Pestalozzi BC, Probst-Hensch N, Clavien PA, Jochum W, Soltermann A, Moch H, Kristiansen G: Expression of the extracellular matrix protein Periostin in liver tumors and bile duct carcinomas. Histopathology 56:600-606, 2010 32. Roberts AB, Sporn MB: Physiological actions and clinical applications of transforming
growth factor-h (TGF-h). Growth Factors 8:1 – 9, 1993
33. Sasaki H, Lo KM, Chen LB, Auclair D, Nakashima Y, Moriyama S, Fukai I, Tam C, Loda M, Fujii Y: Expression of Periostin, homologous with an insect cell adhesion molecule, as a prognostic marker in non-small cell lung cancers. Jpn J Cancer Res 92:869-873, 2001 34. Schmidt, NO, Przylecki, W, Yang, W, Ziu, M, Teng, Y, Kim, SU et al: Brain tumor tropism
33 Neoplasia 7: 623–629, 2005
35. Seung U Kim: Neural stem cell-based gene therapy for brain turmors. Stem Cell Rev and Rep. 7:130-140. 2011
36. Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF: Acquired expression of Periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 24:3992-4003, 2004
37. SHI De-zhi, HU Wei-xing, LI Li-xin, CHEN Gong, WEI Dong and GU Pei-yuan: Pharmacokinetics and the bystander effect in CD::UPRT/5-FC bi-gene therapy of glioma. Chinese Medical Journal 122(11):1267-1272, 2009
38. Smith JS, Jenkins RB: Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci 5:D213-31. 2000
39. Soltysova A, Altanerova V, Altaner C: Cancer stem cells. Neoplasma. 52(6):435-40. 2005 40. Springer CJ, Niculescu-Duvaz I: Prodrug-activating systems in suicide gene therapy. J Clin
Invest 105: 1161-1167, 2000
41. Tafani M, Di Vito M, Frati A, Pellegrini L, De Santis E, Sette G et al: Pro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma. J Neuroinflammation 8: 32, 2011
42. Takeshita S, Kikuno R, Tezuka K, Amann E: Osteoblast specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Bio Chem J 294:271-278, 1993
43. Teodorczyk M, Martin-Villalba A: Sensing invasion: cell surface receptors driving spreading of glioblastoma. J Cell Physiol 222: 1–10, 2010
44. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114(2):97-109. 2007
45. Wang W, Ma JL, Jia WD, Xu GL: Periostin: a putative mediator involved in tumour resistance to anti-angiogenic therapy? Cell Biol Int 35: 1085–1088, 2011
34 Cell Stem. Cell 10: 111–112, 2012
47. Wozniak, M. A., Modzelewska, K., Kwong, L. and Keely, P. J: Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 1692, 103-119, 2004
48. Yamanaka R, Saya H: Molecularly targeted therapies for glioma. Ann Neurol 66(6):717-29, 2009
49. Yunta M, Lazo PA : Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell Signal 15: 559–564. 2003
50. Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS: Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 6(12):1819-29. 2008
51. Zhao, J. and Guan, J. L: Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28: 35-49, 2009
52. Zheng, H., Fu, G., Dai, T., & Huang, H: Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. Journal of Cardiovascular Pharmacology 50: 274–280, 2007
53. Zhu M, Fejzo MS, Anderson L, Dering J, Ginther C, Ramos L, Gasson JC, Karlan BY, Slamon DJ: Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol 119:337-344, 2010
35 -국문요약-
신경줄기세포에 대한 이동성 유도물질들의 특성 분석
아주대학교 대학원 의생명과학과 김 정 미 ( 지도교수: 이 명 애 ) 종양-굴성을 가진 신경줄기세포는 종양을 표적화 하는 치료적 방법에 용이 할 수 있다. 이러한 종양-굴성을 향상시킬 수 있는 CM-1과 CM-2는 이전에 인간 뇌조직의 microarray를 통해 과-발현되는 것이 확인 되었다. 이전 연구는 여러 환자의 뇌종양에서 이동성 유도물질로서 CM-1와 CM-2가 일반적으로 높게 발현되며, 이 물질들이 신경줄기세포의 이동성을 상당히 향상시킴을 확인했다. 또한 이전의 결과는 우리는 F3 신경줄기세포뿐만 아니라 C6 쥐 신경교종 세포와 인간의 신경교종 조직에서 배양된 세포에 존재하는 CM-1의 수용체로 인테그린 αvβ5을 확인했다. 이에 따라 뇌 암줄기세포들도 CM-1가 높은 농도로 존재하는, 종양 덩어리 쪽으로 모이게 만들었다. 이에 따라 우리는 In vivo, 쥐의 뇌종양 모델을 설립하였고, CM-1에 의해 유도되어 이동한 F3.CD에서 분비된 cytosine deaminase는 전구약물인 5-fluorocytosine와의 작용을 통해 종양의 크기가 줄어듦을 확인하였다. 또한 이 실험을 통해 방관자 효과 및 신경줄기세포 이동성 향상이 생존 시간의 연장에 상당히 효과적임을 밝혔다. 다른 이동성 유도물질인 CM-2는36
CD63 수용체에 의해 세포 이동을 유도하는 것으로 알려져 있다. CM-2에 의한 신경줄기세포의 이동성 유도에 있어서 분자적 메커니즘을 조사하기 위해 migration assay를 수행하였고, 이를 통해 인테그린 β1, PI3K 신호 경로가 CM-2에 의한 신경 줄기 세포 이동의 유도와 연루되어 있다는 것을 확인했다. 또한, CD63 knockouted F3 세포와 pREP4 F3 세포에서 FAK의 타이로신 397자리에 인산화를 확인해본 결과, CD63이 발현되지 않는 신경줄기세포에서 CM-2에 의한 FAK 인산화는 pREP4 F3 세포에 비해 상당히 감소하였다. 종합적으로, 본 내용은 이동성 유도 물질들에 의해 종양-표적화 능력이 향상된 인간 신경 줄기 세포가 독창적인 암 치료법으로 이용될 수 있음을 시사한다.
핵심어 : Neural stem cell, Tumor tropism, Stem cell therapy, Glioblastoma multiforme (GBM), Mobility inducers for neural stem cell