저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Identification of the receptor for
cellular internalization of an
anti-nucleic acid antibody, 3D8 scFv
by
Hye-Jin Kim
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Identification of the receptor for
cellular internalization of an
anti-nucleic acid antibody, 3D8 scFv
by
Hye-Jin Kim
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of Master of Biomedical Sciences
Supervised by
Myung-Hee Kwon, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
i
- ABSTRACT -
Identification of the receptor for cellular internalization of an
anti-nucleic acid antibody, 3D8 scFv
A subset of anti-DNA antibodies (Ab) can internalize in a variety of cells. An anti-nucleic acid antibody, murine 3D8 single chain variable fragment (scFv) Ab, has been known to be an internalizing antibody that enters the cells by caveolae/lipid-raft-mediated endocytosis. However, cell surface receptor for 3D8 scFv internalization remains unknown. Here, we studied for cell surface receptor of 3D8 scFv for cellular internalization. 3D8 scFv bound to both cell surface heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) that are highly negatively charged molecules known as endocytic receptors for a variety of ligands. Treatment of soluble HS or CS reduced cell surface binding of 3D8 scFv to intact HSPGs. Intracellular co-localizations of three molecules were observed between 3D8 scFv-HSPGs-caveolin 1, and 3D8 scFv-CSPGs-caveolin 1 in HeLa cells. Furthermore, internalization of 3D8 scFv was significantly reduced in HS/CS-deficient CHO cells (pgsA-745), compared to in wild-type CHO cells. These data provide the evidence for both HSPGs and CSPGs as internalizing receptor of 3D8 scFv.
ii
TABLE OF CONTENTS
ABSTRACT ··· i TABLE OF CONTENTS ··· ii LIST OF FIGURES ··· iv I. INTRODUCTION ··· 1II. MATERIALS AND METHODS ··· 4
A. Materials ··· 4
B. Cells and cell cultures ··· 5
C. Purification of scFv proteins ··· 5
D. Sodiumdodecyl sulfate-polyacrylamide gel electrophoresis ··· 6
E. Competitive ELISA ··· 6
F. Flow cytometry ··· 7
G. Confocal microscopy ··· 8
III. RESULTS ··· 12
A. Purify the 3D8 and HW6 proteins ··· 12
B. 3D8 internalization depends on HSPGs and CSPGs ··· 14
C. 3D8 scFv△ pA binds to cell surface HSPGs and CSPGs ··· 17
iii
D. Internalized 3D8 scFv△ pA co-localizes with HSPGs, CSPGs, and caveolin 1 ···· 21
IV. DISCUSSION ··· 24
V. CONCLUSION ··· 27
REFERENCES ··· 28
국문요약 ··· 31
iv
LIST OF FIGURES
Figure 1. Production of scFv proteins ··· 13
Figure 2. Effects of soluble GAGs on 3D8 scFv△ pA internalization ··· 15
Figure 3. 3D8 scFv internalization in wild type and pan-GAG deficient
pgsA-745 mutant CHO cells ··· 16
Figure 4. Binding of 3D8 scFv△ pA to HSPGs and CSPGs ··· 18
Figure 5. Binding of 3D8 scFv△ pA to cell surface HSPGs in the presence of
soluble GAGs ··· 20
Figure 6. Intracellular co-localization between 3D8 scFv△ pA, HSPGs,
1
I. INTRODUCTION
Anti-DNA antibodies (Abs) are of biomedical interest because they are associated with autoimmune diseases in human (Kim et al., 2006). Anti-DNA antibodies are naturally present in healthy humans, but are preferentially found in patients with autoimmune diseases, particularly systemic lupus erythematosus (Jang and Stollar, 2003). They constitute a subgroup of antinuclear antibodies that bind single-stranded DNA, double-stranded DNA, or both (Hahn, 1998). Some of anti-DNA antibodies have been shown to penetrate cells (Yanase et al., 1997; Seddiki et al., 2001; Hansen et al., 2007; Jang et al., 2009).
Yanase et al. reported that monoclonal anti-DNA antibody (H7) derived from lupus-prone mice can cross the cellular membrane by cell surface binding to brush border myosin (myosin 1) (Yanase et al., 1997). Seddiki et al. demonstrated that calreticulin may act as a cell surface receptor for penetrating anti-DNA monoclonal antibody, F14.6 (Seddiki et al., 2001). In addition, the anti-DNA antibody fragment 3E10 Fv penetrates into living cells with specific nuclear localization and absence of toxicity (Hansen et al., 2007). Penetration of 9D7 monoclonal anti-dsDNA autoantibody into Jurkat cells is not mediated by endocytosis. However, Song et al. observed that 9D7 penetrates into cells through the electrostatic interaction of arginine residues with the negatively charged sulfated polysaccharides on the cell surface (Song et al., 2008).
In addition to the above mentioned anti-DNA Abs, there are numbers of molecules which can cross the cellular membrane. These molecules have been reported to interact with
2
cell surface heparan sulfate proteoglycans (HSPGs) which are negatively charged molecules on the cell surface (Tyagi et al., 2001; Elson-Schwab et al., 2007; Poon and Gariepy, 2007). Proteoglycans (PGs) are glycoproteins that contain one or more covalently-linked glycosaminoglycans (GAGs) attached through a hydroxyl bond to core protein (Esko et al., 2009). PGs are present at the cellular surface and in the extracellular matrix and secretory vesicles (Sarrazin et al., 2011). PGs can be classified into two types depending on sort of saccharides: heparan sulfate PGs (HSPGs) and chondroitin sulfate PGs (CSPGs). The linkage region of GAGs consists of xylose, galactose, and glucuronic acid and this region is identical for HS and CS chains. The chain elongation continues through the addition of N-acetylglucosamine-glucuronic acid disaccharides in the case of HS synthesis while addition of N-acetylgalactosamine-glucuronic acid disaccharides in the case of CS synthesis. GAGs are modified after or during backbone synthesis. Modifications occurring in the synthesis of HS chain are: N-deacetylation/N-sulfation of N-acetylglucosamine; epimerization of glucuronic acid to iduronic acid; sulfation at the 2-O position of iduronic acid; sulfation at 6-O and 3-6-O positions of N-acetylglucosamine and in the synthesis of CS chain are: epimerization of glucuronic acid to iduronic acid; sulfation at the 2-O position of idurocin acid; sulfation at the 4-O position of glucuronic acid; sulfation at the 6-O position of N-acetylgalactosamine and PGs have structural diversity through these modifications (Esko and Selleck, 2002; Hacker et al., 2005). Because of this structural diversity, HSPGs can interact with various ligands such as integrin, chemokine, and morphogen and have multiple activities in cells and tissues (Sarrazin et al., 2011). The structure of CSPGs is identical to
3
HSPGs, though the saccharide is different. CSPGs have been studied in central nervous system a great part of research. Only a few is known for involvement of cell surface CSPGs in endocytosis (Hurt-Camejo et al., 1990; Yang et al., 2010).
3D8 is a single chain variable fragment (scFv) is a recombinant anti-DNA Ab composed of variable region of heavy chain (VH), variable region of light chain (VL), and (G4S1)3 linker. 3D8 scFv has been isolated from hybridoma of autoimmune disease MRL/lpr mice, can across the plasma membrane by caveolae/lipid-raft-mediated endocytosis and accumulate in the cytosol of cells. Although the endocytic pathway of 3D8 scFv was identified, its cellular internalizing receptor remains unknown. Here, we investigated whether PGs act as true internalizing receptors for 3D8 scFv internalization.
4
II. MATERIALS AND METHODS
A. Materials
Heparin (Hp, #H3149), dextran sulfate (a synthetic analogue of Hp: DS, #D7037), chondroitin sulfate A (CS-A: #C9819), dermatan sulfate (CS-B: #C3788), chondroitin sulfate C (CS-C: #27043), non-enzymatic cell dissociation solution (#031M0935), rabbit IgG (#I5006), TRITC-goat anti-rabbit IgG (#T6778), ρ-nitrophenyl phosphate (ρ-NPP: #N2765) were obtained from Sigma (St.Louis, MO, USA). Mouse anti-heparan sulfate (10E4: #H1890) was purchased from USBiological (Swampscott, MA, USA), Alexa Fluor 488-goat anti-mouse IgM (μ chain-specific: #A21042), Alexa Fluor 647-goat anti-rabbit IgG (#A21244), AP-Streptavidine (#434322) were purchased from Invitrogen (Grand Island, NY, USA). Mouse anti-chondroitin sulfate (#ab11570) was purchased from Abcam. Alkaline phosphatase (AP) conjugated-goat anti-rabbit IgG (#31341) and Hoechst 33342 (#62249) was purchased from PIERCE. Mouse anti-caveolin-1 (7C8: #sc-53564) and TRITC conjugated-anti-mouse IgG (#sc-3796) was obtained from SantaCruz. VECTASHIELD® Mounting medium (#H-1000) was purchased from Vector Laboratories. Polyclonal rabbit anti-3D8 scFv was produced by immunization in our laboratory. The HIV-Tat peptide (amino acids 48-60 plus a biotin, Biotin-GRKKRRQRRRPPQ) was synthesized from Peptron (Daejeon, Korea).
5
B. Cells and Cell cultures
Wild type Chinese hamster ovary (CHO)-K1 cells (#CCL-61) and pan-GAG-deficient pgsA-745 mutant CHO cell (#CRL-2242) were purchased from the American Type Culture Collection (Manassas, VA, USA). Wild type and pan-GAG-deficient pgsA-745 mutant CHO cells were cultured in Ham’s F-12 medium supplemented with 10% FBS, and antibiotics (100U/ml penicillin and100 μg/ml streptomycin). Human epithelial cervical carcinoma-derived HeLa cells were grown in Dulbecco’s modified Eagle’s medium with the same supplements as above. Cells were cultured in a humidified 5% CO2 incubator at 37
o C.
C. Purification of scFv proteins
pIg20 vector was used to express scFv proteins with both His6 tag and
Staphyplococcal protein A tag (pA). pIg20△ pA was constructed to express scFv without pA
by inserting a stop codon between His6 tag and pA.pIg20-3D8scFv, pIg20△ pA-3D8 scFv, and pIg20△ pA-HW6 scFv were transformed into Escherichia coli BL21(DE3) pLysE cells (Novagen). The transformants were cultured at 37oC in Luria-Bertani medium containing ampicillin (100 μg/ml) and chloramphenicol (25 μg/ml) until the absorbance at 600nm reached 0.8. Isopropyl 1-thio-β-D-galactopyranoside (0.5 mM) was added to culture medium to induce the expression of proteins. Cells were then cultured at 23oC for 18 h with shaking at 140 rpm. The culture supernatant was collected by centrifugation at 8,000 rpm for 30 min at 4oC and filtered through a 0.45 μm cellulose acetate filter (Sartourius Stedim biotech) to
6
remove cellular debris. The filtered culture supernatant was loaded to IgG-Sepharose 6 Fast Flow column (GE Healthcare) or HiTrap Protein L-agarose column (GE Healthcare). The column was washed with PBST (Phosphate-buffered saline, pH 7.4 plus 0.1% Tween 20) and 5 mM of ammonium acetate (pH 5.0). Proteins were eluted from the column with 0.1 M of acetic acid (pH 3.4). The eluted proteins were concentrated by centrifugation at 5,000 rpm at 4oC using Vivaspin 20 (molecular weight cut off 10,000 Da: Sartorius Stedim biotech), and then buffer was changed to PBS (pH 6.0). The concentration of each protein was determined based on the molar extinction coefficient at 280 nm, which were calculated from the amino acid sequence.
D. Sodiumdodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Purified proteins were diluted in 5X sample buffer (60 mM Tris, pH 6.8, 50% glycerol, 2% SDS, 14.4 mM 2-mercaptoethanol, and 0.1% bromophenol blue in H2O) and boiled for 10 min at 100oC. Protein samples (~ 5 μg) were submitted to electrophoresis on 12% polyacrylamide gels for approximately 2 h at 100 V. Proteins were visualized with Coomassie brilliant blue.
E. Competitive ELISA
For detection of binding of 3D8 scFv△ pA to soluble GAGs, microtiter plates were coated with 10 μg/ml of CS-A or Hp as antigen at 4o
7
(Tris-buffered saline, pH 7.4 plus 0.1% Tween 20) containing 3% (w/v) bovine serum albumin (BSA) for 2 h at room temperature (RT). Plates were then added with various concentrations (0 – 100 μg/ml) of Hp and 3D8 scFv△ pA(20 μg/ml) or various concentrations (0 – 100 μg/ml) of CS-A and 3D8 scFv△ pA (20 μg/ml), respectively. Then, plates were incubated for 1 h at RT. After washing with TBST three times, the bound 3D8 scFv△ pA was detected with anti-His6 tag antibody and then AP-goat anti-mouse IgG antibody. After washing, ρ-NPP solution (1 mg/ml in 0.1 M glycine, 1 mM ZnCl2, and 0.1 mM MgCl2, pH 10.4) was added and absorbance at 405 nm was measured with a microplate reader (Molecular Devices).
F. Flow cytometry
For detection of the internalized 3D8 scFv△ pA in the presence of soluble GAGs, 10 μM of 3D8 scFv△ pA was pre-incubated with soluble GAGs (Hp, DS, A, B, and CS-C: 100 μg/ml) for 30 min at 37o
C. HeLa cells were detached with a non-enzymatic cell dissociation solution from cell culture dish and 6 x 105HeLa cells were treated with pre-mixture and incubated for 6 h at 37oC. After incubation, cells were washed with PBS three times by centrifugation at 1,500 rpm for 3 min at 4oC. After washing, cells were treated with trypsin-EDTA for 10 min at 37oC. Cells were fixed with 4% (w/v) paraformaldehyde/ PBS for 20 min at RT. Then cells were permeabilized with permeabilization buffer (1% BSA, 0.1% saponin, and 0.1% sodium azide in PBS) for 1 h at 4oC. After PBS washing, cells were incubated with rabbit anti-3D8 scFv antibody (1: 200) diluted in permeabilization buffer for
8
1 h at 4oC. Cells were washed with PBS three times and incubated with Alexa Flour 647-conjugated anti-rabbit IgG (1: 400 diluted in permeabilization buffer) for 1 h at 4oC. After washing with PBS three times, cells were resuspended in 4% (w/v) paraformaldehyde/ PBS and analyzed with flow cytometer (BD FACS Canto II).
For detection of 3D8 scFv internalization in wild type and pan-GAG-deficient pgsA-745 mutant CHO cells, cells were detached with a non-enzymatic cell dissociation solution from cell culture dish and 1 x 106 cells were incubated with 10 μM of 3D8 scFv for the indicated times at 37oC. After incubation, cells were washed with PBS three times by centrifugation at 1,500 rpm for 3 min at 4oC. After washing cells were treated with trypsin-EDTA for 10 min at 37oC. Cells were fixed with 4% (w/v) paraformaldehyde/ PBS for 20 min at RT. Then cells were permeabilized with permeabilization buffer for 1 h at 4oC. After PBS washing, cells were incubated with rabbit anti-3D8 scFv antibody (1: 200) diluted in permeabilization buffer for 1 h at 4oC. Cells were washed with PBS three times and incubated with Alexa Fluor 647-conjugated anti-rabbit IgG (1: 400 diluted in permeabilization buffer) for 1 h at 4oC. After washing with PBS three times, cells were resuspended in 4% (w/v) paraformaldehyde/ PBS and analyzed with flow cytometer (BD FACS Canto II).
G. Confocal microscopy
For detection of binding of 3D8 scFv△ pA to cell surface HSPGs in the presence of soluble GAGs, HeLa cells were seeded in 24-well plate with cover slips at 4 x 104 cells/ well.
9
3D8 scFv△ pA (10 μM) was pre-incubated with soluble GAGs (Hp and CS-A: 100 μg/ml) for 30 min at 4oC. The pre-mixture was treated to HeLa cells and incubated for 1 h at 4oC. Cells were washed with PBS five times in shaker and then fixed in 4% (w/v) paraformaldehyde for 10 min at RT. After three times washing with PBS, cells were incubated with the mixture of rabbit anti-3D8 scFv antibody (1: 200) and mouse anti-heparan sulfate antibody (1: 200) diluted in the surface buffer (0.5% BSA and 2 mM EDTA in PBS, pH 8.5) at 4oC overnight. Cells were then washed with PBS five times and incubated with the mixture of TRITC-conjugated anti-rabbit IgG (1: 200) and Alexa Fluor 488-conjugated anti-mouse IgM (μ chain-specific: 1: 200) diluted in the surface buffer for 1 h at RT. After five times washing with PBS, nuclei were stained with Hoechst 33342 (20 μg/ml).After washing with PBS once, cells were mounted in Vectashield, and then analyzed using Zeiss LSM 710 laser confocal microscope equipment with a 40 X 1.2 NA water immersion objective.
For detection of interaction of 3D8 scFv△ pA and cell surface PGs, HeLa cells were incubated with 10 μM of 3D8 scFv△ pA for 1 h at 4o
C. Cells were washed with PBS five times in shaker and then fixed in 4% (w/v) paraformaldihyde for 10 min at RT. After three times washing with PBS, cells were incubated with either the mixture of rabbit anti-3D8 scFv antibody (1: 200) and mouse anti-heparan sulfate antibody (1: 200), or the mixture of rabbit anti-3D8 scFv antibody (1: 200) and mouse anti-chondroitin sulfate antibody (1: 200) diluted in the surface buffer at 4oC overnight. After washing with PBS five times and cells were incubated with the mixture of TRITC-conjugated anti-rabbit IgG (1: 200) and Alexa
10
Fluor 488-conjugated anti-mouse IgM (μ chain-specific: 1: 200) diluted in the surface buffer for 1 h at RT. After five times washing with PBS, nuclei were stained with Hoechst 33342. After washing with PBS once, cells were mounted in Vectashield, and then analyzed using Zeiss LSM 710 laser confocal microscope equipment with a 40 X 1.2 NA water immersion objective.
For examination of intracellular co-localization, HeLa cells were incubated with 10 μM of 3D8 scFv△ pA for the indicated times at 37o
C. Following incubation, cells were fixed with 4% (w/v) paraformaldehyde/ PBS for 10 min at RT and permeabilized with permeabilization buffer for 10 min at RT. After washing with PBS three times, cells were incubated with either the mixture of rabbit anti-3D8 scFv (1: 200), mouse anti-caveolin 1 antibody (1: 200) and mouse anti-heparan sulfate antibody (1: 100), or the mixture of rabbit anti-3D8 scFv (1: 200), mouse anti-caveolin 1 antibody (1: 200), and mouse anti-chondroitin sulfate antibody (1: 100) diluted in permeabilization buffer for overnight at 4oC. After washing with PBS five times, cells were incubated with the mixture of Alexa Flour 647-conjugated anti-rabbit IgG (1: 200), TRITC-647-conjugated anti-mouse IgG (1: 200), and Alexa Flour 488-conjugated anti-mouse IgM (μ chain-specific) (1: 200) diluted in permeabilization buffer for 1 h at RT. After five times washing with PBS, cells were mounted in Vectashield, and then analyzed using Zeiss LSM 710 laser confocal microscope equipment with a 40 X 1.2 NA water immersion objective.
For detection of the internalized 3D8 scFv in wild type and pan-GAG-deficient pgsA-745 mutant CHO cells, cells were seeded in 24-well plate with cover slips at 4 x 104
11
cells/ well. After 1 day, cells were incubated with 10 μM of 3D8 scFv for 2 h at 37o
C. After incubation, cells were washed with PBS five times in shaker. Cells were then fixed and permeabilized. After PBS washing, cells were incubated with rabbit IgG (10 μg/ml) diluted in permeabilization buffer for 1 h at RT. Cells were washed with PBS five times and incubated with FITC-conjugated anti-rabbit IgG (1: 200 diluted in permeabilization buffer) for 1 h at RT. After five times washing with PBS, nuclei were stained with Hoechst 33342. After washing with PBS once, cells were mounted in Vectashield, and then analyzed using Zeiss LSM 710 laser confocal microscope equipment with a 40 X 1.2 NA water immersion objective.
12
III. RESULTS
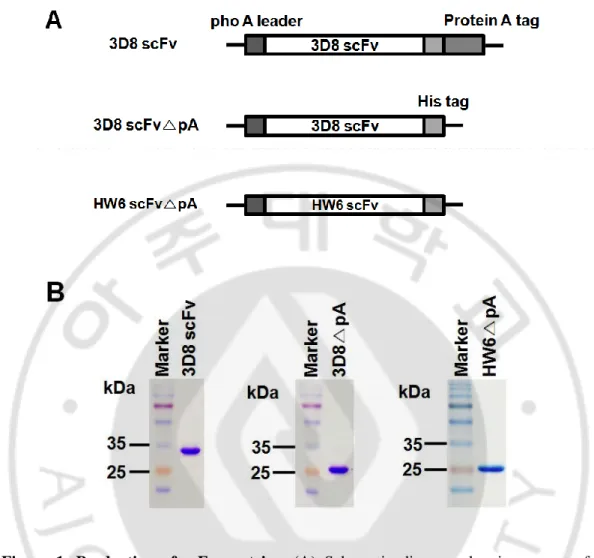
A. Purify the 3D8 and HW6 proteins.
pIg20 vector was used to express scFv proteins with both His6 tag and
Staphyplococcal protein A tag (pA). pIg20△ pA was constructed to express scFv without pA
by inserting a stop codon upstream of pA (Fig. 1A). 3D8 and HW6 proteins were produced from bacterial expression system. The purity of purified proteins was ~90% on SDS-PAGE gels (Fig. 1B). The yields of 3D8 scFv, 3D8 scFv△ pA, and HW6 scFv△ pA were about 4-6
13
Figure 1. Production of scFv proteins. (A) Schematic diagram showing a part of the
expression vectors for the indicated proteins. (B) SDS-PAGE of purified proteins. The plasmids encoding the 3D8 scFv (~ 34 kDa), 3D8 scFv△ pA (~ 27 kDa), and HW6 scFv△ pA (~ 28 kDa) were transformed into E.coli and purified from the culture supernatant
using IgG-Sepharose column or Protein L-agarose column. Proteins (5μg) were run on a 12% polyacrylamide gel and visualized with Coomassie brilliant blue.
14
B. 3D8 internalization depends on HSPGs and CSPGs.
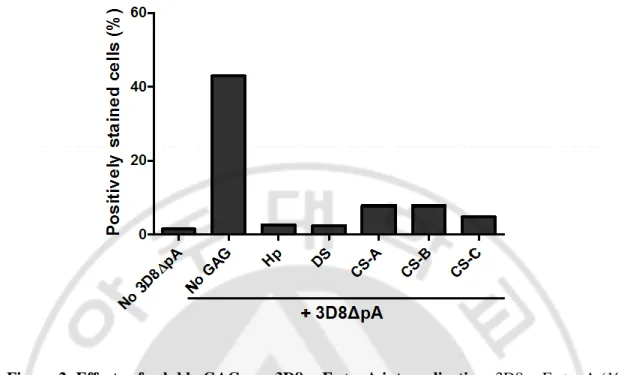
Previous works by another groups have reported that cell-penetrating peptide and cationic lipids enter the cells by interacting with HSPGs (Goncalves et al., 2005; Poon and Gariepy, 2007; Song et al., 2008). Moreover, previous our work has shown that 3D8 scFv internalization was reduced in the presence of soluble Hp that is a high-sulfated HS (Dreyfuss et al., 2009) in HeLa cells (Jang et al., 2009). Here, we examined whether anionic cell surface CSPGs as well as HSPGs can act as internalizing sites for 3D8 scFv. When HeLa cells were incubated with 3D8 scFv△ pA in the presence of soluble GAG, internalization of 3D8 scFv△ pA was significantly inhibited (Fig. 2). Our results suggest that cell surface HSPGs and CSPGs are required for 3D8 scFv internalization.
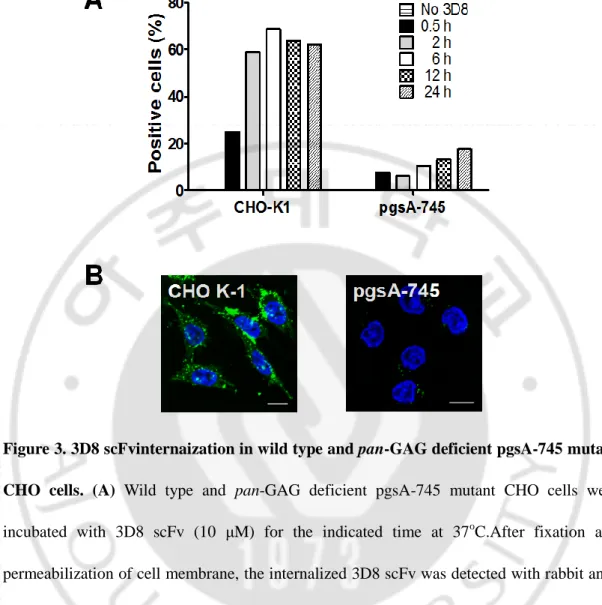
Next, we carried out flow cytometry with wild type and pan-GAG-deficient pgsA-745 mutant CHO cells. When wild type and pan-GAG-deficient pgsA-pgsA-745 mutant CHO cells were incubated with 3D8 scFv, internalization of 3D8 scFv was severely impaired in pan-GAG-deficient pgsA-745 mutant CHO cells compared with that in wild type CHO cells (Fig. 3A). This data was consistent with that by confocal microscopy (Fig. 3B). These results support the data shown in Fig. 2.
15
Figure 2. Effects of soluble GAGs on 3D8 scFv△ pA internalization. 3D8 scFv△ pA (10
μM) was pre-incubated with soluble GAGs such as Hp, DS, CS-A, CS-B, or CS-C (100 μg/ml) for 30 min at 37o
C. HeLa cells were incubated with the pre-mixture for 6 h at 37oC. After fixation and permeabilization of cell membrane, the internalized 3D8 scFv△ pA was detected with rabbit anti-3D8 scFv antibody and Alexa Flour 647-conjugated anti-rabbit IgG, prior to flow cytometry.
16
Figure 3. 3D8 scFvinternaization in wild type and pan-GAG deficient pgsA-745 mutant CHO cells. (A) Wild type and pan-GAG deficient pgsA-745 mutant CHO cells were
incubated with 3D8 scFv (10 μM) for the indicated time at 37oC.After fixation and permeabilization of cell membrane, the internalized 3D8 scFv was detected with rabbit anti-3D8 scFv antibody and Alexa Fluor 647-conjugated anti-rabbit IgG, prior to flow cytometry.
(B) Wild type and pan-GAG deficient pgsA-745 mutant CHO cells were incubated with 3D8
scFv (10 μM) for 2 h at 37oC.After fixation and permeabilization of cell membrane, the internalized 3D8 scFv (green) was detected with rabbit IgG and FITC-conjugated anti-rabbit IgG, prior to confocal microscopy. Bar, 10 μm.
17
C. 3D8 scFv△ pA binds to cell surface HSPGs and CSPGs.
To confirm the binding of 3D8 scFv△ pA to cell surface HSPGs and CSPGs, we performed competitive ELISA. 3D8 scFv△ pA was pre-incubated with Hp as a competitor and then this pre-mixture was added to CS-A-coated plate. The binding of 3D8 scFv△ pA to CS-A was decreased in an Hp concentration-dependent manner (Fig. 4A). In reverse, when we used Hp as an antigen and CS-A as a competitor, binding of 3D8 scFv△ pA to Hp was decreased in a CS-A concentration-dependent manner (Fig. 4B). In confocal microscopy, co-localization of 3D8 scFv△ pA, cell surface HSPGs, and CSPGs was observed on the surface of HeLa cells treated with 3D8 scFv△ pA (Fig. 4C and D).
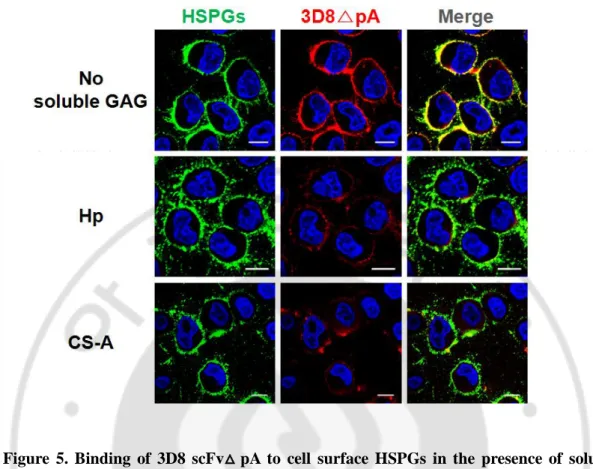
To investigate whether HSPGs compete with CSPGs for 3D8scFv△ pA-binding on the cell surface, we incubated HeLa cells with 3D8 scFv△ pA in the presence of soluble Hp or CS-A. Binding of 3D8 scFv△ pA to cell surface was markedly reduced in the presence of soluble Hp or CS-A (Fig. 5). Overall, these results show that 3D8 scFv△ pA bind to both of cell surface HSPGs and CSPGs on HeLa cells.
19
Figure 4. Binding of 3D8 scFv△ pA to HSPGs and CSPGs. (A) Competitive ELISA.
CS-A (10 μg/ml) was coated on ELISCS-A plate. 3D8 scFv△ pCS-A (20 μg/ml) was pre-incubated with Hp (0 - 100 μg/ml) for 30 min at RT. The 3D8 scFv△ pA bound to CS-A was detected with mouse anti-His6 tag antibody, and then AP-conjugated anti-mouse IgG. (B) Competitive
ELISA. Hp (10 μg/ml) was coated on plate. 3D8 scFv△ pA (20 μg/ml) was pre-incubated
with CS-A (0 - 100 μg/ml) for 30 min at RT. The 3D8 scFv△ pA bound to Hp was detected with mouse anti-His6 tag antibody, and then AP-conjugated anti-mouse IgG. Experiments were done in triplicate. (C and D) Confocal microscopy. HeLa cells were incubated with HW6 scFv△ pA (10 μM) as negative control and 3D8 scFv△ pA (10 μM) for 1 h at 4o
C. Then, cells were incubated with the mixture of Alexa Flour 488-conjugated anti-mouse IgM and TRITC-conjugated anti-rabbit IgG. Binding of scFv△ pA proteins (red) to cell surface HSPGs (C, green) or CSPGs (D, green) was visualized by confocal microscopy. Bar, 10 μm.
20
Figure 5. Binding of 3D8 scFv△ pA to cell surface HSPGs in the presence of soluble GAGs. HeLa cells were incubated with 3D8 scFv△ pA (10 μM) in the absence (upper panel)
or in the presence (middle or lower panel) of Hp (100 μg/ml) or CS-A (100 μg/ml) for 1 h at 4oC. Then, cells were incubated with the mixture of Alexa Flour 488-conjugated anti-mouse IgM and TRITC-conjugated anti-rabbit IgG. The 3D8 scFv△ pA (red) bound to cell surface was detected by confocal microscopy. Bar, 10 μm.
21
D. Internalized 3D8 scFv△ pA co-localizes with HSPGs, CSPGs, and caveolin 1.
Previous our group has demonstrated that 3D8 scFv internalization occurs by caveolae/lipid-raft-mediated endocytosis in HeLa cells (Jang et al., 2009). Accordingly, we speculated that the internalized 3D8 scFv△ pA with either HSPGs and CSPGs would
co-localize with caveolin1, caveolar structural protein, in cytosol. HeLa cells were incubated with 3D8 scFv△ pA and then we detected the internalized 3D8 scFv△ pA, PGs, and caveolin1 by confocal microscopy. Intracellular co-localization of 3D8 scFv△ pA and
HSPGs was detected on 6 h and 12 h incubation, but not on 2 h (Fig.6A). Intracellular co-localization of 3D8 scFv△ pA and CSPGs was observed on 2 h, 6 h, and 12 h incubation (Fig. 6B). Representative images show the intracellular co-localization of 3D8 scFv△ pA-PGs (yellow), 3D8 scFv△ caveolin 1 (magenta), PGs-caveolin1 (cyan), or 3D8 scFv△ pA-PGs-caveolin 1 (white) during endocytosis. These results show that internalization of 3D8 scFv△ pA is mediated by cell surface HSPGs and CSPGs. Besides, this result is consistent with previous data that 3D8 scFv internalizes through caveolae/lipid-raft-mediated endocytosis.
23
Figure 6. Intracellular co-localization between 3D8 scFv△ pA, HSPGs, CSPGs, and caveolin 1. HeLa cells were treated with 10 μM of 3D8 scFv△ pA for 2 h, 6 h, and 12 h at
37oC. After fixation and permeabilization of cell membrane, cells were incubated with the mixture of three antibodies of Alexa Flour 488-conjugated anti-mouse IgM, Alexa Flour 647-conjugated anti-rabbit IgG, and TRITC-647-conjugated anti-mouse IgG. The co-localization of 3D8 scFv△ pA-PGs (yellow), 3D8 scFv△ pA-caveolin 1 (magenta), PGs-caveolin 1 (cyan), or 3D8 scFv△ pA-PGs-caveolin 1 (white) was detected by confocal microscopy. Under
Detail, the enlarged images of the indicated region show co-localization in detail. Bar, 10 μm
24
IV. DISCUSSION
A subset of monoclonal anti-DNA antibodies can internalize cells and then translocate the nucleus in variety of cells (Yanase et al., 1994; Zack et al., 1996; Ternynck et al., 1998; Lee et al., 2007). Up to date, function of HSPGs and CSPGs as an endocytic receptor for the internalization of anti-DNA Abs has not been studied. Here, we report first that cell surface HSPGs and CSPGs are true internalizing receptor for anti-nucleic acid antibody, 3D8 scFv.
So far several membrane components, such as calreticulin, myosin 1, and equilibrative nucleoside salvage transporter (ENT), have been suggested as potential cell surface receptors for the internalization of anti-DNA Abs. Anti-dsDNA mAb was also reported not to require a cell-membrane receptor since cell-penetration was not inhibited by endocytosis inhibitors (Song et al., 2008). It was from the findings that calreticulin (for mAbs F14.6 and H9.3) (Seddiki et al., 2001) or myosin 1 (for mAb H7) (Yanase et al., 1997) has been immunocaptured from the cells treated with the anti-DNA Abs, and 3E10 mAb was unable to penetrate into ENT-deficient cells (Hansen et al., 2007). Moreover, 9D7 anti-dsDNA mAb was reported not to require a cell-membrane receptor since cell-penetration was not inhibited by endocytosis inhibitors (Song et al., 2008). Therefore, it seems that different anti-DNA Abs may preferentially take distinct pathways of penetration.
Human and murine anti-DNA Abs are cross-reactive with HSPGs, the major GAG in glomerular basement membranes (Faaber et al., 1986; Naparstek et al., 1990). Mice
25
immunization with HS induced systemic lupus erythematosus-like disease (Ofosu-Appiah et al., 1998). Hp-affinity chromatography has been widely used for the purification of DNA-binding proteins, such as transcription factors, steroid hormone receptors, and histones (Belting, 2003). Despite accumulated documents for cross-reactivity of DNA-binding proteins to HS, the involvement of cell surface HSPGs in the uptake of anti-DNA Abs have not been demonstrated before our study. Moreover, previous our work showed that 3D8 scFv internalization was inhibited in the presence of soluble Hp in HeLa cells (Jang et al., 2009). Based on these studies, we hypothesized that 3D8 scFv, a basic protein (pI = 9.15), enters the cells by interacting with cell surface HSPGs and CSPGs which are negatively charged molecules on the plasma membrane. Our result showed that 3D8 scFv△ pA internalization was inhibited in the presence of soluble CS as well as Hp (Fig. 2) and 3D8 scFv△ pA bound to cell surface CSPGs as well as HSPGs to a similar extent (Fig. 4). From these data, it is clear that 3D8 scFv△ pA interacts with both cell surface CSPGs as well as HSPGs without any preference before it penetrates into cells.
HSPGs have been proposed to act either as true internalizing receptors or as co-receptors for temporary cell surface attachment in the internalization of a variety of macromolecules because it has been shown that the internalization of macromolecules such as DNA, cationic polymers, and cell-penetrating peptides is dependent on the presence of the intact cell surface HSPGs. Recently a direct evidence for role as a true internalizing receptors of HSPGs was provided by the observation of translocation of intact cell surface HSPGs (syndecan and glypican) to endocytic vesicles induced by anti-HS scFv (AO4B08), depending on the interaction with HS 2-O-sulfation (Wittrup et al., 2009). Like anti-HS scFv,
26
3D8 scFv internalized along with HSPGs, supported by the observation of co-localization of 3D8 scFv with HSPGs on cell surface and inside cells during endocytosis (Fig. 4C and 6A). Compared to numerous documents for endocytosis via binding of macromolecules upon HSPGs, only a few is known for involvement of cell surface CSPGs in endocytosis. Low density lipoprotein (LDL) interacts with CSPGs, internalized, and degraded in human macrophage (Hurt-Camejo et al., 1990). Yang et al have shown that intracellular translocation and cytotoxicity of penetratin-directed mitochondria-disrupting peptides was reduced by the treatment of soluble CS or chondroitinase, and was positively correlated to expression level of CS on cell surface. They suggested that CS overexpression in tumor cells is an important molecular portal that mediates the preferential cytotoxicity of Antp-directed peptides. However, in those molecules, direct evidence for intracellular co-localization with CSPGs has not been shown. (Yang et al., 2010). In present our study, intracellular co-localization between three molecules of HSPGs-3D8 scFv△ pA-caveolin 1 and CSPGs-3D8 scFv△ pA-caveolin 1 were observed (Fig. 6), indicating that both cell surface HSPGs and CSPGs act as true receptor for 3D8 scFv△ pA internalization in HeLa cells. It is worthy to note that our studies showed that cell surface CSPGs as well as HSPGs serve as a true endocytic receptor for 3D8 scFv.
Our finding that anti-nucleic acid Ab enters cells through both HSPGs and CSPGs as true endocytic receptors on cell surface could contribute to the understanding for role of cell surface HSPGs and CSPGs in developing delivery materials to cells.
27
V. CONCLUSION
We provide the evidence for both HSPGs and CSPGs as internalizing receptor of an anti-nucleic acid antibody, 3D8 scFv.
28
REFERENCES
1. Belting M: Heparan sulfate proteoglycan as a plasma membrane carrier. Trends
Biochem Sci 28: 145-151, 2003
2. Dreyfuss JL, Regatieri CV, Jarrouge TR, Cavalheiro RP, Sampaio LO, Nader HB: Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An
Acad Bras Cienc 81: 409-429, 2009
3. Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD, Tor Y: Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem 282: 13585-13591, 2007
4. Esko JD, Kimata K, Lindahl U: Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology (eds. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME) Cold Spring Harbor (NY), 2009
5. Esko JD, Selleck SB: Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435-471, 2002
6. Faaber P, Rijke TP, van de Putte LB, Capel PJ, Berden JH: Cross-reactivity of human and murine anti-DNA antibodies with heparan sulfate. The major glycosaminoglycan in glomerular basement membranes. J Clin Invest 77: 1824-1830, 1986
7. Goncalves E, Kitas E, Seelig J: Binding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry 44: 2692-2702, 2005
8. Hacker U, Nybakken K, Perrimon N: Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol 6: 530-541, 2005
9. Hahn BH: Antibodies to DNA. N Engl J Med 338: 1359-1368, 1998
10. Hansen JE, Tse CM, Chan G, Heinze ER, Nishimura RN, Weisbart RH: Intranuclear protein transduction through a nucleoside salvage pathway. J Biol Chem 282: 20790-20793, 2007
29
11. Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Wiklund O, Bondjers G: Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J Lipid Res 31: 1387-1398, 1990
12. Jang JY, Jeong JG, Jun HR, Lee SC, Kim JS, Kim YS, Kwon MH: A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci 66: 1985-1997, 2009
13. Jang YJ, Stollar BD: Anti-DNA antibodies: aspects of structure and pathogenicity.
Cell Mol Life Sci 60: 309-320, 2003
14. Kim YR, Kim JS, Lee SH, Lee WR, Sohn JN, Chung YC, Shim HK, Lee SC, Kwon MH, Kim YS: Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J Biol Chem 281: 15287-15295, 2006
15. Lee EJ, Jang EJ, Lee E, Yu J, Chung HY, Jang YJ: Cell-penetrating autoantibody induces caspase-mediated apoptosis through catalytic hydrolysis of DNA. Bioorg
Med Chem 15: 2016-2023, 2007
16. Naparstek Y, Ben-Yehuda A, Madaio MP, Bar-Tana R, Schuger L, Pizov G, Neeman ZV, Cohen IR: Binding of anti-DNA antibodies and inhibition of glomerulonephritis in MRL-lpr/lpr mice by heparin. Arthritis Rheum 33: 1554-1559, 1990
17. Ofosu-Appiah W, Sfeir G, Viti D, Burashnikova E: Induction of systemic lupus erythematosus-like disease in mice by immunization with heparan sulfate. Cell
Immunol 183: 22-31, 1998
18. Poon GM, Gariepy J: Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem Soc Trans 35: 788-793, 2007
19. Sarrazin S, Lamanna WC, Esko JD: Heparan sulfate proteoglycans. Cold Spring
Harb Perspect Biol 3, 2011
20. Seddiki N, Nato F, Lafaye P, Amoura Z, Piette JC, Mazie JC: Calreticulin, a potential cell surface receptor involved in cell penetration of anti-DNA antibodies. J Immunol
30
166: 6423-6429, 2001
21. Song YC, Sun GH, Lee TP, Huang JC, Yu CL, Chen CH, Tang SJ, Sun KH: Arginines in the CDR of anti-dsDNA autoantibodies facilitate cell internalization via electrostatic interactions. Eur J Immunol 38: 3178-3190, 2008
22. Ternynck T, Avrameas A, Ragimbeau J, Buttin G, Avrameas S: Immunochemical, structural and translocating properties of anti-DNA antibodies from (NZBxNZW)F1 mice. J Autoimmun 11: 511-521, 1998
23. Tyagi M, Rusnati M, Presta M, Giacca M: Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem 276: 3254-3261, 2001
24. Wittrup A, Zhang SH, ten Dam GB, van Kuppevelt TH, Bengtson P, Johansson M, Welch J, Morgelin M, Belting M: ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: evidence for heparan sulfate epitope specificity and role of both syndecan and glypican. J Biol Chem 284: 32959-32967, 2009
25. Yanase K, Smith RM, Cizman B, Foster MH, Peachey LD, Jarett L, Madaio MP: A subgroup of murine monoclonal anti-deoxyribonucleic acid antibodies traverse the cytoplasm and enter the nucleus in a time-and temperature- dependent manner. Lab
Invest 71: 52-60, 1994
26. Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP: Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest 100: 25-31, 1997
27. Yang H, Liu S, Cai H, Wan L, Li S, Li Y, Cheng J, Lu X: Chondroitin sulfate as a molecular portal that preferentially mediates the apoptotic killing of tumor cells by penetratin-directed mitochondria-disrupting peptides. J Biol Chem 285: 25666-25676, 2010
28. Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH: Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J
31 - 국문요약 -
항-핵산항체 (3D8 scFv)의 세포 내 유입에 관여하는
세포 표면 수용체 분자 규명
아주대학교 대학원 의생명과학과 김혜진 (지도교수: 권명희) 일부의 항-핵산항체는 다양한 종류의 세포 내로 유입 할 수 있다. 3D8 single chain variable fragment (scFv)는 caveolae/lipid-raft 세포 내 이입 경로를 통해서 세포 내로 유입 할 수 있는 항-핵산항체이다. 그러나 3D8 scFv의 세포 내 유입에 관여하는 수용체는 아직 밝혀지지 않았다. 본 연구에서는 3D8 scFv의 세포 내 유입 수용체를 알아보고자, 공촛점현미경 분석법을 수행하여 다음을 관찰하였다. HeLa세포에서 3D8 scFv는 여러 가지 리간드 (ligand)의 수용체로 알려져 있는 세포막 헤파란황산 단백당 (heparan sulfate proteoglycans: HSPGs)과 콘드로이틴황산 단백당 (chondroitin sulfate proteoglycans: CSPGs)에 결합하였다. 3D8 scFv와 가용성 헤파란황산 또는 콘드로이틴황산을 함께 세포에 처리하였을 때에 3D8 scFv과 세포 표면 헤파란황산 단백당과의 결합이 저해되었다. 3D8 scFv를 세포에 처리한 후 세포 안에서 세 가지 분자들의 공존 즉, 3D8 scFv-헤파란황산 단백당-카베올린32 1 (caveolin 1)의 공존 및 3D8 scFv-콘드로이틴 황산단백당-카베올린 1의 공존이 관찰되었다. 또한 3D8 scFv의 세포 내 유입이 세포막 헤파란황산 단백당과 콘드로이틴 황산단백당이 결여된 CHO 세포주에서 상당히 감소되었다. 이러한 결과들은 헤파란황산 단백당과 콘드로이틴황산 단백당이 3D8 scFv의 세포 내 유입 수용체임을 보여준다. 핵심어: 항-핵산항체, 3D8 scFv, 세포내 이입 수용체, 헤파란황산 단백당, 콘드로이틴황산 단백당