Attribution-NonCommercial-NoDerivs2.0 KOREA You are free to :
Share — copy and redistribute the material in any medium or format Under the follwing terms :
Attribution — You must give appropriate credit, provide a link to the license, and
indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial — You may not use the material for commercial purposes.
NoDerivatives — If you remix, transform, or build upon the material, you may not distribute the modified material.
You do not have to comply with the license for elements of the material in the public domain or where your use is permitted by an applicable exception or limitation.
This is a human-readable summary of (and not a substitute for) the license.
Disclaimer
Dissertation for the degree of Doctor of Philosophy
Disease Model for Meniscus Degeneration:
Clinical Inflammatory and Degenerative Tear Model
By
Do Young Park
Department of Anatomy
Disease Model for Meniscus Degeneration:
Clinical Inflammatory and Degenerative Tear
Model
By
Do Young Park
A Dissertation of submitted to the Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of DOCTOR OF PHILOSOPHY
Supervised by
Haeyoung Suh-Kim M.D., Ph.D.
Department of Biomedical Sciences
The Graduate School, Ajou University
Disease Model for Meniscus Degeneration:
Clinical Inflammatory and Degenerative Tear
Model
By Do Young Park
A Dissertation of submitted to the Graduate School of Ajou University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy
Approved by: Date
Byoung-Hyun Min, Committee chair, Professor,
Department of Orthopedic Surgery, School of Medicine, Ajou University
Date
Jae-Ho Lee, Professor,
Department of Biochemistry & Molecular Biology, School of Medicine, Ajou University
Date
Jae Ho Cho, Professor,
Department of Orthopedic Surgery, School of Medicine, Ajou University
Date
Jin-Goo Kim, Professor,
Department of Orthopedic Surgery, Konkuk University Medical Center
Date
Haeyoung Suh-Kim, Thesis Adviser, Professor,
i
Acknowledgements
It has truly been a great honor and a privilege to have had a chance to devote myself into the field of orthopedic basic research over the last 5 years. I would first and foremost like to thank my mentor, Dr. Byoung-Hyun Min. All the things that I have learned, from science to surgery, I owe it to him. I would also like to thank Professor Haeyoung Suh-Kim, who has provided guidance through the whole process. Research-wise, I would like to thank Professor Sora Park, Professor Byung-Hyune Choi, Professor Young Jick Kim, Professor Jae-Ho Lee, and Dr. Jin-Goo Kim, for providing scientific guidance for this thesis. I also thank Dr. Jae Ho Cho, who has been my mentor since my residency, providing support in every step. This work would not have been possible without the help of all my co-workers, especially Dr. Boram Song, Dr. Minh-Dung Truong, Mijin Kim, Xiang Yun Yin, and Hee Woong Yun.
I would also like to thank my parents and Dr. Jae Young Park, who has always supported me. Finally, I dedicate this work to my wife, Dr. In Kyong Yi, and my daughter Joo Won Park, who have made this and everything else possible.
ii
ABSTRACT
The meniscus is a fibrocartilaginous intra-articular structure of the knee that functions as a load bearing surface, offering protection of articular cartilage and stability of the knee joint. The meniscus degenerates due to multiple factors, such as trauma, overuse, overweight, and osteoarthritis (OA). While meniscus degeneration is common and present in multiple forms, current treatment options are extremely limited to partial meniscectomy and meniscus allograft transplantation. Current procedures fall short of restoring meniscus functions and preventing OA progression. Many hurdles remain to improve current treatment, and such hurdles stem from the little known pathogenesis and absence of a disease model. This study aims to devise a disease model for meniscus degeneration. First, we sought to evaluate inflammatory changes and related degeneration of the meniscus, along with cartilage and synovium, against polyethylene wear particles in a unicompartmental knee arthroplasty model, thereby establishing a clinical inflammation model. Secondly, we sought to characterize the degeneration process of meniscus root tears in osteoarthritic knees, with emphasis on fibrocartilage and calcification, thereby establishing a clinical degenerative tear model.
iii
In chapter I, we hypothesized that ultra-high molecular weight polyethylene
(UHMWPE) particles per se would interact with intra-articular tissue, which by acting as inflammatory reservoirs, would subsequently induce OA changes in vitro and in vivo. UHMWPE particles were generated by a previously published micro-cutting process method. In vitro experiment was carried out using an inverted culture system. Three cell types were used; chondrocytes, meniscal fibrochondrocytes, and synoviocytes. Each cell type was cultured with two different concentrations of UHMWPE particles. Pro-inflammatory cytokine production, phagocytosis, and apoptosis were analyzed. In vivo experiment was done by injecting two concentrations of UHMWPE particles into normal and murine OA model knee joints. In vitro experiment showed that pro-inflammatory cytokine and mediator (IL-1β, IL-6, TNF-α, Nitric Oxide, and Prostaglandin E2) production, phagocytosis of particles, and apoptosis were increased in all cell types. In vivo experiment showed degeneration of cartilage and meniscus, as well as synovitis after UHMWPE particle injection. UHMWPE wear particles per se exert toxic effects in cartilage, synovium, and meniscus of the knee joint resulting in pro-inflammatory cytokine release, phagocytosis of particles and apoptosis. This study suggests a clinical inflammatory model based on UKA for meniscus degeneration.
iv
posterior root tears among osteoarthritic knees with emphasis on fibrocartilage and calcification. Samples of medial meniscus posterior roots were harvested from cadavers and patients during knee replacement surgery and grouped as follows; normal reference, no tear, partial tear, and complete tear. Degeneration was analyzed with histology, immunohistochemistry, and PCR. Uniaxial tensile tests were performed on specimens with and without fibrocartilage. Quantifiable data was statistically analyzed by Kruskal-Wallis test with Dunn’s comparison test. Thirty, 28, and 42 samples harvested from 99 patients were allocated in no tear, partial tear, and complete tear group, respectively. Modified Bonar tendinopathy scores for each group were 3.97, 9.31, and 14.15, respectively, showing higher degree of degeneration associated with tear extent (p<0.05 for all groups). Characterization of root matrices revealed increase in fibrocartilage according to tear extent. Tear margins revealed fibrocartilage in 59.3% of partial tear and 76.2% of complete tear samples with distinctive cleavage-like morphology. Root tears with similar morphology were induced within fibrocartilaginous areas during uniaxial tensile testing. Even in the no tear group, 56.6% of samples showed fibrocartilage in the anterior margin of root, adjacent to the meniscus. Increased staining area of calcification and expression of ENPP1 gene were observed in the complete tear group compared to no tear group (p<0.0001). Fibrocartilage and calcification increased in medial meniscus posterior roots, associated with the degree of tear. Both findings, which impair the
v
ligament’s resistance to tension, may play a pivotal role during pathogenesis of degenerative meniscal root tears in OA knees.
Keywords: meniscus; meniscus degeneration; UHMWPE; unicompartmental knee arthroplasty; fibrocartilage; disease model.
vi TABLE OF CONTENTS Acknowledgements ... i ABSTRACT ... ii TABLE OF CONTENTS ... vi LIST OF FIGURES ... ix Background ... 1 1.1. Meniscus ... 2 1.2. Meniscus Degeneration ... 4
1.3. Unmet Needs in Meniscus Degeneration ... 4
1.4. Aim of Study ... 5
CHAPTER I: Inflammatory and degenerative responses of meniscal fibrochondrocytes, chondrocytes, and synoviocytes to polyethylene wear particles in a unicompartmental knee arthroplasty model ... 6
2.1. Introduction ... 7
2.2. Material and Methods ... 9
2.2.1.UHMWPE Wear Particle Production ... 9
2.2.2.Cell Harvesting and Culture ... 12
2.2.3.Analysis of Inflammation ... 13 2.2.4.Phagocytosis ... 14 2.2.5.Confocal Microscopy ... 14 2.2.6.Apoptosis ... 15 2.2.7.Animals ... 15 2.2.8.Histological Assessment ... 16
vii
2.2.9.Statistical Analysis ... 17
2.3. Results ... 18
2.3.1.Inflammatory cytokine and mediator production in all cell types increase after co-culture with UHMWPE... 18
2.3.2.Intra-articular cells exhibit phagocytosis toward UHMWPE particles .... 20
2.3.3.Apoptosis after co-culture of intra-articular cells and UHMWPE ... 23
2.3.4.In Vivo Study ... 25
2.4. Discussion ... 29
CHAPTER II: Degeneration of meniscus roots are accompanied by fibrocartilage formation, which may precede meniscus root tears in osteoarthritic knees 35 3.1. Introduction ... 36
3.2. Methods ... 39
3.2.1.Experimental design and tissue samples ... 39
3.2.2.Gross morphology analysis and length measurement of the meniscus root41 3.2.3.Histology and immunohistochemistry ... 41
3.2.4.Real-time quantitative polymerase chain reaction ... 42
3.2.5.Uniaxial Tension Test ... 44
3.2.6.Imaging ... 44
3.2.7.Statistical Analysis ... 45
3.3. Results ... 46
3.3.1.Demographic data ... 46
3.3.2.Flattening and increase in length was observed in osteoarthritic meniscus roots ... 46
viii
3.3.3.Histological degeneration increased in meniscus root tear groups ... 48
3.3.4.Characterization of root matrix revealed increase of fibrocartilage components in tear specimens ... 52
3.3.5.Tears of meniscus roots were predominantly found within fibrocartilaginous areas ... 55
3.3.6.Root tears were induced within fibrocartilage areas during uniaxial tensile test ... 58
3.3.7.Calcification increased in meniscus root tear specimens ... 60
3.4. Discussion ... 63
Conclusions ... 68
ix
LIST OF FIGURES BACKGROUND
Figure 1.1. Microstructure of the Meniscus. ... 3
CHAPTER I
Figure 2. 1. UHMWPE Wear Particle Generation.. ... 11 Figure 2. 2. Inflammatory cytokine and mediator changes after UHMWPE
co-culture ... 17 Figure 2. 3. Phagocytosis index analysis by flow cytometry.. ... 12 Figure 2. 4. Confocal microscopy of chondrocyte, meniscal fibrochondrocyte,
and synoviocyte co-cultured with UHMWPE ... 20 Figure 2. 5. Apoptosis after co-culture of intra-articular cells and UHMWPE by
TUNEL assay.. ... 22 Figure 2. 6. Representative histopathological results of all groups.. ... 24 Figure 2. 7. Quantitative representation of histological results ... 22
CHAPTER II
Figure 3. 1 Representative gross pictures of groups according to the extent of root tear. ... 40 Figure 3. 2 Gross specimen of normal cadaveric medial meniscus root ... 47 Figure 3. 3 Histological analysis revealed increased degeneration in meniscus
root tear groups... 50 Figure 3. 4 Tenascin C immunohistochemistry for root degeneration evaluation..51 Figure 3. 5 Characterization of root matrix revealed increase in fibrocartilage
x
Figure 3. 6 Tear margin analysis ... 56
Figure 3. 7 Lipoid degeneration in complete tear group ... 57
Figure 3. 8 Uniaxial tensile test of root specimens. ... 59
Figure 3. 9 Calcification analysis of meniscal root tear specimens ... 61
Figure 3. 10 Imaging and histological slides of a case of heterotrophic ossification in the medial meniscus root. ... 62
LIST OF TABLES Table 1 Real-time PCR primer sequence. ... 43
1
2
1.1. Meniscus
Menisci are semilunar, fibrocartilaginous structures between the femoral condyles and tibia plateau within the knee joint. The primary function of the meniscus is load dissipation, thereby offering protection to the articular cartilage. Secondary functions include stabilization of the knee joint and nutrient supply and distribution to adjoining intra-articular structures [1]. The microstructure of the meniscus is specifically designed to perform these tasks. Collagen fibers are zonally organized as shown in Fig. 1.1 [2]. The outer meniscal rim constitutes largely of circumferential fibers, which resist ‘hoop stress’, or extrusion forces induced by femoral condylar loads. In the inner meniscus, radially coursing collagen fibers are found, which directly dissipate compressive loads towards the outer rim. Radial tie fibers, which course through the middle of the tissue towards the outer capsule, hold the meniscus to the adjoining capsule. Surface fibers are arranged in a closely knit fashion, offering toughness along with low frictional properties [1].
Biochemically, the meniscus consists of primarily collagen type 1, followed by collagen type 2, which can be found in the inner zone and surface. Aggrecan and other articular cartilage related proteins can be found in the inner zone. Cells of the meniscus are not well defined, but are termed fibrochondrocytes that show both fibrotic and chondrogenic features. Fibrochondrocytes within the meniscus also show spatial distribution, with inner tissue showing more chondrogenic cells [3].
3
Menisci have distinct blood supply, with the inner one third of the tissue being completely avascular, while the outer third showing more vascularity compared to the middle third. The meniscus can be divided into ‘zones’ depending on the vascularity, with the inner, middle, and outer one thirds termed white-white, white-red, and red-red zones, respectively. The repair potential after injury is consequently dependent on the vasculature, in which the inner, white-white zone has very low spontaneous repair potential [1].
4
1.2. Meniscus Degeneration
Meniscus, like many other musculoskeletal tissue, degenerates due to a number of factors. Known inciting factors include aging, trauma, obesity, and osteoarthritis. The incidence of meniscus degeneration is extremely common, present in 70-90% of symptomatic osteoarthritic knees. The clinical presentation varies, ranging from pain, loss of motion, effusion. Meniscus degeneration is represented as intra-meniscal high signal intensities, tears, maceration, and meniscal extrusion in MRI. Degenerative changes of the meniscus lead to increased decrease in meniscus compression modulus, and subsequent femur-tibia contract pressure. As a result, it is a key factor in osteoarthritis progression [4].
1.3. Unmet Needs in Meniscus Degeneration
Current treatment for meniscus degeneration includes meniscectomy and meniscal allograft transplantation. Meniscectomy is the most common orthopedic procedure performed in the US. This procedure involves removal of grossly torn or damaged meniscus tissue. While some symptomatic improvement have been reported, it is well known that removal of meniscus tissue increases tibiofemoral contact pressure, thereby accelerating osteoarthritis. Meniscal allograft transplantation, which involves replacing the degenerated or damaged meniscus with a meniscus allograft, also offers some functional improvement. Yet like meniscectomy, there is currently little evidence that
5
the transplanted allograft functions as a normal meniscus and delay osteoarthritic progression.
Unlike articular cartilage treatments, almost no treatment advancements have been made over the last 20 years. This may be due to a number of reasons, such as complexity of meniscus tissue, less importantly perceived by orthopedic surgeons, and absence of a disease model. A disease model is crucial in both understanding a disease, and devising novel treatment strategies based on pathogenesis of the disease. Currently, the pathogenesis of meniscus degeneration remains unclear. Degeneration in current clinical settings is also unknown. No widely accepted animal model exist for meniscus degeneration.
1.4. Aim of Study
The goal of this thesis is to devise a disease model for meniscus degeneration based on clinical inflammation and degenerative tear.
Chapter I
Evaluate meniscus changes, along with cartilage and synovium, against polyethylene wear particles in a unicompartmental knee arthroplasty model.
Chapter II
Characterize degeneration of meniscus root tears in human osteoarthritic knees, with emphasis on fibrocartilage and calcification.
6
CHAPTER I: Inflammatory and degenerative
responses of meniscal fibrochondrocytes,
chondrocytes, and synoviocytes to polyethylene
wear particles in a unicompartmental knee
arthroplasty model
7
2.1. Introduction
Ultra-high molecular weight polyethylene (UHMWPE) is the bearing material of choice when it comes to total knee arthroplasties (TKA) as well as partial knee replacements. Continuous motion of the joint creates UHMWPE particles by abrasive cutting, fatigue, or chemical-mechanical mechanisms [5]. These wear particles are primarily phagocytosed by macrophages, which lead to release of inflammatory cytokines such as IL-1b, IL-6, TNF-a , and PGE2. Proinflammatory cytokines ultimately stimulate osteoclast precursors leading to periprosthetic osteolysis and resultant joint replacement failure [6]. This scenario has been extensively studied in TKR and total hip replacements. UHMWPE particle-induced inflammatory response in partial arthroplasties, however, is unknown. Moreover, studies on the interaction of such particles with other synovial joint structures such as cartilage, meniscus, and synovium are lacking. These interactions occur in partial knee replacements such as unicompartmental knee arthroplasty (UKA) or patellofemoral arthroplasty (PFA), in which only one compartment of the knee is replaced.
While partial joint replacements such as UKA and PFA are on the rise, survival rates of these implants have fallen short of TKA [7, 8]. One of the most important failure modes in UKA is progression of OA in the non-operated compartment [7, 9]. Some authors have hypothesized this mode of failure to be a result of transfer of increased forces to the uninvolved compartment [10].
8
A few studies, however, support the possibility of a biological process involving UHMWPE wear particles in the progression of OA at the non-operated compartments of UKA. Possibilities of inflammatory reaction by intra-articular cell sources other than macrophages in the knee have been suggested. Castillo et al. found that chondrocytes act as non-professional phagocytes, capable of engulfing cell debris as well as latex particles in vitro [11]. Chang et al. have shown that chondrocytes are capable of phagocytosing UHMWPE particles which lead to elevations of OA associated cytokines such as NO and PGE2 in vitro [12]. Yet the link between UHMWPE wear particles and OA in the knee joint has not been established.
In an effort to clarify the interaction between UHMWPE wear particles and the non-replaced compartments in partial joint replacement surgeries, we hypothesized that UHMWPE particles per se would interact with intra-articular tissue, which by acting as inflammatory reservoirs, would subsequently induce OA related changes in vitro and in vivo. Our goal was to assess the inflammatory response, phagocytic activity, as well as degree of apoptosis in chondrocytes, synoviocytes, and meniscal fibrochondrocytes in the presence of UHMWPE particles in vitro. We have also sought to assess the in vivo response of cartilage, synovium and meniscus after intra-articular injection of UHMWPE particles in a murine model.
9
2.2. Material and Methods
2.2.1. UHMWPE Wear Particle Production
A modified version of the micro-cutting procedure for generation of UHMWPE particles was constructed [13]. Briefly, photolithography patterning and etching was done to create a silicon wafer surface with micro-cutting edges as shown in Figure 2.1A. As for UHMWPE, standard UKA bearing material (Zimmer, Warsaw, Indiana, USA) was used. Bearing materials were cut to make cylinder pins in the size of 6mm in diameter and 25mm in length. Linear reciprocating wear test cycles were carried out on the silicon wafer surface using a custom made wear tester (Pacific Engineering, Yeosu, Korea) under a contact pressure of 3MPa, a stroke length of 19mm, a frequency of 1.5Hz, and a sliding speed of 57mm/sec in purified water mixed with 12.6mg/ml bovine serum albumin (Probumin, Millipore, MA, USA). After the wear test, UHMWPE particles were collected by repeated rinsing with purified water. Digestion of albumin and other endotoxins was done by addition of 5N NaOH solution and kept at 65C for 24 hours. Particles were collected on a 0.1μm pore size membrane by a vacuum filtration process. Particles were sterilized under ultra-violet light for 48 hours before use. Diameters and aspect ratio (AR) were calculated on 500 particles in 5 different occasions on an identical sample by digital image processing software (Image J, National Institute of Health) [13, 14]. Particles averaged 5.21mm ± 1.85mm (mean ± SD) in length and 1.58±0.42 (mean ± SD) in aspect ratio (AR) (Figure 2.1B). Size distribution was as follows; 6.77% for <0.1µm, 43.62% for <1.0µm, and 97.65% for
10
<10µm. Particles were tested for endotoxins by Limulus Ameboyte Lysate kit (Sigma-Aldrich, St. Louis, MO). Results were negative for endotoxins (<0.01uE) [13, 15-17]. Particles were sterilized under ultra-violet light for 48 hours before use.
11
Figure 2. 1. UHMWPE Wear Particle Generation. (A) Microscopic view of the silicon
wafer surface. Each micro-cutting edge was 5mm apart, with 1.3mm in height. (B) Scanning electron microscopy (SEM) of UHMWPE wear particles generated by micro-cutting process.
12
2.2.2. Cell Harvesting and Culture
Knee joints were harvested from 8week old male Wistar rats (Orient Bio, Seongnam, South Korea), after euthanasia. Articular cartilage from the femoral condyles, infra-patellar synovial membrane, and both menisci from each knee were dissected and minced followed by digestion with 0.2% collagenase type II (Gibco, USA) for cartilage, and 0.2% collagenase type I (Gibco, USA) for synovium and menisci in different culture media for 4 hours. Cartilage, synovium, and menisci were suspended in DMEM (Hyclone, USA), α-MEM (Hyclone, USA), and DMEM F12 (Gibco, USA) media, respectively. In each medium, 10% fetal bovine serum and antibiotics were added. The suspended solutions were centrifuged at 1700rpm for 10 minutes. The supernatant was drained and chondrocytes, synoviocytes, as well as meniscal fibrochondrocytes were each seeded and expanded. Passage 2 cells were chosen and seeded in 24-well culture plates in densities of 1 x 105 cells /well. The ratios of particle number to cell number were chosen as 0:1 (negative control), 1:1 (low dose), and 10:1 (high dose), respectively. Lipopolysaccharide (LPS) is known to incite cytokine and mediator production, while decreasing proteoglycan synthesis in intra-articular cells [18, 19]. It was used as a positive control, in a concentration of 1µg/ml. An inverted cell culture system was used to maximize contact between cells and particles [20]. Cultures were carried out for 3 days.
13
2.2.3. Analysis of Inflammation
Inflammatory cytokines, IL-1β, IL-6, TNF-α, and mediators, nitric oxide (NO) and prostaglandin E2 (PGE2), all related with OA progression, were chosen for analyses [21]. Cytokine concentrations were measured with rat IL-1 β (Rat IL-1 β Platinum ELISA), IL-6 (Rat IL-6 Platinum ELISA) and TNF- α (Rat TNF- α Platinum ELISA) ELISA kits (eBioscience, CA, USA). The supernatants from each well were diluted 1:2 and 50µl of diluted supernatants were taken and added in the polyclonal antibody pre-coated plates, together with standard solutions. Protocols provided by the manufacturer were followed.
For NO analysis, NO Assay Kit (abcam, Cambridge, UK) was used. Briefly, the supernatants from each well were diluted 1:10, and 85μl of each sample was added in the provided 96-well plate. Protocol provided by the manufacturer was followed.
For PGE2 analysis, PGE2 EIA kit (Cayman Chemical, MI, USA) was used. Supernatants of each culture well were diluted 20 fold with EIA buffer solution. Fifty μl of diluted samples and standards were added in the pre-coated 96 well plate. Protocol provided by the manufacturer was followed.
14
2.2.4. Phagocytosis
Phagocytosis Index Measurement by Flow Cytometry. Phagocytosis of UHMWPE particles were identified by measuring the phagocytosis index [22]. Phagocytosis index is a calculation based on changes in cell granularity (side scattering), which increases if cells phagocytose UHMWPE particles14. Cells from each well were trypsinized (Trypsin-EDTA, Gibco, NY, USA) and cell suspensions were centrifuged. Supernatants were discarded and cells were suspended in 350µl of phosphate buffered saline (PBS) and 150µl of propidium iodide (PI) solution (Invitrogen, NY, USA) [23]. Cells were incubated at 37C for 5 minutes, avoiding light exposure. Flow cytometry analysis was done.
2.2.5. Confocal Microscopy
In order to visualize phagocytosis of each cell type, confocal microscopy was taken from the high dose groups. We have taken advantage of UHMWPE’s auto-fluorescent properties under FITC wavelengths [23]. Cells were trypsinized and fixed with 4 % paraformaldehyde-PBS for 30 minutes at room temperature. They were counterstained with PI solution for 2 minutes at room temperature and centrifuged for 5 minutes at 1000rpm. After suspension in PBS, the cells were dropped on a slide, cover slipped and observed with confocal microscopy. Digitalized confocal images were processed with UltraVIEW system (PerkinElmer, MA, USA).
15
2.2.6. Apoptosis
TUNEL assay. Analysis of apoptosis was done for each group as previously described using APO-BrdU TUNEL assay kit (Invitrogen, NY, USA) by flow cytometry [24]. Extra care was taken to preserve the cell-UHMWPE pellets between washing steps, which have the propensity to float.
2.2.7. Animals
For our in vivo study, 10 week old male Wistar rats (Orient Bio, Seongnam, South Korea), weighing approximately 300g, were used. The experimental protocol was approved by the Animal Care and Use Committee of Ajou University. The rats were divided randomly into the following groups. In groups 1 and 2, UHMWPE were injected in normal knees at two different doses (Low; 107 particles/100ml, High; 108 particles/100ml). In groups 3, 4, and 5, surgical OA models were made as positive controls. This was based on the observation that most patients receiving UKA have established OA in the knee, albeit single compartmental cartilage degeneration [7]. For groups 4 and 5, UHMWPE injections at two different doses were made after establishment of OA (6 weeks after surgical OA induction). For the groups requiring injection (Groups 1, 2, 4, 5), UHMWPE were dispersed in PBS according to their respective dosages. Intra-articular injections were carried with BD Ultra-Fine needle Insulin Syringe (Becton, Dickinson and Company). Surgical OA models (Groups 3, 4, 5) were made as follows. Rats were anesthetized via intramuscular injection with a mixture
16
of Zoletil (50mg/ml; Virbac laboratories, Carros, France) and xylazine hydrochloride (Rompun; Bayer, Ansan, Korea) (Zoletil: Rompun = 1:2). Both legs were shaved and aseptically prepped in a supine position. On the left knee, a medial parapatellar approach arthrotomy was done to expose the knee joint. The medial collateral ligament and the mid portion of the medial meniscus were transected as described previously [25]. The wound was closed layer by layer using 4-0 nylon. A sham operation was performed in the right knee. For groups 1 and 2, the end points were 6 weeks after injection. For Group 3, the end point was 12 weeks after OA surgery. For Groups 4 and 5, injections of UHMWPE were given after 6 weeks of OA surgery, and the end point was 6 weeks after the injection. After the respective end points, animals were sacrificed and knee joints were harvested. Specimen were fixed in 4% paraformaldehyde for 1 week, and decalcified with 0.5% nitric acid for 24 hours. Each right knee served as a control.
2.2.8. Histological Assessment
Processing and paraffin embedding were done for each specimen. Serial 10μm sagittal sections were made from the lateral compartment of the knee, so as to include the lateral femoral condyle, lateral meniscus, and synovial tissue. Eight sections obtained at 20μm intervals were placed on microscopy slides. Four slides were stained with Safranin-O/Fast Green (SAFO) for cartilage and meniscus analysis, while the other four were stained with hematoxylin/eosin (HE) for synovium analysis. The slides were mounted with Permount mounting solution (Fisher Scientific, PA, USA). For cartilage degeneration analysis, Osteoarthritis Research Society International (OARSI) OA
17
grading system was used (grade 0 to 6; normal, grade 0; surface intact, grade 1; surface discontinuity, grade 2; vertical fissures, grade 3; erosion, grade 4; denudation, grade 5; deformation, grade 6) [26]. For histopathologic analysis of knee meniscus, the grading system proposed by Pauli et al. was used (0 to 18 points; normal tissue, 0 to 4 points; mild degeneration, 5 to 9 points; moderate degeneration, 10 to 14 points; severe degeneration, 15 to 18 points) [27]. For analysis of synovitis, we used the histological score proposed by Krenn et al. (0 to 9 points; no synovitis, 0 to 1 point; low-grade synovitis, 2 to 4 points; high grade synovitis, 5 to 9 points) [28]. Each scoring was measured by 3 independent researchers.
2.2.9. Statistical Analysis
Data obtained by the above methods were processed by one-way ANOVA analysis. A p-value <0.05 was considered to be statistically significant.
18
2.3. Results
2.3.1. Inflammatory cytokine and mediator production in all cell types increase after co-culture with UHMWPE
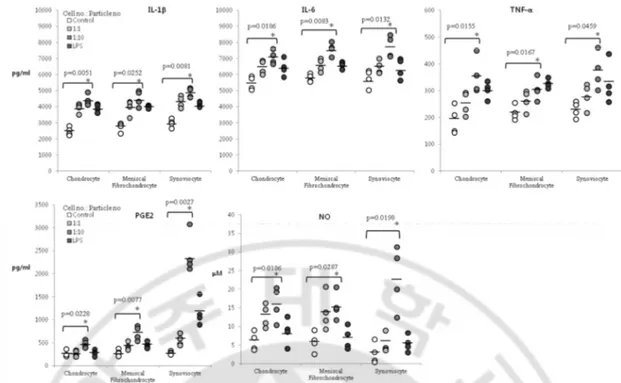
Production of IL-1b was increased in high dose groups of chondrocytes, meniscal fibrochondrocytes, and synoviocytes compared to controls (p=0.0051, p=0.0252, p=0.0081, respectively). Production of IL-6 was also increased in high dose groups of the above three cells (p=0.0186, p=0.0083, p=0.0132, respectively). TNF-a, another cytokine measured, also showed increase in production for high dose groups in all cell types (p=0.0155, p=0.0167, p=0.0459) (Figure 2.2). As for mediator production, PGE2 increased for high dose groups in all cell types when compared to controls (p=0.0228, p=0.0077, p=0.0027). Synoviocytes, in particular, showed a 5-fold increase of PGE2 production in high dose group when compared to the control group. For NO, increased levels were observed in all high dose groups for all cell types when compared to controls (p=0.0186, p=0.0287, p=0.0198). Again, the high dose group of synoviocytes demonstrated a 5-fold increase compared to the control group (Figure 2.2).
19
Figure 2. 2. Inflammatory cytokine and mediator changes after UHMWPE co-culture. Dot
plot representing concentration of inflammatory cytokines IL-1b, IL-6, TNF-a, and inflammatory mediators PGE2 and NO after co-culture of chondrocytes, meniscal fibrochondrocytes, and synoviocytes with UHMWPE. Differences among groups with p ≤ 0.05 are enumerated.
20
2.3.2. Intra-articular cells exhibit phagocytosis toward UHMWPE particles
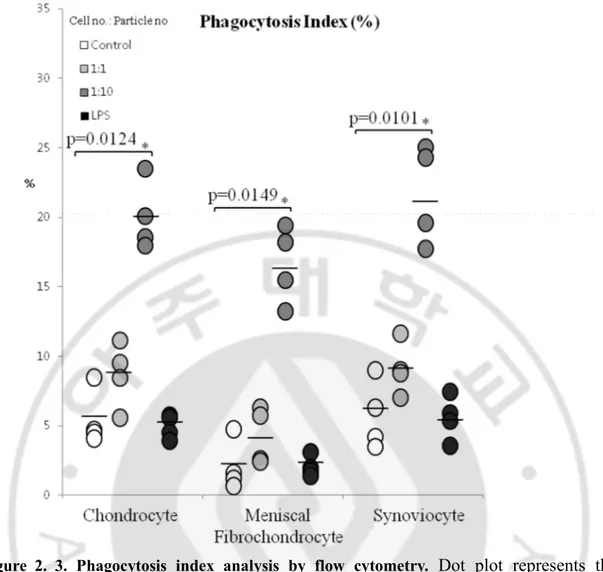
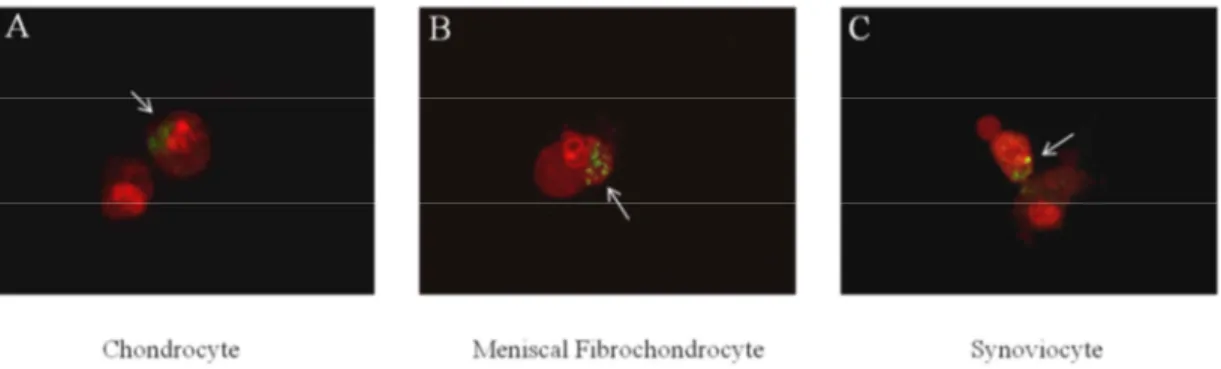
Phagocytosis index increased in a particle dose dependent manner in all cell types. High dose treated groups showed the largest percentage of increase for chondrocytes, meniscal fibrochondrocytes, and synoviocytes (p=0.0124, p=0.0149, p=0.0101, respectively) (Figure 2.3). Confocal images confirmed the above result. In each cell type, UHMWPE wear particles were observed inside the cell. Features of apoptosis, such as chromatin condensation and membrane blebbing were also noted (Figure 2.4 A, B, C).
21
Figure 2. 3. Phagocytosis index analysis by flow cytometry. Dot plot represents the
percentage of cells showing phagocytic activity, determined by increase of side scatter by FACS analysis.
22
Figure 2. 4. Confocal microscopy of chondrocyte, meniscal fibrochondrocyte, and
synoviocyte co-cultured with UHMWPE. Images of PI stained cells were merged with
FITC fluorescence images. UHMWPE particles, which show FITC fluorescence (white arrows), are seen inside each cell type, suggestive of phagocytosis. Chromatin condensation, are noted in all cell types, suggestive of apoptotic activity (A) Chondrocytes showing engulfment of UHMWPE. (B) Meniscal fibrochondrocytes showing engulfment of UHMWPE. (C) Synoviocytes showing engulfment of UHMWPE. Note the membrane blebbing, suggestive of apoptotic activity. x600
23
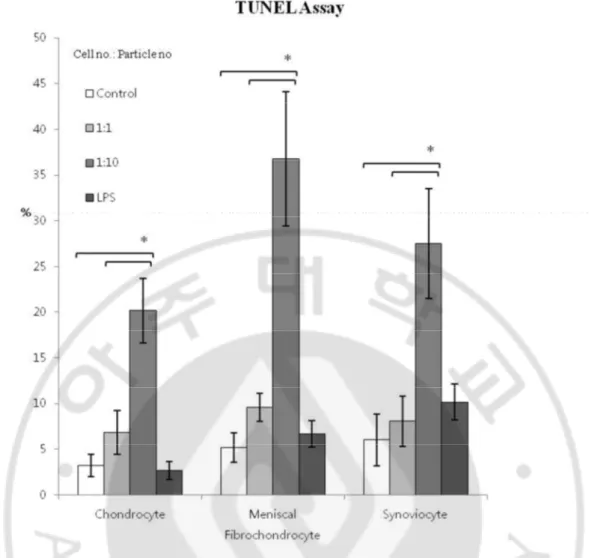
2.3.3. Apoptosis after co-culture of intra-articular cells and UHMWPE
TUNEL assay results showed a higher percentage of TUNEL positive cells in high dose groups of the 3 cells when compared to controls (p=0.0081, p=0.0049, p=0.0132). These results suggest apoptosis occurs in all cell types when co-cultured with UHMWPE particles (Figure 2.5).
24
Figure 2. 5. Apoptosis after co-culture of intra-articular cells and UHMWPE by TUNEL
25
2.3.4. In Vivo Study
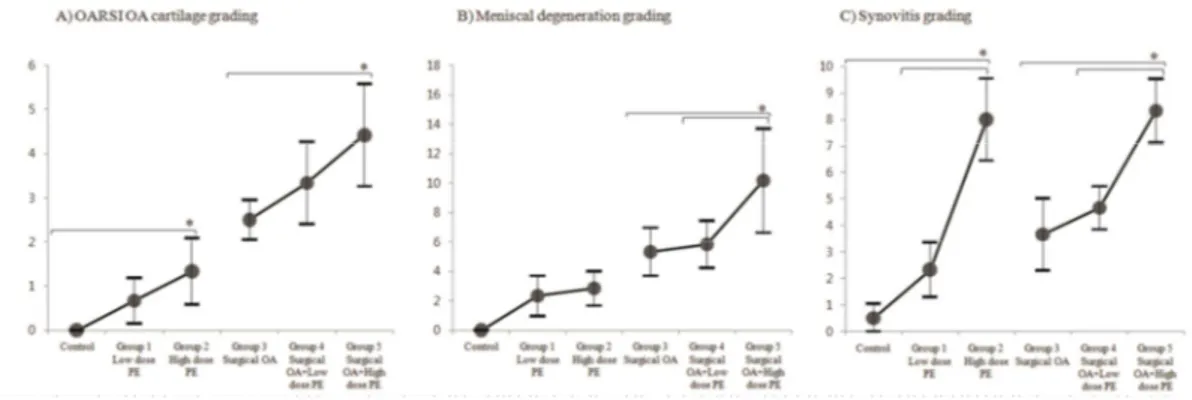
Controls of all groups (right knees of each specimen treated with PBS injection and/or sham surgery) displayed no morphological differences among each other. In cartilages, group 1 showed early OA changes such as edema and clustering of chondrocyte in the superficial zone. Group 2 showed decreased matrix staining, in addition to disorientation of chondron columns. Both group 1 and 2 showed no change in the smoothness of cartilage surface but lessened matrix formation was observed; this was especially noticeable in group 2. Group 3, the surgically-induced OA group, showed matrix fissures. Group 4 showed matrix loss. Group 5 exhibited extensive surface denudation. For menisci, groups 1 and 2 showed early degenerative changes such as increase in cellularity and matrix staining when compared to controls. Groups 3 and 4 showed surface fibrillation and hypocellular areas. Group 5 exhibited hypocellularity with disorganization of collagen fibers. For synovium, groups 2 and 5 (both high dose UHMWPE injected groups) showed typical high grade inflammatory OA synoviopathy, with giant cells encircling UHMWPE (Figure 2.6) [29]. Quantifications of histological changes are represented in figure 2.7. The respective intra- and inter-observer ICC of each scoring system were as follows; OARSI OA scoring (0.78/0.91), meniscus scoring (0.79/0.89), and synovitis scoring (0.83/0.95). For cartilage, groups 2 and 5 each showed more degeneration related changes compared to control and group 3, respectively (p=0.024, p=0.0049). For meniscus, group 5 resulted in higher degeneration scores compared to group 3 and 4 (p<0.001, p<0.001). As for synovium, group 2 showed increased synovitis over control and group 1 (p<0.001,
26
p<0.001). Group 5 also showed increased synovitis scores over group 3 and 4 (p<0.001, p<0.001) (Figure 2.7).
27
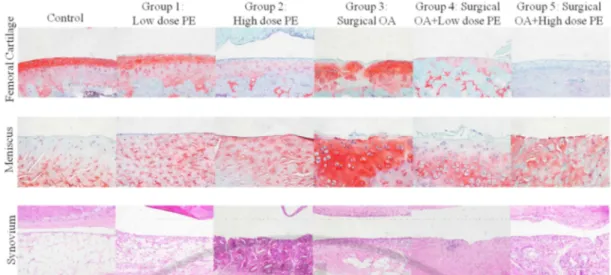
Figure 2. 6. Representative histopathological results of all groups. Upper row shows
cartilage slides, stained in SAFO. Meniscus slides, also stained in SAFO are shown in the middle row. Synovium slides, stained in HE, are shown in the lower row. X200
28
Figure 2.7. Quantitative representation of histological results. (A) Quantitative
representation of cartilage status by OARSI scoring. (B) Quantitative representation of degenerative changes of menisci. (C) Quantitative representation of synovitis. Error bars represent 95% confidence intervals.
29
2.4. Discussion
Arthritic change in the remaining articular compartment after partial joint replacement surgery such as UKA is an important, emerging concern. While previous theory suggests increased postoperative mechanical loading in the remaining compartment as the cause of this phenomenon, our results provide a biological explanation caused by UHMWPE particles as another reason for OA in the remaining compartment. Since all intra-articular tissues such as cartilage, meniscus, and synovium are known to be involved in the pathogenesis of OA, this study aimed at the analysis of the responses of such tissues to UHMWPE particles. To the best of our knowledge, this is the first report to investigate the relationship between UHMWPE particles and individual intra-articular tissue responses eventually leading to OA. We first demonstrated cellular response to UHMWPE particles; phagocytosis of particles, apoptosis, and inflammatory cytokine production, in vitro. Finally, we were able to induce, as well as progress OA changes in a rat model by injecting UHMWPE particles.
Our histopathological results demonstrate that UHMWPE particles may be an inciting factor for OA in the remaining cartilage after UKA. UHMWPE particles per se induced OA changes in the normal rat knee, while exacerbating the disease progression in OA models. Significant differences were observed in high dosage UHMWPE groups, in not only in vivo, but also in vitro. This suggests an existence of a critical particle dose to cause degradation of intra-articular tissues and initiate OA related changes [22]. Inflammatory synoviopathy with giant cells, in addition, were observed in the high dose groups only (group 2 and 5), indicating a stronger inflammatory process involved.
30
Another interesting histological finding was found on normal knees with UHMWPE injection (groups 1 and 2). While the morphological integrity of articular cartilage and meniscus were fairly maintained, considerable changes in the matrices of these tissues were found. Such particle-tissue interactions inflicting matrix changes may have accelerated tissue damage in the OA models groups (groups 4 and 5). Overall, deformation of inner matrices of cartilage and meniscus were notable in all UHMWPE injected groups (groups 1, 2, 4, and 5).
Considerable amount of studies indicate that proinflammatory cytokines (IL-1β, IL-6, TNF-α) and mediators (NO and PGE2) play a key role during inflammation and tissue destruction in OA [30]. We have shown an increase of cytokines and mediator production in chondrocytes, meniscal fibrochondrocytes, and synoviocytes. Our results suggest that these cells can each act as inflammatory reservoirs, capable of producing cytokines and mediators when coming into contact with UHMWPE particles. All cells exhibited such responses in a particle dose dependent manner. Synoviocytes, in particular, secreted more inflammatory mediators than the other two cells. Zysk et al. have also observed strong synovial inflammatory responses after polyethylene particle injection in mice [31]. We therefore expect the synovium to play a lead role in such inflammatory response.
Synoviocytes and chondrocytes have already been reported to have phagocytosis capability. This study shows that meniscal fibrochondrocytes also have this ability. Castillo et al. previously reported that chondrocytes act as “non professional phagocytes” capable of engulfing latex particles and cartilage detritus [11]. Chang et al. reported that chondrocytes engulfed UHMWPE particles, and subsequently secreted inflammatory
31
factors such as NO and PGE [12]. This study shows that meniscal fibrochondrocytes and synoviocytes also show similar trends with regard to UHMWPE particles, ultimately resulting in apoptosis.
Apoptosis is regarded as one of the main, inherent mechanisms of OA. Trauma, inflammation, and aging all cause chondrocyte apoptosis, reducing matrix synthesis; this eventually induces cartilage damage. Moreover, it disrupts cell-matrix interaction and consequently ignites the apoptotic cascade again, initiating a destructive vicious cycle [32]. Our TUNEL assay results show a UHMWPE dose dependent increase of apoptosis in all 3 cells. This process was also visualized by chromatin condensation and apoptotic blebbing in confocal images. This vicious cycle of apoptosis may be escalated as the concentration of UHMWPE particles increases, due to particle accumulation in the intra-articular space. Long-term wear of UHMWPE is thus expected to have serious adverse effects on the articular cartilage.
Some limitations exist in our study. The single injection-murine model used in here may not entirely reflect the chronic production of wear particles in patients with joint replacements [10]. Many prosthetic wear animal models exist [5]. Continuous intramedullary infusion of polyethylene particles by osmotic pumps in a murine model has been achieved by several authors [33, 34]. We did not place this device in the knee joint, due to concerns that the pumps themselves would cause irritation. If possible, a sophisticated animal model incorporating a more continuous infusion of UHMWPE in the knee joint should be utilized in future studies.
Particle size and shape used in this study may not entirely reflect particles generated after partial arthroplasties. First of all, UHMWPE particle size differs according to the
32
manufacturing/ isolation process (i.e. simulator or in vivo retrieval), anatomical region of joint prosthesis (i.e. total hip arthroplasty or total knee arthroplasty) and endpoint of study (i.e. osteolysis, macrophage response, in vivo tissue response etc.). UHMWPE particles isolated from in vitro hip simulators appear to be predominantly submicron and round, with occasional fibrils [35]. Studies utilizing microcutting technique as the manufacturing process, the method also used in our study, produce a mean diameter size comparable to our results [12, 13]. Polyethylene retrieval studies from actual patients during revision knee arthroplasty offer the most accurate representation of the actual particle size produced in the clinical setting. Shanbhag et al. reported wear particle analysis data from total knee replacements and the mean size was 1.7±0.7µm, with ranges from 0.1µm to 200µm [14]. The distribution of particles were as follows; 36% <1µm, 90% <3µm10. Compared to our data, there is about <4µm difference in mean size, while the distribution difference is <10% for <1µm sized particles. Secondly, as for particle shape, our overall AR was 1.58±0.42 (mean ± SD). Globular particles constituted 79.3% of the measurements (AR 1-2.39) while elongated fibular shape particles constituted 19.23% of the measurements (AR > 2.4-5). A retrieval study from hip revision arthroplasties reported an AR of 1.17±0.06 to 3.59±0.50 [36]. Our AR is comparable to a retrieval study from TKA patients (AR 1.7) [14]. Morphologically more elongated particles with a higher AR are more commonly found in hip arthroplasty retrieval/ simulator studies rather than knee arthroplasty related studies.
Controversy exists on the ‘critical’ size and shape of UHMWPE wear particles capable of inciting inflammatory reactions. Some authors suggest that particle size of 0.2-0.8µm is the most ‘critical’ to induce biologic responses [37, 38]. Hallab et al.
33
discusses that the internalization of small particles in the nanometer sizes (<150nm) occurs by endocytosis for particles, whereas larger particles (>150nm to 10µm) are phagocytosed in the traditional manner inside phagosomes of macrophages. They found that the most inflammatory particles were not the smallest particles tested (i.e., 0.3-0.7 µm), but rather they were the larger 10-13µm particles barely able to be phagocytosed by the cells [39]. Other authors have observed that polyethylene debris too large to be ingested provoke foreign body giant cell reactions [40, 41]. This process itself is capable of inciting production of mediators that stimulate osteolysis [40, 41]. These larger sized particles have been found in in vivo human tissue from revision arthroplasties [42]. As for shape, Sieving et al. have reported that sharp, elongated shape particles activated a more pronounced inflammatory response in a murine air pouch model [36]. These results, however, have yet to be confirmed in a synovial joint environment. In summary, we believe that both small (<1 µm) and large (>10µm) particles induce inflammatory reactions in the knee joint. Our data show a near even distribution between <1µm and >1µm sized particles. Both phagocytosis of small particles as well as foreign body giant cell reaction in the synovium were shown in the confocal microscopic images and histological images, respectively. The size of UHMWPE particles used in this study covered both the small, phagocytosable particles and large, indigestible particles, both of which are related to in vivo inflammatory reactions. Further studies are required to elucidate the effect of particle size and shape in OA induction described in our study.
Clinical relevance of our study is that it provides an alternate, biochemical explanation for the development of OA and early failure in partial arthroplasties. Both
34
clinicians and patients should note that UHMWPE particles not only cause osteolysis and implant loosening, but may also cause OA in the non-replaced compartments, leading to revision surgery [9]. Advancements of bearing materials may, in turn, reduce revision rates of partial joint arthroplasties, which currently fall behind total joint replacements [7, 9].
UHMWPE wear particles per se exert detrimental effects on chondrocytes, synoviocytes, and meniscal fibrochondrocytes of the knee joint by phagocytosis of particles and apoptosis, resulting in pro-inflammatory cytokine release. Relationship between UHMWPE wear particles and OA was observed in a murine model, suggesting that UHMWPE wear particles play a role during OA development in partially replaced joints.
35
CHAPTER II: Degeneration of meniscus roots
are accompanied by fibrocartilage formation,
which may precede meniscus root tears in
osteoarthritic knees
36
3.1. Introduction
Meniscus roots are ligamentous structures that connect the meniscus to tibial subchondral bone within the knee joint [43]. They complete the meniscus ring complex by dissipating loads to the tibia. Consequences of tears in the meniscus root are devastating, resulting in meniscal extrusion and hoop strain failure, biomechanically similar to total meniscectomy status [44-46]. Subsequent increase in tibiofemoral contact pressure leads to rapid progression of osteoarthritis (OA) [44, 47, 48]. These tears are frequently diagnosed, presenting in 10% to 21% of all arthroscopic meniscus related operations [49-51]. The actual incidence of meniscus root tears (MRTs), however, is believed to be much higher than previously reported. This is due to the recent interest regarding these tears, together with difficulty in imaging diagnosis, with reported MRI accuracies as low as 72.9% [47, 49, 51-53]. Two types of MRTs have been described, one being traumatic avulsion of the root occurring in young patients [43, 54]. The other, much more prevalent type consists of non-traumatic, degenerative MRTs occurring in elderly patients with strong association to OA [43, 50, 52]. Degenerative MRTs are not well characterized, and therefore are loosely defined as tears occurring in association with long-standing degeneration within the knee joint, often resulting in irreparable tissue [49, 52]. Degenerative MRTs overwhelmingly occur in the medial meniscus posterior root (MMPR) due to the highest force uptake during weight bearing in this area, as well as restricted mobility, which is further worsened by OA related joint stiffness [45-47]. Recent research regarding MRTs focuses on early, accurate diagnosis, as well as surgical treatment by repair, in efforts to conserve the meniscus unit and
37
articular cartilage integrity [52]. These efforts are hindered due to the lack of information on the tissue status of degenerative roots and the overall pathogenesis of degenerative medial meniscus posterior root tears (DMMPRTs).
Degenerative tears have been reported in various other tendons, including rotator cuff and Achilles tendons [55-58]. Tendons and ligaments are organized to withstand tensile forces, consisting of spindle shaped fibroblasts within longitudinally aligned type I collagen fibers. Degeneration of these structures, generally referred to as ‘tendinopathy’ or ‘ligamentopathy’, are caused by intrinsic factors such as aging and systemic diseases, as well as extrinsic factors such as repeated minor strains, including shear and compression [59]. Fibrocartilage, characterized by chondrocyte-like round cells within a matrix containing both type I and II collagen, is generally absent in the tendon stroma, except near the enthesis4. Some tendons and ligaments that cope with compressive forces, however, adapt to compression by fibrocartilage metaplasia in the stroma. Fibrocartilaginous adaptation in tendons improves resistance to compression while reducing resistance to tension. Fibrocartilage metaplasia in tendons is often associated with degeneration and tears, as demonstrated in supraspinatus and Achilles tendon tears [55-58]. Other histopathological changes associated with degenerative tears that exert similar deleterious effects include matrix remodeling by matrix metalloproteinases, collagen disruption, vascular proliferation, and calcification [60-62]. The microstructure of meniscus roots differs from the meniscus. It consists of fibrous connective tissue architecture similar to ligaments and tendons [43, 63]. The above observations concerning degenerative tears of tendons and ligaments may therefore be extrapolated to meniscus roots, given the similarities in microstructures between roots and tendons.
38
In general, meniscus roots primarily function in tension, holding the meniscus from extruding during weight bearing [63]. MMPRs, however, have been reported to function in multiple vectors due to their unique anatomical position. Apart from tension, they are also subjected to torsion, bending, and compression [63, 64]. Compressive loads are increased in this location during the process of OA, due to joint space narrowing, anterior cruciate ligament (ACL) deficiency, and impingement [65, 66]. We hypothesized that fibrocartilage is likely to be associated with DMMPRTs, given both the microstructural similarities between meniscus roots and other ligament or tendinous structures, as well as biomechanical insults during the process of degeneration. The aim of this study was to characterize degeneration of MMPRs in osteoarthritic knees in association with the degree of tear, with emphasis on fibrocartilage and calcification.
39
3.2. Methods
3.2.1. Experimental design and tissue samples
All protocols in this study were approved by our institutional review board. This study was prospectively designed, using tissue samples of MMPRs obtained from patients with primary, degenerative OA undergoing total or unicondylar knee replacement surgery. Patients with rheumatoid arthritis, inflammatory joint disease other than OA, enthesopathy, or prior history of knee surgery and trauma were excluded. MMPR samples with bony avulsions, samples damaged during surgery, and severely macerated samples grossly indistinguishable from adjoining joint structures were also excluded from further analysis. During surgery, gross morphology of the ACL was recorded and categorized as previously described [67]. MMPRs were harvested together with the tibial bone plate and the posterior horn of medial meniscus, in order to preserve its architecture. Samples were categorized into three groups according to tear extent; ‘no tear’ (NT), ‘partial tear’ (PT), and ‘complete tear’ (CT) group (Figure 1). We have defined ‘tear’ as having a gross radial tear in the meniscus root. Longitudinal tears, intrasubstance tears, microscopic tears, and radial tears of the medial meniscus posterior horns were not categorized as ‘tears’. For histological reference purpose, 5 normal MMPR samples were obtained from 3 cadavers (2 male and 1 female) in the same manner as the surgical specimens and were labeled as ‘normal reference’ (NR) group (Figure 3.1). Mean age of cadaveric donors was 53.6 (range 47-61). All cadaveric samples were obtained from joints without gross intra-articular pathology within 3 months of embalming.
40
Figure 3. 1 Representative gross pictures of groups according to the extent of root tear.
Normal, cadaveric reference root (A), no tear (B), partial tear (C), and complete tear (D). Arrows indicate the area of interest, and asterisks indicate normal ‘shiny white fibers’. Note the loss of ‘shiny white fibers,’ with flattening and lengthening of root in no tear (B) and partial tear (C) groups harvested from osteoarthritic knees, indicating compression insults. Tear margins of the complete tear group (D) show flat, glistening fibrocartilaginous tissue adhered to the tibia.
41
3.2.2. Gross morphology analysis and length measurement of the meniscus root
Gross morphological features of each sample were recorded before fixation. Whole length of the root, from the end of the meniscus posterior horn to tibial insertion, was measured in the NR, NT and PT groups using a digital vernier caliper (500, Mitutoyo, Japan). The length of the CT group was not measured due to the irregularity of the tear margins.
3.2.3. Histology and immunohistochemistry
All specimens were longitudinally cut in half, parallel to the transverse plane of the knee. One half was cryopreserved for gene and biomechanical analysis, and the other half was fixated in 10% formalin for 1 week. Processing and paraffin embedding were done for each histological specimen. Mid-longitudinal sections of 8 μm thickness were cut from each specimen and placed on slides. Safranin-O/Fast Green (SAFO), Hematoxylin/Eosin (HE), Picrosirius Red (PR), Alizarin Red (AR), and Von Kossa (VK) stains were performed. Immunohistochemical analysis was done with collagen type I Collagen I Ab, ab34710, abcam, Cambridge, MA) and collagen type II (Anti-Collagen II Ab, ab34712, abcam, Cambridge, MA) (extracellular matrix analysis), S-100 (S-S-100 Ab, GTX15521, GeneTex, Irvine, CA) (chondrocyte-like cell detection), and Tenascin-C (Anti-Human Tenascin-C Mouse IgG MoAb, 10335, IBL, Japan) (ligament degeneration) using 3, 3'-diaminobenzidine (DAB) substrate method. All regions of interest (ROIs) for histology were set as the area 5mm into the root, starting from the
42
meniscus-root junction. This region, nearby the meniscus posterior horn, is where radial tears of MMPRs are mostly found [49]. For degeneration scoring, the modified Bonar tendinopathy scoring system was used, which evaluates cell morphology, collagen arrangement, cellularity, vascularity and ground substance grade (tendon with most pathology scores maximum of 20 points) [68]. Immunohistochemistry and AR images were quantified as the percentage of positively stained area versus whole area via image deconvolution methods previously described (ImageJ, NIH) [69].
3.2.4. Real-time quantitative polymerase chain reaction
Ten randomly selected samples from each group (NT, PT, CT) were analyzed using qRT-PCR. Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) and 1 μg of total RNA was reverse transcribed into cDNA using AMV (Roche, Mannheim, Germany). Reaction mixtures contained 1X SYBR Green Reaction Mix (Roche, Mannheim, Germany), 20ng cDNA, and 10 pM of each primer. After an initial incubation for 10 min at 95℃, the reactions were carried out for 30 -40 cycles at 95℃ for 10 sec and 60 or 62℃ for 30 sec by CFX96 Touch™ Real -time PCR Detection System (Bio-rad, Hercules, CA). Primers used in analysis included COL1A1 and COL2A1 for root matrix analysis and ecto-nucleotide pyrophosphatase/phosphodiesterase1 (ENPP1) for calcification analysis [70-72]. The information of primers is given in Table 1. Relative gene expression of samples was normalized to GAPDH as an internal control and calculated by comparative CT method.
43
Table 1 Real-time PCR primer sequence
Primer Sequence Length
(bp) Temp. (oC) Accession no. GAPDH (F) CGCTCTCTGCTCCTCCTGTT 81 62 NM_002046.5 (R) CCATGGTGTCTGAGCGATGT COL1A1 (F) GTCGAGGGCCAAGACGAAG 143 60 XM_006721703.1 (R) CAGATCACGTCATCGCACAAC COL2A1 (F) CGTCCAGATGACCTTCCTACG 122 60 XM_006719242.1 (R) TGAGCAGGGCCTTCTTGAG ENPP1 (F) GAAACGCCTCCTACCCTCTT 352 62 NM_006208.2 (R) ATCCTGGCCAGAAAAATGTG
44
3.2.5. Uniaxial Tension Test
After histological analysis, 10 samples without fibrocartilage and 10 samples with fibrocartilage were chosen for biomechanical testing and tear induction. Tensile specimen strips of 1cm width and 400μm thickness were obtained using cryo-section techniques previously described [73]. During sectioning, a 10μm thickness section was obtained before and after the 400μm section for histological analysis to reflect the initial status of each tissue being tested. Each 400μm-thick specimen was trimmed to 1cm width parallel to the main ligament fiber orientation, so as to leave no surface damage on the edges. Specimens were also tailored to exclude synovial infiltration and adipose tissue. Uniaxial tension tests were carried out using an Instron uniaxial tension testing machine (H5KT, Tinius Olsen, Horsham, PA) using a modified version of previously described methods [73]. Briefly, samples were thawed at room temperature in Ringer’s solution. After alignment, samples were preloaded under 0.2 N. Preconditioning was applied as described previously [73]. Video recordings were done to determine the site of tear initiation. Samples were stretched at a constant rate of 1mm/min until failure. Ultimate tensile strengths (Mpa) were calculated from the stress-strain curves. After failure, samples were immediately fixed and processed for histological analysis to evaluate the site of tear.
45
All patients received standing AP and lateral radiographs of both knees as well as standing hip-knee-ankle radiographs before surgery. Kellgren Lawrence (KL) grade and mechanical axis angle was measured for each radiograph [74]. Patients with signs of ectopic calcifications on radiographs received CT scans (Brilliance, 3rd Generation 64-Channel Scanner, Philips, Netherlands) of the knee joint before surgery.
3.2.7. Statistical Analysis
All data is presented as means ± standard deviation (SD), with the exception of semi-quantitative ordinal rank data, which is presented as medians with 25%-75% quartile ranges. Histological scoring was done by two separate researchers blinded to the results on two separate occasions. Intra-class correlation coefficients for intra and inter-observer agreement were assessed. Data from each experimental group were compared either with Mann-Whitney U test or Kruskal-Wallis test with Dunn’s multiple comparison test. Statistical significance was set as p<0.05. P values were categorized as p<0.05(*), p<0.01(**), and p<0.001(***) in graphs.
46
3.3. Results
3.3.1. Demographic data
One hundred MMPR samples from 99 patients were used in this study. Total of 30, 28, and 42 MMPR samples were allocated into NT, PT, and CT groups, respectively. The mean age of patients of each group were 67.93 (range 58-83, SD 6.52), 65.81 (range 50-82, SD 8.09), and 68.71 (range 55-85, SD 7.51), respectively (p>0.05 between all groups). Gender of patients (Male:Female) for each sample group were 7:23, 9:19, and 9:33, respectively. The KL grades of knees for each group were 15 G3/ 15 G4 for NT, 10 G3/ 18 G4 for PT, and 11 G3/31 G4 for CT (p>0.05 between all groups). The mechanical axis angles for NT, PT, and CT groups were 9.41 (SD 7.25), 10.2 (SD 4.03), and 9.99 (SD 5.7) degrees varus, respectively (p>0.05 between all groups). The gross ACL status for each knee, shown in the order of ‘normal’, ‘abnormal’, and ‘deficient’ ACL, was 11, 12, and 7 for NT, 5, 13, and 10 for PT, and 4, 20, and 18 for CT group (p<0.05 for NT vs. CT).
3.3.2. Flattening and increase in length was observed in osteoarthritic meniscus roots
MMPRs of NR samples matched previously published descriptions of normal roots, with relatively short, 3-dimensional insertions into the tibial plateau [52]. Prominent ‘shiny white fibers’, a group of posterior supplemental fibers continuous with the main, dense posterior root attachment, were also present (Figure 3.2) [52]. MMPRs were flat
47
in shape with loss of ‘shiny white fibers’ in the majority of the NT (17/30, 56.67%) and PT (28/28, 100%) samples compared to NR samples (Figure 3.1) [52]. As for the CT group, tear margins consisted of glistening, white amorphous tissue, rather than ligament bundles. All tear margins from the CT group showed flattening and tissue adhesion to the tibia. Lengthening of root was also observed for NT and PT groups, compared to the NR group. The mean length of root for NR, NT, and PT were 9.52mm (SD 0.62), 13.23mm (SD 3.81), and 14.49mm (SD 3.19), respectively (NR vs. PT, p=0.0057) (Figure 3.1, Figure 3.2).
Figure 3. 2 Gross specimen of normal cadaveric medial meniscus root. (A) Posterior view
(B) Superior view and (C) Inferior view. Note the oblique course of the root. (D) Normal enthesis microstructure of medial meniscus root in reference cadaveric specimen. (E) Meniscus root junction, (F) ligamentous root, Safranin-O staining, and (G) H&E staining x100. No fibrocartilage was seen in the ligamentous portion of the root. Arrow indicates root region. B; Bone, M; Meniscus, R; Root, UFC; Uncalcified fibrocartilage, TM; Tide mark, CFC; Calcified fibrocartilage, SB; Subchondral bone.
48
3.3.3. Histological degeneration increased in meniscus root tear groups
Normal histology of MMPR is represented in Figure 3.2. Normal histology of the root resembled that of normal ligament or tendon, with absence of fibrocartilage throughout the matrix except in the enthesis. In the NT group, cells showed increased roundness with round nucleus compared to NR group (Figure 3.3.A (j), Figure 3.2.G). Some separation of individual collagen fiber bundles was observed with overall intact architecture (Figure 3.3.A (d), (m)). Occasional cluster of vessels and mucin were seen within the root matrix (Figure 3.3.A (d), (g), (j)). In the PT group, chondrocyte-like cells were observed, with conspicuous lacunae (Figure 3.3.A (k)). Cellularity-wise, hyper-cellularity was observed with some hypo-cellular areas in the PT group samples (Figure 3.3.A (k)). Collagen architecture of PT samples was disorganized, with frequent intrasubstance tears and diminished polarization compared to NR group (Figure 3.3.A (b), (n), Figure 3.2.F). Increase in vasculature was observed, with capillary clusters present throughout the matrix compared to NR group (Figure 3.3.A (h), (k), Figure 3.2.G). Mucin confluent with disorganized collagen fibers was also observed throughout the matrix (Figure 3.3.A (e), (h), (k)). In the CT group, hypertrophic chondrocyte-like cells, often in clusters, dominated the cellular morphology (Figure 3.3.A (l)). Cellularity was decreased compared to PT group, with frequent acellular areas (Figure 3.3.A (l)). Number of capillaries was decreased, or mostly absent, compared to PT group (Figure 3.3.A (i), (l)). Collagen architecture was completely disorganized, with severely diminished polarization compared to NR group (Figure 3.3.A (o)). Ground substance was found throughout the sections (Figure 3.3.A (f)). Modified Bonar tendinopathy
49
scores for each group were 4 (1-6), 8.5 (6-12.5), and 13.5 (11.63-15.50), for NT, PT, and CT groups, respectively, showing higher degree of degeneration in that order (NT vs. PT, p=0.0274, PT vs. CT, p=0.0302, NT vs. CT, p<0.001) (Figure 3.3.B). Subscore analysis revealed increased degeneration in all parameters for CT and PT groups when compared to the NT group (NT vs. CT, p<0.001 for all parameters, NT vs. PT, p<0.05 for all parameters). Ground substance scores, in particular, increased in the order of NT, PT, and CT (NT vs. PT, p=0.0186, PT vs. CT, p=0.0161, NT vs. CT, p<0.001) (Figure 3.3.C). The respective intra- and inter-observer ICC of Bonar scoring system for this study were 0.73 and 0.84. Tenascin-C immunohistochemistry revealed similar results, with increased degenerative staining patterns in PT and CT compared to NT (Figure 3.4) [75].
50
Figure 3. 3 Histological analysis revealed increased degeneration in meniscus root tear groups. (A) Representative histological pictures of each group stained with Safranin-O, H&E
and Picrosirius Red. Note the increase in chondrocyte like cells and ground substance in the tear groups. Hypercellularity and increase in vasculature were observed in PT, where as hypocellularity and decrease in vasculature were observed in CT specimens. Collagen architecture was disorganized in PT and CT specimens. Magnifications are noted on the left side (B) Quantification of degeneration by modified Bonar scores showed increase in degeneration in the order of NT, PT, and CT. (C) Subscore analysis showed increase in all degeneration parameters for PT and CT, compared to NT. Asterisk legends are as follows; above NT (NT vs. PT), above PT (PT vs. CT), above CT (NT vs. CT).